Abstract
Background
Clinical trials traditionally use time‐to‐first‐event analysis embedded within the composite endpoint of cardiovascular death (CVD), myocardial infarction (MI), or stroke. However, many patients have >1 event, and this approach may not reflect overall experience. We addressed this by analyzing all cardiovascular events in TRACER.
Methods and Results
TRACER randomized 12 944 patients with non‐ST‐segment elevation acute coronary syndromes to placebo or to protease‐activated receptor 1 antagonist vorapaxar with a median follow‐up of 502 days (interquartile range, 349 to 667). Analysis of vorapaxar's effect on recurrent CVD, MI, or stroke was prespecified using the Wei, Lin, and Weissfeld approach. Vorapaxar did not reduce the first occurrence of the primary endpoint of CVD, MI, stroke, revascularization, or rehospitalization for recurrent ischemia, but reduced the secondary composite endpoint of CVD, MI, or stroke (14.7% vorapaxar vs. 16.4% placebo; hazard ratio [HR], 0.89; 95% confidence interval [CI], 0.81 to 0.98; P=0.02; number needed to treat [NNT], 81). Recurrent secondary events occurred in 2.7% of patients. Vorapaxar reduced overall occurrences of ischemic events, first and subsequent (HR, 0.88; 95% CI, 0.80 to 0.98; P=0.02; NNT, 51). Also, there was a trend indicating that vorapaxar reduced the expanded endpoint, including revascularization and rehospitalization for recurrent ischemia (HR, 0.92; 95% CI, 0.84 to 1.01; P=0.09). Vorapaxar increased overall occurrences of moderate and severe Global Use of Strategies to Open Occluded Coronary Arteries bleeding (HR, 1.42; 95% CI, 1.21 to 1.66; P<0.001) and Thrombolysis in Myocardial Infarction clinically significant bleeding (HR, 1.550; 95% CI, 1.403 to 1.713; P<0.001).
Conclusions
Vorapaxar reduced overall occurrences of ischemic events, but increased bleeding. These exploratory findings broaden our understanding of vorapaxar's potential and expand our understanding of the value of capturing recurrent events.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique identifier: NCT00527943.
Keywords: acute coronary syndromes, recurrent events, vorapaxar
Introduction
Most clinical trials incorporate “time to first event” into their primary analyses and do not take into account the effect of therapy after the initial event. Therefore, the disease burden and effectiveness and safety of a therapy may not represent the totality of the patient experience. The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial randomized nearly 13 000 patients with non‐ST‐segment elevation acute coronary syndromes (NSTE ACS) to the protease‐activated receptor 1 antagonist vorapaxar or placebo. The study was terminated prematurely, after enrollment completion and the target endpoint was reached, following an unplanned, independent safety review of excess bleeding hazard. Randomization to vorapaxar was associated with no significant effect on the first occurrence of the primary composite endpoint of cardiovascular death (CVD), myocardial infarction (MI), stroke, recurrent ischemia with rehospitalization, or urgent coronary revascularization (hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.85 to 1.01; P=0.07). There was, however, a nominal reduction of the prespecified, secondary, time‐to‐event composite endpoint of CVD, MI, or stroke (HR, 0.89; 95% CI, 0.81 to 0.98; P=0.02).1–2
In the current report, we further explored the effect of randomized treatment with placebo or vorapaxar on overall event history of CVD, MI, or stroke. We also explored the effect of treatment on overall occurrences of bleeding events.
Methods
The TRACER trial's design,3 endpoint definitions, and primary results have been reported.2 In summary, this double‐blind, placebo‐controlled, event‐driven, international trial in 37 countries compared placebo with vorapaxar (40‐mg loading dose followed by 2.5 mg daily) in patients with NSTE ACS at high risk for ischemic events. Patients were planned to be followed for at least 1 year. All patients who experienced nonfatal events were recommended to continue the randomized treatment.
The TRACER study was approved by institutional review committees and all patients gave informed consent.
Statistical Analysis
We analyzed overall occurrences of the composite ischemic endpoints and composite bleeding endpoints as described above. As prespecified in the protocol, analyses of efficacy endpoints included all randomized subjects with events accrued from randomization to last follow‐up. Analyses of bleeding endpoints included subjects with at least 1 dose of randomized treatment with events accrued while on treatment.
Baseline characteristics were presented for patients who experienced none, 1, or multiple composite endpoints. To investigate the effect of vorapaxar on occurrences of events, we employed the stratified Cox model proposed by Wei, Lin, and Weissfeld (the WLW model).4 The WLW model allows us to consider the ordering of events as separate events (ie, strata) of interest, as well as the different types of events that may occur in the same patient (eg, MI or stroke). A subject is simultaneously included in the model for time to first event of interest, time to second event of interest, etc., up to the maximum number of events observed for a subject in a study. In the model for time to Kth event (K=1, 2, etc.), a subject's time starts at randomization and ends at either (1) the time when the subject's Kth event has occurred or (2) the time of death or last follow‐up. In the latter case, the subject would be censored. With this approach, we investigated the effect of vorapaxar on the first and each subsequent event as well as the common treatment effect across the entire event history. In addition, we estimated the number needed to treat (NNT) all events as well as the first event. HRs and corresponding CIs are reported.
