Abstract
Background
Left ventricular (LV) dyssynchrony is related to adverse outcomes in systolic heart failure, but its prognostic importance in asymptomatic population is not known. Our objective was to assess the prognostic implications of LV mechanical dyssynchrony in a large multiethnic population before the occurrence of global LV dysfunction.
Methods and Results
A total of 1392 participants in the Multi‐Ethnic Study of Atherosclerosis (MESA; mean age: 64.7 years; 46% were women) with cardiac magnetic resonance imaging at baseline were followed for a median duration of 8.3 years. Harmonic phase imaging analysis was used to derive systolic circumferential strain. Greater standard deviation of time to peak systolic strain (SD‐TPS) indicates greater dyssynchrony. With SD‐TPS as a continuous variable, Cox proportional hazards analysis was used to assess hazards ratio after adjusting for demographics, cardiovascular risk factors, LV mass‐to‐volume ratio, and ejection fraction. Using the 75th percentile of SD‐TPS as a cutoff, Kaplan–Meier analysis was performed between 2 categorical groups for each gender. Higher values of dyssynchrony in women predicted major adverse cardiovascular events, defined as myocardial infarction, heart failure, stroke, and death (hazard ratio: 1.01 per 1‐ms increment in SD‐TPS, P=0.015), hard coronary events (hazard ratio: 1.05 per 1‐ms increment in SD‐TPS, P=0.026), and cerebrovascular events (hazard ratio: 1.03 per 1‐ms increment in SD‐TPS, P=0.013). In contrast, dyssynchrony in men was not predictive of events. Kaplan–Meier analyses in women revealed increased event occurrence in the higher dyssynchrony group, but this was not the case in men.
Conclusions
In an asymptomatic cohort, greater LV dyssynchrony determined by cardiac magnetic resonance imaging predicts adverse cardiovascular outcome in women but not in men.
Clinical Trial Registration
URL: http://clinicaltrials.gov. Unique identifier: NCT00005487.
Keywords: cardiac magnetic resonance imaging, cardiovascular events, left ventricular dyssynchrony, prognosis
Introduction
Conventionally, individual cardiovascular risk factors define the likelihood of cardiovascular adverse events. Left ventricular ejection fraction (LVEF), a measure of global systolic function, has been used to predict future cardiovascular events.1 LVEF, however, is an insensitive indicator of ventricular function and is influenced largely by ventricular geometry and hemodynamic conditions. Increasingly, various parameters have been identified to better risk‐stratify asymptomatic individuals over and above the traditional risk factors and LVEF.2–5 In the Multi‐Ethnic Study of Atherosclerosis (MESA) study, higher left ventricular (LV) mass,6 concentric remodeling defined as an increased LV mass‐to‐volume ratio (LVMR),6 the LV global function index,7 and decreased myocardial circumferential strain8 have been documented as risk predictors for adverse cardiac events.
Synchronous contraction of LV myocardium is imperative for optimal heart function. LV dyssynchrony as a measure of regional myocardial dysfunction is considered a marker of heart disease and has been linked to age, increased LV mass, and low regional myocardial perfusion.9 Previous studies in human and animal models have demonstrated that the mechanical behavioral heterogeneity of LV dyssynchrony is associated with changes in myocardial perfusion and LV remodeling causing further deterioration of chamber function.10–11 Improved clinical outcomes following reverse remodeling with cardiac resynchronization therapy in patients with systolic heart failure further strengthen this viewpoint.12 In addition, mechanical dyssynchrony in patients with cardiomyopathy has been associated with increased risk of ventricular arrythmogenesis by promoting electrical heterogeneity.13–15 Although the role of LV dyssynchrony in determining clinical outcome among patients with heart failure has been increasingly recognized, the ability of this parameter to predict major adverse cardiovascular events (MACE) in a large, asymptomatic, multiethnic population has not been studied.
There are clear differences between women and men in terms of cardiac structure, function, and myocardial remodeling in response to cardiovascular risk exposure.16–18 In addition, there is diversity between women and men in dyssynchrony induction and/or progression. In the MESA cohort, dyssynchrony is primarily associated with myocardial interstitial fibrosis in women, whereas in men, dyssynchrony is associated with replacement fibrosis (ie, scar) (R. Sharma, MD, and J.A.C. Lima, MD, unpublished data, 2014). Whether these sex differences in cardiac structure and function, as well as in the pathogenesis of myocardial mechanical dyssynchrony, have any bearing on risk prediction remain unexplored.
Cardiovascular magnetic resonance (CMR) has emerged as a comprehensive imaging modality in the analysis of cardiac anatomy and function. Tagged CMR imaging is a sensitive method for the detection of regional myocardial deformation, and the standard deviation of time to peak systolic circumferential strain (SD‐TPS) is the most commonly used index of LV dyssynchrony.9,19
Our goal was to study the prognostic implications of LV dyssynchrony measured by tagged CMR imaging in predicting MACE among asymptomatic women and men before the development of global LV dysfunction. We also evaluated risk prediction of LV dyssynchrony for cardiovascular events other than MACE, including hard coronary events, all coronary events, and cerebrovascular events.
Methods
Study Population
MESA is a prospective cohort study designed to examine the characteristics and underlying mechanisms of development and progression of subclinical cardiovascular disease in asymptomatic individuals. Overall, 6814 MESA participants aged 45 to 84 years from 4 different ethnic backgrounds (white, black, Hispanic, and Chinese) were enrolled. Individuals with known cardiovascular disease or prior cardiac symptoms were excluded. On enrolment, study participants underwent extensive baseline evaluation including clinical questionnaires, physical examinations, and laboratory tests at 6 participating centers in the United States. CMR with tagged imaging was performed in 1774 randomly selected individuals for the assessment of circumferential strain. Clinical characteristics of these subcohorts were comparable to the entire study population.8,20 Of these, 1392 participants who had more than 8 of 12 myocardial segments analyzable for time to peak systolic strain are included in the study. The study protocol was approved by the institutional review board at each participating site, and written informed consent was obtained from each participant.
Tagged CMR Studies
After the acquisition of scout and cine images, as per standard protocol, tagged CMR images were acquired using whole‐body 1.5‐T scanners with ECG‐triggered segmented K‐space fast spoiled gradient‐echo pulse sequence during breath hold. Three tagged short‐axis slices (base to apex) were obtained using spatial modulation of magnetization encoding gradients. The following protocol was used for tagged CMR study: field of view, 40 cm; slice thickness, 7 to 8 mm; repetition time, 6 ms; echo time, 3.0 ms; flip angle, 10° to 12°; phase‐encoding views, 128 with 6 phase‐encoding views per segment; temporal resolution, 21 to 40 ms; tag spacing, 7 mm.
Assessment of Left Ventricular Geometry
Using semiautomated Medis software (MASS 4.2), endocardial and epicardial borders were contoured at end diastole. The difference between the epicardial and endocardial areas for all slices was multiplied by slice thickness and section gap, the product of which was then multiplied by specific gravity of the myocardium to determine ventricular mass. The papillary muscle mass was excluded from the LV mass measurements. Concentric remodeling was defined using a parameter derived as ratio of LV mass to end‐diastolic LV volume.6,21–22
Left Ventricular Dyssynchrony Analysis
Short‐axis slices at 3 levels (basal, midventricular, and apex) and 4 regions (septum, anterior, posterior, and lateral) were analyzed using harmonic phase imaging (HARP 1.15; Diagnosoft). This method allows fast assessment of myocardial strain during a cardiac cycle.23 Time intervals from end diastole to peak systolic circumferential strain were measured. As described by Rosen et al,9 the SD‐TPS in 12 segments (3 slices×4 regions) was used as the index of myocardial dyssynchrony (Figure 1). Greater values of SD‐TPS were interpreted as reflecting greater dyssynchrony. This parameter is equivalent to the measure obtained by the echocardiography method described by Yu et al,24 Maximal time difference (between earliest and latest segmental peak strain) was also considered as a secondary parameter of LV dyssynchrony.
Figure 1.

Tagged cardiac magnetic resonance study with a sample representation of circumferential strain curve. The x‐axis in the strain curve represents time in milliseconds.
Segments without well‐defined peak circumferential strain due to significant noise were considered as not interpretable. Results of 382 participants (21%) were excluded because of poor‐quality or missing slice acquisition or registration. Overall, 1392 studies having more than 8 segments analyzable for time to peak systolic strain were included in the analyses.
Clinical Follow‐up and Event Monitoring
Participants were followed for a median duration of 8.3 years (interquartile range: 7.5 to 8.6 years) from their baseline examination. In addition to the scheduled MESA examination, a telephone interviewer contacted each participant or representative by telephone every 9 to 12 months to inquire about all cardiovascular outpatient diagnoses and procedures, interim hospital admissions, and deaths. To verify self‐reported diagnoses, copies of all death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses were requested. Next‐of‐kin interviews for out‐of‐hospital cardiovascular deaths were performed. Medical records were successfully acquired from 99% of hospitalized cardiovascular events and 97% of outpatient diagnostic visits.
Adjudication of Events
Reviewers classified myocardial infarction as definite, probable, or absent based primarily on combinations of symptoms (eg, chest pain), ECG abnormalities, and cardiac biomarker levels. Coronary heart disease death was classified as present or absent based on hospital records and interviews with families. Definite fatal coronary heart disease required myocardial infarction within 28 days of death, chest pain within the 72 hours before death, or a history of coronary heart disease and the absence of a known nonatherosclerotic or noncardiac cause of death. Adjudicators graded angina, based on their clinical judgment, as definite, probable, or absent. Definite and probable angina required clear documentation of chest pain or anginal equivalent. Definite angina also required objective evidence of reversible myocardial ischemia or obstructive coronary artery disease (eg, positive stress test or ≥70% coronary artery obstruction). Stroke was defined as documented focal neurologic deficit lasting 24 hours or until death. When neurological deficits persisted for <24 hours, clinically relevant lesion documentation on brain imaging was required. Patients with focal neurological deficits secondary to brain trauma, tumor, infection, or other nonvascular cause were excluded. Reviewers classified congestive heart failure (CHF) as definite or probable based on CHF symptoms such as shortness of breath or edema. In addition to symptoms, probable CHF required CHF diagnosed by a physician and patient receiving medical treatment for CHF. Definite CHF required 1 objective criterion or more, such as pulmonary edema or congestion by chest x‐ray, dilated ventricle or poor LV systolic function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction denoting increased LV filling pressure. The documented LVEF at the diagnosis of CHF was obtained from medical records. Participants not meeting any criteria, including only a physician's diagnosis of CHF without any other evidence, were considered as having no CHF.
Two independent physicians from the MESA events committee reviewed all medical records for end point classification and assignment of incidence dates. The reviewers were blinded to the MESA CMR results and used prespecified criteria. Disagreements between reviewing physicians for event classification were adjudicated by members of the events committee. If differences persisted, the full events committee made the final classification.
Primary outcome was defined as MACE including myocardial infarction, definite heart failure, stroke, and all‐cause death. Secondary outcomes were defined as (1) hard coronary events classified as myocardial infarction, resuscitated cardiac arrest, and death from coronary disease; (2) composite of all coronary events, additionally including definite angina and probable angina followed by revascularization; and (3) cerebrovascular events including both fatal and nonfatal stroke and transient ischemic attack.