Multivariable analyses were carried out to identify patients who were at risk of having >1 event. Baseline characteristics, randomized treatment, and interactions between the 2 treatment groups were included in the model (Appendices A and B). Factors were selected through forward, backward, and step‐wise selection processes. P values were not adjusted for multiple comparisons. Analyses were performed using SAS software (version 9.0; SAS Institute Inc., Cary, NC).
Results
The flow of patients in the TRACER trial and the occurrences of single and recurrent events are shown in Figure 1. The key secondary composite endpoint of CVD, MI, or stroke occurred in 822 of 6473 patients (12.7%) receiving vorapaxar versus 910 of 6471 patients (14.1%) receiving placebo (HR, 0.89; 95% CI, 0.81 to 0.98; P=0.02) during the follow‐up period of 502 days (interquartile range, 349 to 667).
Figure 1.
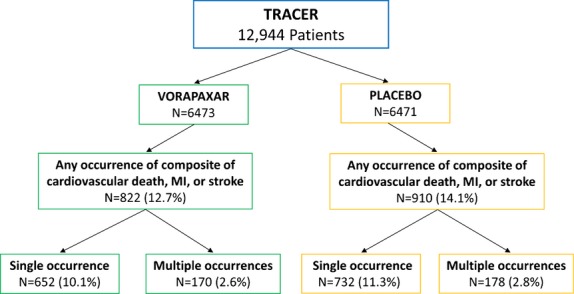
Patient flows and occurrences of ischnemic events. MI indicates myocardial infarction.
Single Occurrence
As noted in Table 1 and Figure 1, the number of patients with just 1 occurrence of the composite endpoint of CVD, MI, or stroke was 1384 (652 vorapaxar [10.1%] and 732 placebo [11.3%]; odds ratio, 0.8765; 95% CI, 0.7838 to 0.9803; P=0.03).
Table 1.
Baseline Characteristics for Patients With No Occurrence, a Single Occurrence, or Multiple Occurrences of Key Secondary Endpoints
| No Occurrence (n=11 212) | Single Occurrence (n=1384) | Multiple Occurrence (n=348) | |
|---|---|---|---|
| Age, y, mean (SD) | 64.1 (9.8) | 66.0 (10.4) | 68.5 (10.3) |
| Female, n (%) | 3127 (27.9) | 406 (29.3) | 99 (28.4) |
| Race (%) | |||
| White | 9589/11 181 (85.8) | 1156/1381 (83.7) | 294/347 (84.7) |
| Black | 256/11 181 (2.3) | 46/1381 (3.3) | 10/347 (2.9) |
| American Indian | 29/11 181 (0.3) | 5/1381 (0.4) | 1/347 (0.3) |
| Asian | 925/11 181 (8.3) | 105/1381 (7.6) | 26/347 (7.5) |
| Pacific Islander | 27/11 181 (0.2) | 4/1381 (0.3) | 1/347 (0.3) |
| Multiracial | 355/11 181 (3.2) | 65/1381 (4.7) | 15/347 (4.3) |
| Region (%) | |||
| North America | 2855/11 212 (25.5) | 425/1384 (30.7) | 124/348 (35.6) |
| Latin America | 709/11 212 (6.3) | 112/1384 (8.1) | 27/348 (7.8) |
| Europe I | 5142/11 212 (45.9) | 575/1384 (41.5) | 122/348 (35.1) |
| Europe II | 1319/11 212 (11.8) | 135/1384 (9.8) | 33/348 (9.5) |
| Asia | 822/11 212 (7.3) | 90/1384 (6.5) | 24/348 (6.9) |
| Australia/New Zealand | 365/11 212 (3.3) | 47/1384 (3.4) | 18/348 (5.2) |
| Body weight, kg, mean (SD) | 82.5 (17.7) | 82.5 (18.9) | 81.9 (18.7) |
| BMI*, mean (SD) | 28.5 (5.2) | 28.8 (5.7) | 28.8 (5.3) |
| Hypertension (%) | 7776/11 207 (69.4) | 1060/1384 (76.6) | 292/348 (83.9) |
| Hyperlipidemia (%) | 6877/11 204 (61.4) | 928/1384 (67.1) | 257/348 (73.9) |
| Diabetes mellitus (%) | 3319/11 206 (29.6) | 578/1384 (41.8) | 173/348 (49.7) |
| Previous stroke (%) | 427/11 207 (3.8) | 88/1384 (6.4) | 38/348 (10.9) |
| Previous myocardial infarction (%) | 3067/11 206 (27.4) | 536/1384 (38.7) | 188/348 (54.0) |
| Peripheral arterial vascular disease (%) | 692/11 205 (6.2) | 173/1384 (12.5) | 71/348 (20.4) |
| Past tobacco use (%) | 3590/11 205 (32.0) | 456/1384 (32.9) | 140/348 (40.2) |
| Current tobacco use (%) | 3106/11 205 (27.7) | 363/1384 (26.2) | 67/348 (19.3) |