Statistical Analysis
Baseline participant characteristics and demographics are presented as mean±SD or percentage as appropriate. SD‐TPS as a measure of dyssynchrony was used as an independent variable. Separate analysis was performed for men and women. Using SD‐TPS as a continuous variable, Cox proportional hazard regression was performed to evaluate the probability distributions from the time of magnetic resonance imaging scan to cardiovascular or cerebrovascular events. Adjustment covariates were age, race, systolic blood pressure, antihypertensive medication, smoking status, diabetes mellitus, heart rate, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, body mass index, level of education, C‐reactive protein, LVMR, and LVEF. Similar analysis was performed for maximal time difference as an independent variable. To avoid “overfitting” of the models, parsimonious Cox proportional hazard analyses were also performed using the enter method and forward stepwise regression method with a P value of 0.05 as a cutoff. The Shapiro‐Wilk test for normalcy distribution revealed a right skewed distribution of dyssynchrony parameter SD‐TPS (P<0.001) consistent with the inclusion of “healthy population” for enrollment in the study. Consequently, the 75th percentile of SD‐TPS for the full study cohort was used instead of mean+2SD as a cutoff for dyssynchrony. The Kaplan–Meier estimates for time‐to‐event distributions were computed between the 2 SD‐TPS groups for each sex. The log‐rank test was used to evaluate the difference of the time‐to‐event distributions between the 2 groups.
To assess whether LV dyssynchrony as a marker of regional myocardial dysfunction is related to LV remodeling, multivariable linear regression analysis was performed with LVMR as an independent variable and the natural log‐transformed SD‐TPS as a dependent variable, adjusting for age, sex, race, and heart rate. The 2 variables were used as continuous variables to assess a concentric remodeling–dyssynchrony relationship as a continuum of a pathophysiological process. Differences of the basic characteristics between the 2 sexes were evaluated using Student t tests for continuous variables and the chi‐square tests for categorical variables. The 0.05 significance level was used for all hypothesis tests, and all t tests were 2‐sided. The statistical computations were performed using the STATA software package version 12.0 (StataCorp). The authors had full access to the data and take full responsibility for its integrity.
Results
Baseline Demographics and Participant Characteristics
The entire subcohort of 1392 MESA participants (women: 46.0%) with magnetic resonance imaging tagging studies obtained at study onset was analyzed. The mean ages of women (64.7 years) and men (64.3 years) were comparable. Compared with men, women had a lower prevalence of impaired fasting glucose and diabetes, lower diastolic blood pressure, and higher high‐density lipoprotein cholesterol. Men had lower magnetic resonance imaging–determined LVEF and higher end‐diastolic mass, end‐systolic and diastolic volumes, and LVMR. Detailed distribution and characteristics of these parameters are presented in Table 1. Mean times to peak systolic strain among women and men were 361.34±73.93 ms and 354.24±74.22 ms (P=0.074), respectively, whereas SD‐TPS values were 92.41±27.81 ms (median: 92.15 [interquartile range: 70.76 to 109.84 ms]) and 84.96±26.89 ms (median: 83.49 [interquartile range: 65.39 to 101.59]) (P<0.001), respectively. Maximal time difference values among women and men were 289.95±88.22 ms (median: 280.00 [interquartile range: 210.00 to 350.00 ms]) and 267.46±87.28 ms (median: 245 ms [interquartile range: 210.00 to 315.00 ms]), respectively.
Table 1.
Participants Demographics and Characteristics
| Parameters | Female (n=640) | Male (n=752) |
|---|---|---|
| Age, y | 64.71±9.61 | 64.33±9.81 |
| Race, % | ||
| White | 29.69 | 26.80 |
| Chinese | 15.78 | 13.87 |
| Black | 26.88 | 27.07 |
| Hispanic | 27.66 | 32.40 |
| Diabetes mellitus, %* | ||
| Normal | 73.75 | 65.47 |
| Impaired fasting glucose | 14.69 | 20.13 |
| Untreated | 2.03 | 4.13 |
| Treated | 9.22 | 10.40 |
| Heart rate, bpm* | 64.08±8.98 | 61.60±9.79 |
| Systolic blood pressure, mm Hg | 127.32±22.44 | 126.75±18.87 |
| Diastolic blood pressure, mm Hg* | 68.48±10.03 | 74.84±9.21 |
| Antihypertensive medication, % | 43.12 | 35.63 |
| Body mass index, kg/m2* | 28.16±5.34 | 27.22±4.03 |
| LDL‐cholesterol, mg/dL | 118.74±31.95 | 117.56±30.34 |
| HDL‐cholesterol, mg/dL* | 55.85±14.86 | 45.72±11.89 |
| eGFR, mL/min per 1.73 m2* | 79.31±17.99 | 81.74±18.13 |
| Smoking, %* | ||
| Never | 65.16 | 43.73 |
| Former | 25.94 | 42.80 |
| Current | 8.44 | 13.33 |
| C‐reactive protein, mg/L* | 4.31±5.74 | 2.71±5.29 |
| QRS duration, ms | 88.76±11.70 | 96.99±14.74 |
| Global parameters | ||
| LV ejection fraction, %* | 71.65±6.49 | 66.44±7.85 |
| LV mass indexed to BSA, g/m2* | 70.98±11.92 | 86.37±17.68 |
| LV end‐diastolic volume, mL/m2*,* | 64.04±10.73 | 70.24±15.74 |
| LV end‐systolic volume, mL/m2*,* | 18.32±6.04 | 23.88±9.57 |
| LVMR, g/mL* | 1.12±0.21 | 1.26±0.26 |
BSA indicates body surface area; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LV, left ventricle; LVMR, LV mass‐to‐volume ratio.
P<0.05 for test to assess difference of mean or proportion.
Values indexed to BSA.