| Percutaneous coronary intervention (%) | 2534/11 202 (22.6) | 421/1384 (30.4) | 135/348 (38.8) |
| Coronary artery bypass graft (%) | 1165/11 207 (10.4) | 256/1384 (18.5) | 122/348 (35.1) |
| Statin at enrollment (%) | 9399/11 212 (83.8) | 1169/1384 (84.5) | 304/348 (87.4) |
| Thienopyridine at enrollment (%) | 9788/11 212 (87.3) | 1212/1384 (87.6) | 307/348 (88.2) |
| Troponin or CK‐MB positive (%) | 10 397/11 133 (93.4) | 1322/1377 (96.0) | 331/347 (95.4) |
| Renal insufficiency <30 mL/min (%) | 124/10 622 (1.2) | 37/1306 (2.8) | 29/333 (8.7) |
| ST‐segment depression (%) | 3562/11 212 (31.8) | 485/1384 (35.0) | 152/348 (43.7) |
| ST‐segment elevation (%) | 637/11 212 (5.7) | 76/1384 (5.5) | 23/348 (6.6) |
| TIMI risk score ≥3 (%) | 11 149/11 212 (99.4) | 1380/1384 (99.7) | 348/348 (100.0) |
Data presented as n/N (%), unless otherwise indicated. CK‐MB indicates creatine kinase‐MB; TIMI, Thrombolysis in Myocardial Infarction.
Weight (kg)/height2 (m2).
Multiple Occurrences
As shown in Tables 1 and 2, patient characteristics associated with increased occurrences of CVD, MI, and stroke events were older age; having diabetes; history of hypertension (HTN), MI, or stroke; peripheral arterial vascular disease; impaired renal function; previous percutaneous coronary intervention and coronary artery bypass grafting; and taking thienopyridine or statins at baseline. Most factors associated with risk for first ischemic event were also associated with recurrent events. As shown in Table 3, there were more patients with HTN and more current smokers in the placebo group.
Table 2.
Baseline Characteristics Associated With Multiple Occurrences of CVD, MI, or Stroke
| HR (95% CI) | P Value | |
|---|---|---|
| Heart rate by 10/min | 1.079 (1.035 to 1.124) | 0.0003 |
| Age ≥75 years | 1.461 (1.258 to 1.697) | <0.0001 |
| Multirace | 1.417 (1.081 to 1.857) | 0.0115 |
| Region: North America | 1.298 (1.144 to 1.473) | <0.0001 |
| History of hypertension | 1.156 (1.012 to 1.321) | 0.0323 |
| History of diabetes mellitus | 1.378 (1.228 to 1.546) | <0.0001 |
| History of stroke | 1.266 (1.023 to 1.568) | 0.0302 |
| History of myocardial infarction | 1.377 (1.201 to 1.580) | <0.0001 |
| History of peripheral arterial vascular disease | 1.595 (1.359 to 1.872) | <0.0001 |
| History of percutaneous coronary intervention | 1.160 (1.012 to 1.330) | 0.0334 |
| History of coronary artery bypass grafting | 1.713 (1.479 to 1.983) | <0.0001 |
| Qualifying event: troponin or CK‐MB >upper limit of normal | 1.670 (1.254 to 2.224) | 0.0004 |
| ST‐segment elevation at enrollment | 1.254 (0.991 to 1.587) | 0.0598 |
| ST‐segment depression at enrollment | 1.384 (1.232 to 1.556) | <0.0001 |
| Renal insufficiency: CrCl <30 mL/min | 2.459 (1.818 to 3.325) | <0.0001 |
| Renal insufficiency: CrCl ≤30 to <60 mL/min | 1.382 (1.174 to 1.627) | 0.0001 |
| Killip scale I | 0.600 (0.500 to 0.722) | <0.0001 |
| Taking thienopyridine at enrollment | 1.191 (1.000 to 1.419) | 0.0505 |
| Taking statin at enrollment | 1.193 (1.027 to 1.384) | 0.0206 |
CI indicates confidence interval; CK‐MB, creatine kinase‐MB; CrCl, creatinine clearance; CVD, cardiovascular death; HR, hazard ratio; MI, myocardial infarction.
Table 3.
Baseline Characteristics According to Randomized Treatments for Patients Who Had Multiple Occurrences of CVD, MI, or Stroke
| Vorapaxar (n=170) | Placebo (n=178) | |
|---|---|---|
| Age, y, mean (SD) | 69.6 (10.3) | 67.6 (10.2) |
| Age ≥75 years (%) | 61/170 (35.9) | 52/178 (29.2) |
| Female (%) | 42/170 (24.7) | 57/178 (32.0) |
| Race (%) | ||
| White | 140/170 (82.4) | 154/177 (87.0) |
| Black | 3/170 (1.8) | 7/177 (4.0) |
| American Indian | 1/170 (0.6) | 0/177 (0.0) |
| Asian | 15/170 (8.8) | 11/177 (6.2) |
| Pacific Islander | 1/170 (0.6) | 0/177 (0.0) |
| Multiracial | 10/170 (5.9) | 5/177 (2.8) |
| Body weight (kg), mean (SD) | 81.3 (18.1) | 82.5 (19.2) |
| BMI*, mean (SD) | 28.7 (5.1) | 28.9 (5.5) |
| Hypertension (%) | 135/170 (79.4) | 157/178 (88.2) |
| Hyperlipidemia (%) | 125/170 (73.5) | 132/178 (74.2) |
| Diabetes mellitus (%) | 85/170 (50.0) | 88/178 (49.4) |
| Stroke (%) | 19/170 (11.2) | 19/178 (10.7) |
| Myocardial infarction (%) | 88/170 (51.8) | 100/178 (56.2) |
| Peripheral arterial vascular disease (%) | 40/170 (23.5) | 31/178 (17.4) |
| Past tobacco use (%) | 77/170 (45.3) | 63/178 (35.4) |
| Current tobacco use (%) | 23/170 (13.5) | 44/178 (24.7) |
| Percutaneous coronary intervention (%) | 64/170 (37.6) | 71/178 (39.9) |
| Coronary artery bypass graft (%) | 59/170 (34.7) | 63/178 (35.4) |
| Statin at enrollment (%) | 146/170 (85.9) | 158/178 (88.8) |
| Thienopyridine at enrollment (%) | 154/170 (90.6) | 153/178 (86.0) |
| Troponin or CK‐MB positive (%) | 165/170 (97.1) | 166/177 (93.8) |
| Renal insufficiency CrCl <30 mL/min (%) | 19/164 (11.6) | 10/169 (5.9) |
| ST‐segment depression (%) | 79/170 (46.5) | 73/178 (41.0) |
| ST‐segment elevation (%) | 11/170 (6.5) | 12/178 (6.7) |
| Killip class >II (%) | 11/169 (6.5) | 8/178 (4.5) |
| TIMI risk score ≥3 (%) | 170/170 (100.0) | 178/178 (100.0) |
Data presented as n/N (%), unless otherwise noted. BMI indicates body mass index; CK‐MB, creatine kinase‐MB; CrCl, creatinine clearance; CVD, cardiovascular death; MI, myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Weight (kg)/height2 (m2).
Multiple recurrent events of CVD, MI, and stroke occurred in 2.6% of patients randomized to vorapaxar and in 2.8% of patients randomized to placebo. Overall, patients randomized to vorapaxar experienced fewer occurrences of events during the study than patients randomized to placebo (HR, 0.88; 95% CI, 0.80 to 0.98; P=0.02; NNT, 51). Recurrent MI occurred in 86 (1.3%) of vorapaxar and 101 (1.6%) placebo patients, whereas recurrent stroke occurred in 4 (<0.1%) vorapaxar and 10 (0.15%) placebo patients. Table 4 shows that there is a nonsignificant effect of vorapaxar on overall occurrences of the expanded composite endpoint (HR, 0.92; 95% CI, 0.84 to 1.01; P=0.09; NNT, 62).
Table 4.
Effect of Vorapaxar on Overall Occurrences of the Composite Endpoint of CVD, MI, Stroke, Urgent Revascularization, and Rehospitalization for Ischemic Event
| WLW Marginal Regression Model of All Occurrences of Events | ||
|---|---|---|
| Parameter | HR (95% CI) | Pr>Chi‐Square |
| Vorapaxar effect on | ||
| First event | 0.924 (0.849 to 1.006) | 0.0695 |
| Second event | 1.001 (0.836 to 1.199) | 0.9892 |
| Third event | 0.901 (0.634 to 1.282) | 0.5635 |
| Fourth event | 0.618 (0.309 to 1.236) | 0.1737 |
| Fifth event | 0.272 (0.076 to 0.973) | 0.0452 |
| Sixth event | 0.000 (0.000 to 0.000) | <0.0001 |
| Seventh event | 0.000 (0.000 to 0.000) | <0.0001 |
| Eight event | 0.000 (0.000 to 0.000) | <0.0001 |
| Common vorapaxar effect across multiple events | 0.921 (0.837 to 1.012) | 0.0884 |
CI indicates confidence interval; CVD, cardiovascular death; HR, hazard ratio; MI, myocardial infarction; WLW, Wei, Lin, and Weissfeld.