There were 52 MACE occurrences in women and 131 in men. Among women and men, the numbers of hard cardiac events were 11 and 42, respectively; for all cardiac events, they were 17 and 62; and for cerebrovascular events, including fatal or nonfatal stroke and transient ischemic attack, they were 17 and 42, respectively.
LV Dyssynchrony and Hazard Analyses
After adjusting for covariates, Cox proportional hazard analysis revealed a hazard ratio (HR) of 1.014 (per 1‐ms increment in SD‐TPS, P=0.015) in women for MACE. In contrast, SD‐TPS did not predict MACE in men (P=0.633). Similarly, the HR for hard cardiac events among women was 1.057 (per 1‐ms increment in SD‐TPS, P=0.026) in contrast to men (P=0.307). HR for all cardiac events showed a trend in women with an HR of 1.021 (per 1‐ms increment in SD‐TPS, P=0.108) but no relation in men (P=0.191). For cerebrovascular events, the HR in women was 1.031 (per 1‐ms increment in SD‐TPS, P=0.013). This relationship was not significant in men (P=0.989) (Tables 2 and 3). For all‐cause mortality, unadjusted HRs in women and men were 1.013 (P=0.035) and 1.005 (P=0.183), respectively. After adjustment for covariates, there was attenuation of associations for both women (P=0.250) and men (P=0.670), with loss of statistical significance. HRs of MACE for individual covariates are shown in the Tables 4 and 5. Results of the parsimonious models had similar statistical inferences and are presented in Table 6.
Table 2.
Unadjusted Hazard Ratios of Adverse Outcomes Among Women and Men
| Parameters | Women | Men | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | LR | HR (95% CI) | P Value | LR | |
| MACE | 1.017 (1.007 to 1.026) | <0.001 | 12.45 | 1.001 (0.995 to 1.008) | 0.616 | 0.25 |
| Hard CHD | 1.027 (1.006 to 1.048) | 0.008 | 6.77 | 0.996 (0.985 to 1.008) | 0.578 | 0.31 |
| All CHD | 1.010 (1.000 to 1.033) | 0.050 | 3.75 | 0.997 (0.988 to 1.007) | 0.651 | 0.21 |
| CVA | 1.026 (1.009 to 1.042) | 0.002 | 9.48 | 1.002 (0.986 to 1.018) | 0.767 | 0.09 |
CVA indicates cerebrovascular events; CHD, coronary heart disease; LR, likelihood ratio; MACE, major adverse cardiovascular events.
Table 3.
Adjusted Hazard Ratios of Adverse Outcomes Among Women and Men
| Parameters | Women | Men | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | LR | HR (95% CI) | P Value | LR | |
| MACE | 1.014 (1.0002 to 1.026) | 0.015 | 85.20 | 0.998 (0.991 to 1.005) | 0.633 | 97.94 |
| Hard CHD | 1.057 (1.006 to 1.110) | 0.026 | 49.64 | 0.993 (0.980 to 1.006) | 0.307 | 43.94 |
| All CHD | 1.021 (0.995 to 1.049) | 0.108 | 54.80 | 0.991 (0.983 to 1.003) | 0.191 | 59.84 |
| CVA | 1.031 (1.006 to 1.056) | 0.013 | 41.45 | 1.0001 (0.983 to 1.017) | 0.989 | 34.85 |
HR represents increased risk of event per 1‐ms increment in standard deviation of time to peak systolic circumferential strain. Model adjusted for age, race, systolic blood pressure, antihypertensive medication, smoking status, diabetes mellitus, heart rate, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, body mass index, socioeconomic index as level of education, C‐reactive protein, LV mass‐to‐volume ratio, and LV ejection fraction. CHD indicates coronary heart disease; CI, confidence interval; CVA, cerebrovascular event; HR, hazard ratio; LR, likelihood ratio; MACE, major adverse cardiovascular event.
Table 4.
Cox Proportional Hazard Model for Major Adverse Cardiovascular Events in Women, Using Multivariable Analyses With Enter Method and Forward Stepwise Regression
| Parameters | Univariable Analysis | Multivariable Analysis (Enter Method) | Multivariable Analysis (Stepwise) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Participant demographics | ||||||
| Age, per year | 1.100 (1.06 to 1.13) | <0.01 | 1.094 (1.053 to 1.137) | <0.001 | 1.102 (1.06 to 1.145) | <0.001 |
| Ethnicity* | ||||||
| Black | 1.244 (0.584 to 3.647) | 0.57 | ||||
| Hispanic | 1.08 (0.50 to 2.33) | 0.84 | ||||
| Chinese | 1.775 (0.809 to 3.891) | 0.15 | ||||
| Education, <8th grade | 2.205 (1.177 to 4.131) | 0.01 | 2.205 (1.177 to 4.131) | 0.014 | 2.191 (1.102 to 4.354) | 0.025 |
| Education, graduate | 0.866 (0.370 to 2.029) | 0.74 | ||||
| Cardiovascular risk factors | ||||||
| Diabetes, treated and untreated | 1.78 (0.82 to 3.87) | 0.14 | ||||
| SBP, per mm Hg | 1.02 (1.01 to 1.03) | <0.01 | 1.006 (0.993 to 1.019) | 0.349 | ||
| Body mass index, kg/m2 | 1.00 (0.95 to 1.06) | 0.88 | ||||
| LDL cholesterol, mg/dL | 1.00 (0.99 to 1.01) | 0.83 | ||||
| HDL cholesterol, mg/dL | 0.99 (0.97 to 1.01) | 0.25 | ||||
| Heart rate, per bpm | 1.01 (0.98 to 1.04) | 0.66 | 1.015 (0.984 to 1.048) | 0.331 | 1.011 (0.98 to 1.043) | 0.473 |
| Current smoking | 1.65 (0.73 to 3.72) | 0.23 | 4.973 (2.097 to 11.794) | <0.001 | 5.054 (2.166 to 11.79) | <0.001 |
| Antihypertensive medication | 3.14 (1.74 to 5.65) | <0.01 | 2.258 (1.169 to 4.362) | 0.015 | 2.474 (1.293 to 4.734) | 0.006 |
| C‐reactive protein, per mg/L | 1.04 (1.02 to 1.05) | <0.01 | 1.039 (1.009 to 1.07) | 0.011 | ||
| CMR LV parameters | ||||||
| LV end‐diastolic mass, g | 1.00 (0.99 to 1.01) | 0.55 | ||||
| SD‐TPS | 1.017 (1.007 to 1.026) | <0.01 | 1.011 (1.000 to 1.022) | 0.041 | 1.013 (1.002 to 1.024) | 0.019 |
CI indicates confidence interval; CMR, cardiac magnetic resonance; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein; LV, left ventricular; SBP, systolic blood pressure; SD‐TPS, standard deviation of time to peak systolic circumferential strain.
White is the reference ethnicity.
Table 5.
Cox Proportional Hazard Model for Major Adverse Cardiovascular Events in Men, Using Multivariable Analyses With Enter Method and Forward Stepwise Regression
| Parameters | Univariate Analysis | Multivariable Analysis (Enter Method) | Multivariable Analysis (Stepwise) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Participant demographics | ||||||
| Age, per year | 1.05 (1.03 to 1.07) | <0.01 | 1.063 (1.042 to 1.087) | <0.001 | 1.072 (1.049 to 1.095) | <0.001 |
| Ethnicity* | ||||||
| Black | 1.47 (0.92 to 2.32) | 0.10 | ||||
| Hispanic | 1.35 (0.86 to 2.13) | 0.18 | ||||
| Chinese | 0.49 (0.22 to 1.06) | 0.07 | ||||
| Education, <8th grade | 0.800 (0.442 to 1.449) | 0.46 | ||||
| Education, graduate | 0.378 (0.208 to 0.684) | <0.01 | 0.521 (0.283 to 0.959) | 0.036 | 0.493 (0.27 to 0.899) | 0.021 |
| Cardiovascular risk factors | ||||||
| Diabetes, treated and untreated | 1.85 (1.21 to 2.82) | <0.01 | 1.034 (0.643 to 1.663) | 0.888 | ||
| SBP, per mm Hg | 1.02 (1.01 to 1.03) | <0.01 | 1.007 (0.997 to 1.017) | 0.131 | ||
| Body mass index, kg/m2 | 1.04 (1.00 to 1.08) | 0.08 | ||||
| LDL cholesterol, mg/dL | 1.00 (0.99 to 1.00) | 0.23 | ||||
| HDL cholesterol, mg/dL | 1.00 (0.98 to 1.01) | 0.62 | ||||
| Heart rate, per bpm | 1.02 (1.01 to 1.04) | <0.01 | 1.028 (1.009 to 1.047) | 0.003 | 1.027 (1.009 to 1.046) | 0.003 |
| Current smoking | 2.08 (1.29 to 3.38) | <0.01 | 1.587 (0.986 to 2.553) | 0.057 | 1.684 (1.055 to 2.687) | 0.029 |
| Antihypertensive medication | 1.44 (1.02 to 2.03) | 0.04 | 1.014 (0.692 to 1.486) | 0.940 | ||
| C‐reactive protein, per mg/L | 1.04 (1.03 to 1.06) | <0.01 | 1.015 (0.995 to 1.036) | 0.139 | 1.021 (1.001 to 1.041) | 0.040 |
| CMR LV parameters | ||||||
| LV end‐diastolic mass, g | 1.008 (1.004 to 1.012) | <0.01 | 1.017 (1.007 to 1.026) | <0.001 | 1.01 (1.006 to 1.014) | <0.001 |
| SD‐TPS, ms* | 1.001 (0.995 to 1.008) | 0.62 | 1.000 (0.993 to 1.007) | 0.974 | ||
CI indicates confidence interval; CMR, cardiac magnetic resonance; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein; LV, left ventricular; SBP, systolic blood pressure; SD‐TPS, standard deviation of time to peak systolic circumferential strain.
White is the reference ethnicity.
SD‐TPS for stepwise analyses was not statistically significant.
Table 6.
Parsimonious Cox Proportional Hazard Analyses for Women and Men Using Enter Method and Forward Stepwise Regression Models
| Parameters | Univariable | Multivariable (Enter Method) | Multivariable (Stepwise)* | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Women | ||||||
| MACE | 1.017 (1.007 to 1.026) | 0.000 | 1.011 (1.00 to 1.022) | 0.041 | 1.013 (1.002 to 1.024) | 0.019 |
| Hard CHD | 1.027 (1.006 to 1.048) | 0.008 | 1.026 (1.003 to 1.050) | 0.024 | 1.046 (1.017 to 1.077) | 0.002 |
| All CHD | 1.010 (1.000 to 1.033) | 0.050 | 1.017 (0.999 to 1.035) | 0.061 | 1.020 (1.001 to 1.039) | 0.031 |
| CVA | 1.026 (1.009 to 1.042) | 0.002 | 1.025 (1.006 to 1.043) | 0.009 | 1.029 (1.009 to 1.049) | 0.003 |
| Men | ||||||
| MACE | 1.001 (0.995 to 1.008) | 0.616 | 1.00 (0.993 to 1.007) | 0.974 | N/A* | |
| Hard CHD | 0.996 (0.985 to 1.008) | 0.578 | 0.994 (0.982 to 1.006) | 0.397 | N/A* | |
| All CHD | 0.997 (0.988 to 1.007) | 0.651 | 0.994 (0.984 to 1.004) | 0.278 | N/A* | |
| CVA | 1.002 (0.986 to 1.018) | 0.767 | 1.001 (0.985 to 1.018) | 0.841 | N/A* | |
HR represents increased risk of event per 1‐ms increment in standard deviation of time to peak systolic circumferential strain. Covariables were age, race, systolic blood pressure, antihypertensive medication, smoking status, diabetes mellitus, heart rate, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, body mass index, socioeconomic index as level of education, C‐reactive protein, and end‐diastolic LV mass. CI indicates confidence interval; CHD, coronary heart disease; CVA, cerebrovascular event; HR, hazard ratio; MACE, major adverse cardiovascular event; SD‐TPS, standard deviation of time to peak systolic circumferential strain.