Sequence of Events
Table 5 shows the sequences of events in the order they occurred for patients whose first event was MI or stroke. Figure 2 shows the total number of events for patients whose first events were CVD, MI, or stroke. CVD occurred as a first event in 202 vorapaxar patients (3.1%) and in 208 placebo patients (3.2%). Nonfatal MI or nonfatal stroke occurred before CVD in 170 patients (1.3%), with 90 (1.4%) in the vorapaxar group, and 80 (1.2%) in the placebo group.
Table 5.
Sequence of Ischemic Events in the Order They Occurred
| Sequence of Events | Placebo | Vorapaxar | Total |
|---|---|---|---|
| Subjects whose first event was MI | |||
| MI_CVD | 44 (0.68) | 49 (0.76) | 93 |
| MI_MI_CVD | 10 (0.15) | 14 (0.22) | 24 |
| MI_MI_MI_CVD | 4 (0.06) | 3 (0.05) | 7 |
| MI_MI_MI_MI_CVD | 1 (0.02) | 0 (0.00) | 1 |
| MI_MI_MI_MI_MI_CVD | 1 (0.02) | 0 (0.00) | 1 |
| MI_MI | 51 (0.79) | 43 (0.66) | 94 |
| MI_MI_MI | 9 (0.14) | 10 (0.15) | 19 |
| MI_MI_MI_MI | 2 (0.03) | 5 (0.08) | 7 |
| MI_MI_MI_MI_MI | 1 (0.02) | 0 (0.00) | 1 |
| MI_MI_MI_MI_MI_MI_MI_MI | 1 (0.02) | 0 (0.00) | 1 |
| MI_stroke | 7 (0.11) | 6 (0.09) | 13 |
| MI_stroke_MI | 2 (0.03) | 0 (0.00) | 2 |
| MI_stroke_CVD | 2 (0.03) | 1 (0.02) | 3 |
| Subjects whose first event was stroke | |||
| Stroke_CVD | 7 (0.11) | 10 (0.15) | 17 |
| Stroke_MI | 4 (0.06) | 4 (0.06) | 8 |
| Stroke_MI_CVD | 1 (0.02) | 1 (0.02) | 2 |
| Stroke_MI_MI | 0 (0.00) | 1 (0.02) | 1 |
| Stroke_stroke | 4 (0.06) | 3 (0.05) | 7 |
| Stroke_stroke_CVD | 2 (0.03) | 1 (0.02) | 3 |
| Stroke_stroke_MI | 1 (0.02) | 0 (0.00) | 1 |
| Stroke_stroke_stroke | 3 (0.05) | 0 (0.00) | 3 |
Data presented as n (%). CVD indicates cardiovascular death; MI, myocardial infarction.
Figure 2.
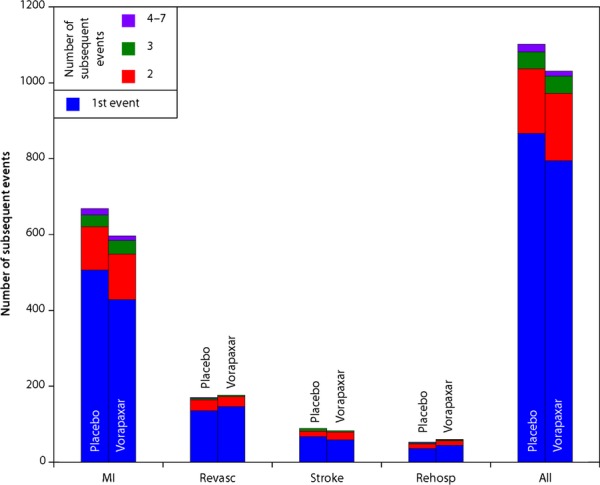
First and subsequent CVD, MI, or stroke among patients randomized to vorapaxar or to placebo. CVD indicates cardiovascular death; MI, myocardial infarction; rehosp, rehospitalization; revasc, revascularization.
Recurrence of the expanded endpoint, including revascularization and rehospitalization for recurrent ischemia, occurred in 237 (3.7%) vorapaxar and 449 (6.9%) placebo patients. Figure 3 shows the first and second events with the expanded endpoint. Recurrent revascularization occurred in 14 vorapaxar and 14 placebo patients, and recurrent hospitalization for recurrent ischemia occurred in 4 vorapaxar and 2 placebo patients.
Figure 3.