Forward stepwise regression analyses in men were not statistically significant.
Kaplan–Meier analyses revealed increased occurrence of MACE in women with greater dyssynchrony (P=0.003) (Figure 2). Similarly, higher event rates in women were noted for hard coronary events (P=0.009) and cerebrovascular events (P=0.023), with a trend toward all coronary events (P=0.093). In contrast, similar relationships were not significant in men, with respective P values of 0.284, 0.249, 0.713, and 0.294 for the above‐mentioned outcomes. Kaplan–Meier analyses for secondary outcomes are represented in Figure 3.
Figure 2.
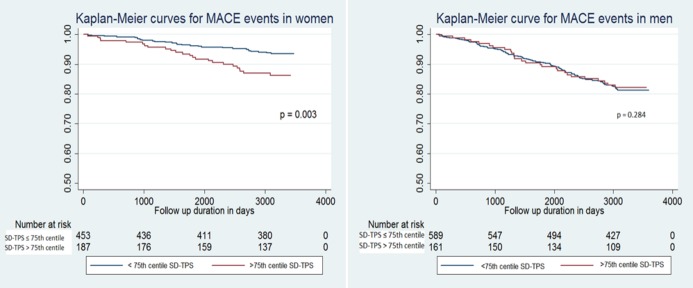
Kaplan–Meier curves for major adverse cardiac event occurrence in women and men. Group 1: ≤75th percentile of SD‐TPS; Group 2: >75th percentile of SD‐TPS. MACE indicates major adverse cardiovascular events; SD‐TPS, standard deviation of time to peak systolic circumferential strain.
Figure 3.
Kaplan–Meier curves for secondary events outcome in women and men. Group 1: ≤75th percentile of standard deviation of time to peak systolic circumferential strain; Group 2: >75th percentile of standard deviation of time to peak systolic circumferential strain.
Results of Cox proportional hazard analyses using maximal time difference as a study variable are presented in Tables 7 and 8. In univariable analyses, maximal time difference was predictive of adverse events in women; however, the relationship was not consistent in multivariable analyses. Hence, maximal time difference performed worse than SD‐TPS in predicting events. Maximal time difference derived as a difference between earliest and latest segmental peak strain is intuitively a “noisier” parameter of LV dyssynchrony than SD‐TPS. Moreover, maximal time difference is suboptimal in representing dyssynchronous contraction of LV myocardium and its improved response to resynchronization therapy.24
Table 7.
Cox Proportional Hazard Analyses for Maximal Time Difference Among Segments Predicting Adverse Outcomes in Women
| Parameters | Women | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| MACE | 1.005 (1.002 to 1.008) | 0.001 | 1.003 (0.999 to 1.007) | 0.084 |
| Hard CHD | 1.007 (1.001 to 1.014) | 0.021 | 1.009 (0.995 to 1.023) | 0.171 |
| All CHD | 1.005 (0.999 to 1.010) | 0.057 | 1.006 (0.997 to 1.015) | 0.144 |
| CVA | 1.007 (1.001 to 1.012) | 0.008 | 1.004 (0.997 to 1.011) | 0.214 |
CHD indicates coronary heart disease; CI, confidence interval; CVA, cerebrovascular event; HR, hazard ratio; MACE, major adverse cardiovascular event.
Table 8.
Cox Proportional Hazard Analyses for Maximal Time Difference Among Segments Predicting Adverse Outcomes in Men
| Parameters | Men | |||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| MACE | 0.999 (0.997 to 1.001) | 0.856 | 0.999 (0.996 to 1.001) | 0.415 |
| Hard CHD | 0.997 (0.994 to 1.001) | 0.234 | 0.996 (0.992 to 1.0007) | 0.114 |
| All CHD | 0.998 (0.995 to 1.0009) | 0.190 | 0.996 (0.993 to 0.999) | 0.046 |
| CVA | 0.999 (0.995 to 1.004) | 0.976 | 1.001 (0.995 to 1.006) | 0.715 |
HR represents increased risk of event per 1‐ms increment in maximal time difference between segmental times to peak strain. For adjustment parameters, please see Table 3. CI indicates confidence interval; CHD, coronary heart disease, CVA, cerebrovascular event; HR, hazard ratio; MACE, major adverse cardiovascular event.
Association of LV Mass‐to‐Volume Ratio and LV Dyssynchrony
Multivariable regression analysis revealed the association of concentric remodeling (LVMR) with log‐transformed SD‐TPS as the dyssynchrony index (β‐coefficient: 0.145 log [ms] per unit increment in g/mL, P<0.001). The added variable plot is presented in Figure 4. Sex‐based analysis revealed a greater association of LVMR with dyssynchrony among men (β‐coefficient: 0.154 log [ms] per g/mL, P=0.001) than among women (β‐coefficient: 0.123 log [ms] per g/mL, P=0.057) (Table 9).
Figure 4.