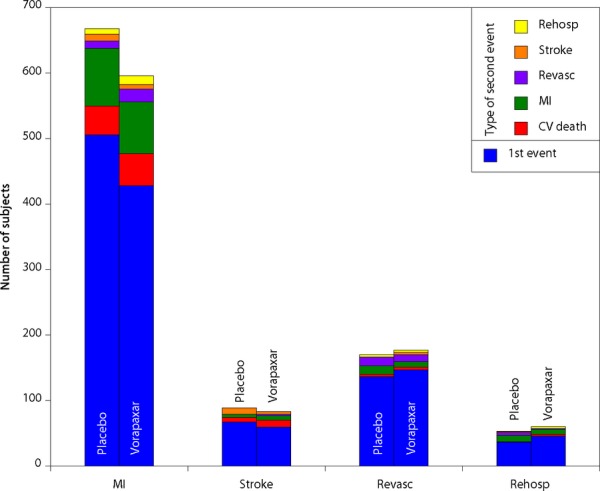
First and second ischemic events, CVD, MI, or stroke among patients randomized to vorapaxar or to placebo. CVD indicates cardiovascular death; MI, myocardial infarction; rehosp, rehospitalization; revasc, revascularization.
Study drug discontinuation was slightly higher in the vorapaxar group, compared to the placebo group (28.2% vs. 26.8%; P=0.07).
Safety
Vorapaxar increased Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) moderate and severe bleeding overall (Table 6; HR, 1.42; 95% CI, 1.21 to 1.66; P<0.001). Recurrent GUSTO moderate or severe bleeding occurred in 0.3% of patients, and a second occurrence of Thrombolysis in Myocardial Infarction (TIMI) clinically significant bleeding occurred in 1.7%, a third occurrence in 0.3%, a fourth in 0.04%, and a fifth in 0.02%. Vorapaxar also increased TIMI clinically significant bleeding (1.55; 95% CI, 1.40 to 1.71; P<0.001). Table 7 shows the effect of vorapaxar on overall occurrences of TIMI clinically significant bleeding.
Table 6.
Patients With Occurrences of Bleeding Endpoints and Their Components
| Vorapaxar (n=6446) (%) | Placebo (n=6441) (%) | |
|---|---|---|
| Patients with occurrence of GUSTO moderate or severe bleeding | ||
| 1 | 362 (5.6) | 274 (4.3) |
| 2 | 27 (0.4) | 16 (0.2) |
| 3 | 2 (0.03) | 0 (0.0) |
| Patients with occurrence of TIMI clinically significant bleeding | ||
| 1 | 878 (13.6) | 678 (10.5) |
| 2 | 149 (2.3) | 66 (1.0) |
| 3 | 30 (0.5) | 11 (0.2) |
| 4 | 5 (0.08) | 0 (0.0) |
| 5 | 3 (0.05) | 0 (0.0) |
Data presented as n (%). GUSTO indicates Global Use of Strategies to Open Occluded Coronary Arteries; TIMI, Thrombolysis in Myocardial Infarction.
Table 7.
Effect of Vorapaxar on Overall Occurrences of Composite TIMI Clinically Significant Bleeding Event
| WLW Marginal Regression Model of All Occurrences of TIMI Clinically Significant Bleeding Events | ||
|---|---|---|
| Parameter | HR (95% CI) | Pr>Chi‐Square |
| Vorapaxar effect on | ||
| First event | 1.427 (1.300 to 1.566) | <0.0001 |
| Second event | 2.428 (1.865 to 3.162) | <0.0001 |
| Third event | 3.207 (1.678 to 6.129) | 0.0004 |
| Fourth event | 8.050 (1.002 to 64.654) | 0.0498 |
| Fifth event | 3.049 (0.312 to 29.796) | 0.3379 |
| Sixth event | 0.000 (0.000 to 0.001) | <0.0001 |
| Common vorapaxar effect across all events | 1.550 (1.403 to 1.713) | <0.0001 |
CI indicates confidence interval; HR, hazard ratio; TIMI, Thrombolysis in Myocardial Infarction; WLW, Wei, Lin, and Weissfeld.
Discussion
In TRACER patients with NSTE ACS, a single component of the key secondary composite endpoint of CVD, MI, or stroke in the time‐to‐first‐event analysis occurred in 10.7% of patients. Multiple recurrent events occurred in 2.7% of patients, meaning that 25.2% of patients included in the time‐to‐first‐event analysis had recurrent events. Vorapaxar reduced first events by 11% (HR, 0.89; 95% CI, 0.81 to 0.98) and overall ischemic events by 12% (HR, 0.88, 95% CI, 0.80 to 0.98).
The effect of vorapaxar on the reduction of total events was greater than previously reported for time‐to‐first‐event analysis,2 with the NNT over 502 days reduced from 81 to 51 for the composite endpoint of CVD, MI, or stroke. These data show the importance of capturing recurrent events to ascertain overall patient benefit.
Vorapaxar increased moderate and severe bleeding overall as well as recurrent GUSTO moderate and severe bleeding and TIMI clinically significant bleeding. However, recurrent bleeding was infrequent, with 0.4% of patients receiving vorapaxar having a recurrent GUSTO moderate‐to‐severe bleed and 2.9% having a recurrent TIMI clinically significant bleed, despite continuing therapy in most patients. Among patients who had GUSTO moderate or severe bleeding, 62% of them (60% of vorapaxar and 67% of placebo) never discontinued treatment. Among patients who had TIMI clinically significant bleeding, 69% (67% vorapaxar and 73% placebo) never discontinued treatment. As a reference, among those who never had a bleeding event, 74% (73% vorapaxar and 74% placebo) never discontinued treatment.