AV plot representing relationship of LV dyssynchrony (SD‐TPS) and concentric remodeling defined as LVMR. *Represents logarithmic transformation of SD‐TPS. The AV plot represents the graphical relationship between the “fitted values” of the study variables after multivariable linear regression. Adjustment parameters were age, sex, race, and heart rate. AV indicates added variable; coef, β‐coefficient; LV, left ventricle; LVMR, left ventricular mass to volume ratio; SD‐TPS, standard deviation of time to peak systolic circumferential strain; t, t statistics (derived as β‐coefficient/SE) of multivariable linear regression analysis.
Table 9.
Association of Concentric Remodeling (LVMR) With Log‐Transformed SD‐TPS as a Dyssynchrony Parameter
| Parameters | Univariable Analyses | Multivariable Analyses | ||
|---|---|---|---|---|
| β‐Coefficient (95% CI) | P Value | β‐Coefficient (95% CI) | P Value | |
| All participants | 0.092 (0.021 to 0.163) | 0.011 | 0.145 (0.070 to 0.220) | <0.001 |
| Women | 0.140 (0.019 to 0.261) | 0.023 | 0.123 (−0.003 to 0.251) | 0.057 |
| Men | 0.152 (0.061 to 0.244) | 0.001 | 0.154 (0.061 to 0.248) | 0.001 |
Unit of β‐coefficient log [ms] per unit increment in g/mL. Multivariable model adjusted for age, race, and heart rate. CI indicates confidence interval; LVMR, left ventricular mass‐to‐volume ratio; SD‐TPS, standard deviation of time to peak systolic circumferential strain.
Discussion
MESA is the first study to utilize cardiac CMR tagging as a sensitive and accurate tool to determine the association of myocardial dyssynchrony with cardiovascular events in a general multiethnic population. There are several conclusions from this analysis. First, LV dyssynchrony confers an elevated risk of incident cardiovascular and cerebrovascular events in a large multiethnic cohort of asymptomatic women. In contrast, this relationship is not significant in men. Second, concentric remodeling (defined as LVMR) correlates with greater LV dyssynchrony, reflecting a greater extent of uncoordinated contraction as the LV remodels and changes geometry in response to pathological stimulation. Importantly, the latter relationship is more prominent in men than in women.
There is diversity between women and men in terms of myocardial response to aging and specific cardiovascular risk exposures.16–17 During the cardiac aging process, women are more likely to preserve myocardial mass and structure, whereas men tend to have greater myocyte cell loss and cellular reactive hypertrophy. These sex‐related differences may also reflect different mechanisms of dyssynchrony induction among the two gender groups.17 Liu et al have noted that women have greater myocardial interstitial fibrosis.18 In the MESA cohort, myocardial interstitial fibrosis in women was significantly associated with LV dyssynchrony parameters independent of myocardial scar or the presence of left‐bundle branch block. Such a relationship was not significant in men, for whom only replacement fibrosis (ie, scar) was associated with dyssynchrony (R.K. Sharma, MD, and J.A.C. Lima, MD, unpublished data, 2014). In the MESA cohort, women had greater interstitial fibrosis than men,18 with correspondingly higher values of SD‐TPS. This finding emphasizes sex differences in the myocardial response to aging and hemodynamic stress. Interestingly, we found that LV dyssynchrony in this asymptomatic cohort was predictive of adverse events only in women. Whether dyssynchrony itself constitutes a pathophysiological mechanism in the cardiovascular disease process or instead signifies a surrogate marker of LV remodeling remains to be defined. Because LV dyssynchrony correlates with myocardial interstitial fibrosis in women and is associated with LVMR, it appears that LV dyssynchrony is a surrogate marker of concentric remodeling and interstitial fibrosis developed in response to increased cardiovascular risk burden. This may explain worse cardiovascular outcome with incremental worsening in the dyssynchrony parameter. This may particularly be relevant for cerebrovascular events.
LV remodeling in response to cardiovascular risk exposure has been related to adverse cardiovascular outcome. Data from the MESA study have previously described concentric remodeling (LVMR per 10% increment) as a predictor of adverse coronary heart disease events with an unadjusted HR of 5.5 (P<0.0001) and an HR of 2.1 (P=0.02) after adjusting for participant demographics and cardiovascular risk factors.6 In addition, other studies have demonstrated the role of LVMR in predicting adverse cardiovascular outcomes.25–28 We found significant correlations of LVMR with the dyssynchrony index. Overall, LVEF in MESA participants at baseline was within normal limits. This may implicate LV dyssynchrony as an incipient marker of LV dysfunction representing subclinical impairment of regional ventricular performance in response to cumulative cardiovascular risk exposure. Moreover, we found that the association between remodeling and dyssynchrony is more prominent in men. This relationship suggests that dyssynchrony in men depends on the development of greater remodeling compared with women.
Methodological Considerations
Our study demonstrates the prognostic significance of subclinical myocardial dyssynchrony and its associated difference by sex in a large multiethnic population of women and men; which to our knowledge is unique. This relationship was studied using tagged CMR, which is a robust measure of myocardial deformation with a high degree of reproducibility.29 In addition, all study participants had close follow‐up and explicit event classification and monitoring as part of the MESA study.
The number of adverse events in the MESA cohort is relatively small. Consequently, reliable evaluation of each type of cardiovascular event will require extended follow‐up in this cohort. Because MESA participants had no known cardiovascular disease at baseline, the participants undergoing CMR examination at baseline represent a relatively healthy sample of the population. Thus, generalization of the study results is limited by selection and survivor bias. This may particularly be relevant for the male population included in this analysis because lower values of dyssynchrony were noticed in men compared with women. In addition, although this study describes the significance of LV dyssynchrony in association with cardiovascular events, a detailed explanation of the underlying mechanism involving event occurrences is beyond the scope of this paper.
Conclusion
Our study demonstrates that LV mechanical dyssynchrony is a prognostic marker of adverse cardiovascular and cerebrovascular events among asymptomatic women in a large, multiethnic cohort. This relationship was not observed in men. In addition, LV dyssynchrony was also noted to be significantly correlated with concentric remodeling of the left ventricle.
Sources of Funding
This research was supported by contracts N01‐HC‐95159 through N01‐HC95168 from the US National Heart, Lung, and Blood Institute. Bayer HealthCare provided Magnevist for the MESA cardiac magnetic resonance study.
Disclosures
R.K.S. is a recipient of a postdoctoral research grant by Unijules Life Sciences Ltd, India. Other authors have no conflict of interest to report.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Multi‐Ethnic Study of Atherosclerosis (MESA) for their valuable contributions. A full list of participating MESA investigators and institutions can be found online (http://www.mesa-nhlbi.org).
References
- 1.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999; 100:999-1008. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS, Ahn C, Kronzon I, Koenigsberg M. Congestive heart failure, coronary events and atherothrombotic brain infarction in elderly blacks and whites with systemic hypertension and with and without echocardiographic and electrocardiographic evidence of left ventricular hypertrophy. Am J Cardiol. 1991; 67:295-299. [DOI] [PubMed] [Google Scholar]
- 3.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2000; 35:1628-1637. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The framingham heart study. Ann Intern Med. 1989; 110:101-107. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990; 322:1561-1566. [DOI] [PubMed] [Google Scholar]
- 6.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008; 52:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO, Burke GL, Liu S, Liu K, Bluemke DA, Lima JA. Left ventricular global function index by magnetic resonance imaging–a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2013; 61:770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, Opdahl A, Almeida AL, Wu CO, Gomes AS, Bluemke DA, Lima JA. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2013; 34:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen BD, Fernandes VR, Nasir K, Helle‐Valle T, Jerosch‐Herold M, Bluemke DA, Lima JA. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009; 120:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990; 259:H300-H308. [DOI] [PubMed] [Google Scholar]
- 11.Prinzen FW, Cheriex EC, Delhaas T, van Oosterhout MF, Arts T, Wellens HJ, Reneman RS. Asymmetric thickness of the left ventricular wall resulting from asynchronous electric activation: a study in dogs with ventricular pacing and in patients with left bundle branch block. Am Heart J. 1995; 130:1045-1053. [DOI] [PubMed] [Google Scholar]
- 12.Linde C, Gold MR, Abraham WT, St John Sutton M, Ghio S, Cerkvenik J, Daubert C. Long‐term impact of cardiac resynchronization therapy in mild heart failure: 5‐year results from the resynchronization reverses remodeling in systolic left ventricular dysfunction (reverse) study. Eur Heart J. 2013; 34:2592-2599. [DOI] [PubMed] [Google Scholar]
- 13.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O'Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009; 119:1220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Andrea A, Caso P, Severino S, Scotto di Uccio F, Vigorito F, Ascione L, Scherillo M, Calabro R. Association between intraventricular myocardial systolic dyssynchrony and ventricular arrhythmias in patients with hypertrophic cardiomyopathy. Echocardiography. 2005; 22:571-578. [DOI] [PubMed] [Google Scholar]
- 15.Fauchier L, Marie O, Casset‐Senon D, Babuty D, Cosnay P, Fauchier JP. Ventricular dyssynchrony and risk markers of ventricular arrhythmias in nonischemic dilated cardiomyopathy: a study with phase analysis of angioscintigraphy. Pacing Clin Electrophysiol. 2003; 26:352-356. [DOI] [PubMed] [Google Scholar]
- 16.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003; 41:217-223. [DOI] [PubMed] [Google Scholar]
- 17.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995; 26:1068-1079. [DOI] [PubMed] [Google Scholar]
- 18.Liu CY, Chang Liu Y, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JA. Evaluation of age related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced t mapping in the multi‐ethnic study of atherosclerosis (MESA). J Am Coll Cardiol. 2013; 62:1280-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009; 11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881. [DOI] [PubMed] [Google Scholar]
- 21.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011; 58:1733-1740. [DOI] [PubMed] [Google Scholar]
- 22.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch‐Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, III, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2005; 112:984-991. [DOI] [PubMed] [Google Scholar]
- 23.Castillo E, Osman NF, Rosen BD, El‐Shehaby I, Pan L, Jerosch‐Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR‐tagging in a multi‐center study: interobserver and intraobserver agreement of fast strain analysis with harmonic phase (harp) mri. J Cardiovasc Magn Reson. 2005; 7:783-791. [DOI] [PubMed] [Google Scholar]
- 24.Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, Kong SL, Lin H, Zhang Y, Sanderson JE. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005; 45:677-684. [DOI] [PubMed] [Google Scholar]
- 25.Ghali JK, Liao Y, Cooper RS. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol. 1998; 31:1635-1640. [DOI] [PubMed] [Google Scholar]
- 26.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991; 114:345-352. [DOI] [PubMed] [Google Scholar]
- 27.Muiesan ML, Salvetti M, Monteduro C, Bonzi B, Paini A, Viola S, Poisa P, Rizzoni D, Castellano M, Agabiti‐Rosei E. Left ventricular concentric geometry during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension. 2004; 43:731-738. [DOI] [PubMed] [Google Scholar]
- 28.Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Cuccurullo F, Mezzetti A. Prognostic value of left ventricular concentric remodeling in uncomplicated mild hypertension. Am J Hypertens. 2004; 17:1035-1039. [DOI] [PubMed] [Google Scholar]
- 29.Donekal S, Ambale‐Venkatesh B, Berkowitz S, Wu CO, Choi EY, Fernandes V, Yan R, Harouni AA, Bluemke DA, Lima JA. Inter‐study reproducibility of cardiovascular magnetic resonance tagging. J Cardiovasc Magn Reson. 2013; 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]