These findings increase our understanding of the overall benefit of vorapaxar in addition to treatment with aspirin in patients with NSTE ACS. Clearly, reducing recurrent events is of major clinical benefit. In addition, reducing the total burden of ischemic events may also reduce costs,5–8 but must be balanced with the increased bleeding.
Combination and Sequence of Events
With a composite endpoint of CVD, MI, or stroke, there are 7 possible sequences of events if only 1 event of each type occurs. If multiple MI or stroke events occur, there are many more possibilities, as shown in Table 5. Patients had up to 7 recurrences of MI and 3 had recurrence of 2 strokes. There was a high dependence of recurrent events, with nonfatal MI or nonfatal stroke occurring before CVD in 1.3% of patients. This stresses the importance of continuing vorapaxar and other therapies when a patient experiences nonfatal events.
Factors associated with increased occurrence of events included most risk factors for ischemic events. Taking a thienopyridine or a statin at enrollment was associated with increased occurrence of events. Other studies with P2Y12 inhibitors have shown the efficacy of prasugrel and ticagrelor in reducing recurrent events, as compared with clopidogrel treatment.6,9 In the PLATelet inhibition and patient Outcomes (PLATO) trial, ticagrelor reduced first and recurrent events, as compared with clopidogrel (HR for time to second event, 0.80; 95% CI, 0.70 to 0.90; P<0.001).6 Most of the treatment effect (83%) was expressed with first events in the composite endpoint of CVD, MI, or stroke. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel/Thrombolysis in Myocardial Infarction (TRITON‐TIMI) trial, prasugrel reduced subsequent events among patients who survived the primary event, as compared with patients taking clopidogrel (HR, 0.65; 95% CI, 0.46 to 0.92; P=0.016).9 Again, most of the treatment effect (70.8%) was on the primary composite endpoint of CVD, MI, or stroke. In the current analysis, the effect of vorapaxar on recurrent events was also mostly on first events (1732 of 2080 first events=83.3%).
Limitations
The WLW survival analysis we used for assessment of recurrent events is well validated,10–11 but the statistical model has a number of limitations, including that the evaluation of treatment effect on recurrent events includes the treatment effect on first events and does not preserve the initial randomization. Recurrent event analyses are also affected by different mortality rates and other postrandomization factors in the 2 randomized treatment groups. Only the former was accounted for by the WLW method. We did not collect information about the size of the recurrent MIs or the disability associated with recurrent strokes.
In this analysis, each of the components of the composite endpoint (CVD, MI, or stroke) was weighted equally. However, the relative importance of the components of the composite may differ between clinical trialists,12 physicians, and patients, and more research is required in this area.
Conclusions
Randomization to vorapaxar nominally reduced the time to first composite event of CVD, MI, or stroke and the risk of recurrent ischemic events. The NNT was reduced from 81 for first events to 51 for recurrent events. Overall recurrent moderate and severe GUSTO bleeding as well as recurrent clinically significant TIMI bleeding was increased with vorapaxar. These exploratory findings increase our knowledge of the overall potential of vorapaxar to reduce ischemic events and of its safety.
Focusing only on time‐to‐first‐event analysis may miss important information about efficacy and risks of a therapy.
Acknowledgment
The authors thank Charlene Nell, Team Support Administrator, Green Lane Cardiovascular Research Unit, for her excellent secretarial assistance.
Authors' Contributions
All authors had full access and contributed equally to the content of the article. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Appendix
Baseline Covariates for Ischemic Events
Demographics: age, sex, and race; vital signs: weight, heart rate, and diastolic blood pressure; medical history: hypertension, diabetes, stroke, transient ischemic attack, angina, myocardial infarction, peripheral artery disease, heart failure, atrial fibrillation, tobacco use, percutaneous coronary intervention, and coronary artery bypass graft; disease status at enrollment: ST‐segment elevation, new T wave, and Killip class; labs at enrollment: troponin and creatinine clearance; medications at baseline: thienopyridine, aspirin, beta‐blocker, statin, and glycoprotein IIb/IIIa.
Appendix
Baseline Covariates for Bleeding Events
Demographics: age, sex, and race; vital signs: weight, heart rate, and diastolic blood pressure; medical history: hypertension, diabetes, stroke, transient ischemic attack, angina, myocardial infarction, peripheral artery disease, heart failure, atrial fibrillation, tobacco use, percutaneous coronary intervention, and coronary artery bypass graft; disease status at enrollment: ST‐segment elevation, new T wave, and Killip class; labs at enrollment: troponin, creatinine clearance, hemoglobin, and white blood cells; medications at baseline: thienopyridine, aspirin, beta‐blocker, statin, and glycoprotein IIb/IIIa.
Sources of Funding
The TRACER trial was funded by Merck & Co, Inc. (Whitehouse Station, NJ; ClinicalTrials.gov Identifier: NCT00527943).
Disclosures
White: research grants from Sanofi‐Aventis, Eli Lilly, The Medicines Company, National Institutes of Health, Pfizer, Roche, Johnson & Johnson, Schering‐Plough, Merck Sharpe & Dohme, AstraZeneca, GlaxoSmithKline (GSK), Daiichi Sankyo Pharma Development, and Bristol‐Myers Squibb (BMS); advisory boards for Merck Sharpe & Dohme, Roche, and Regado Biosciences. Huang: none. Tricoci: consultant agreement and research grant from Merck & Co. Van de Werf: research grant, honoraria for lectures, and advisory board membership from Merck & Co. Wallentin: research grants from AstraZeneca, Merck & Co., Boehringer Ingelheim, BMS/Pfizer, GSK; Speakers' bureau/lecture fees from AstraZeneca, Boehringer Ingelheim, BMS/Pfizer, GSK, and Merck & Co.; honoraria from Boehringer Ingelheim, AstraZeneca, BMS/Pfizer, GSK, and Merck & Co.; consultant/advisory board for Merck & Co., Regado Biosciences, Evolva, Portola, CSL Behring, Athera Biotechnologies, Boehringer Ingelheim, AstraZeneca, GSK, and BMS/Pfizer; and travel support from BMS/Pfizer. Lokhnygina: none. Moliterno: consultant and research funding from Merck & Co. Aylward: research grants from Merck & Co., AstraZeneca, and Sanofi; speaker bureau and advisory board for AstraZeneca, Eli Lilly, Boehringer Ingelheim, Bayer, Johnson & Johnson, and Servier. Mahaffey: full disclosures before August 1, 2013 available at www.dcri.org. Disclosures after August 1, 2013 available at http://med.stanford.edu/profiles/kenneth_mahaffey. Armstrong: research grant from Merck & Co.; consulting for Eli Lilly; and other for AstraZeneca.
References
- 1.Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012; 366:1404-1413. [DOI] [PubMed] [Google Scholar]
- 2.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW. Thrombin‐receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012; 366:20-33. [DOI] [PubMed] [Google Scholar]
- 3.TRA*CER Executive and Steering Commzrittees. The thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRA*CER) trial: study design and rationale. Am Heart J. 2009; 158:327-334. [DOI] [PubMed] [Google Scholar]
- 4.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modelling marginal distributions. J Am Stat Assoc. 1989; 84:1065-1073. [Google Scholar]
- 5.Janzon M, Levin LA, Swahn E. Cost‐effectiveness of an invasive strategy in unstable coronary artery disease; results from the FRISC II invasive trial. The Fast Revascularisation during InStability in Coronary artery disease. Eur Heart J. 2002; 23:31-40. [DOI] [PubMed] [Google Scholar]
- 6.Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, Ardissino D, Maurer G, Morais J, Nicolau JC, Oto A, Storey RF, James SK, Cannon CP. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO study. Circulation. 2013; 127:673-680. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, Robertson DH, Alexander C, Nag S, Cook JR, Demopoulos LA, DiBattiste PM, Cannon CP, Weintraub WS. Cost and cost‐effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non‐ST‐segment elevation myocardial infarction. JAMA. 2002; 288:1851-1858. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney EM, Wang K, Arnold SV, Proskorovsky I, Wiviott S, Antman E, Braunwald E, Cohen DJ. Cost‐effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with Prasugrel‐Thrombolysis in Myocardial Infarction TRITON‐TIMI 38. Circulation. 2010; 121:71-79. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SA, Antman EM, Wiviott SD, Weerakkody G, Morocutti G, Huber K, Lopez‐Sendon J, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON‐TIMI 38 trial. Eur Heart J. 2008; 29:2473-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonardi S, Tricoci P, White HD, Armstrong PW, Huang Z, Wallentin L, Aylward PE, Moliterno DJ, Van de Werf F, Chen E, Providencia L, Nordrehaug JE, Held C, Strony J, Rorick TL, Harrington RA, Mahaffey KW. Effect of vorapaxar on myocardial infarction in the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRA*CER) trial. Eur Heart J. 2013; 34:1723-1731. [DOI] [PubMed] [Google Scholar]
- 11.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid‐lowering statin therapy compared with moderate lipid‐lowering statin therapy after acute coronary syndromes from the PROVE IT‐TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009; 54:2358-2362. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong PW, Westerhout CM, Van de Werf F, Califf RM, Welsh RC, Wilcox RG, Bakal JA. Refining clinical trial composite outcomes: an application to the Assessment of the Safety and Efficacy of a New Thrombolytic‐3 (ASSENT‐3) trial. Am Heart J. 2011; 161:848-854. [DOI] [PubMed] [Google Scholar]


