Abstract
Background
Dipeptidyl‐peptidase 4 (DPP4) inhibitors improve glycemic control in patients with diabetes mellitus by preventing the degradation of glucagon‐like peptide‐1 (GLP‐1). GLP‐1 causes vasodilation in animal models but also increases sympathetic activity; the effect of GLP‐1 in the human vasculature and how it is altered by DPP4 inhibition is not known. DPP4 also degrades the vasodilator brain natriuretic peptide (BNP) to a less potent metabolite. This study tested the hypothesis that DPP4 inhibition potentiates the vasodilator responses to GLP‐1 and BNP in the human forearm.
Method and Results
Seventeen healthy subjects participated in this randomized, double‐blinded, placebo‐controlled crossover study. On each study day, subjects received DPP4 inhibitor (sitagliptin 200 mg by mouth) or placebo. Sitagliptin increased forearm blood flow and decreased forearm vascular resistance without affecting mean arterial pressure and pulse. GLP‐1 and BNP were infused in incremental doses via brachial artery. Venous GLP‐1 concentrations were significantly higher during sitagliptin use, yet there was no effect of GLP‐1 on forearm blood flow in the presence or absence of sitagliptin. BNP caused dose‐dependent vasodilation; however, sitagliptin did not affect this response. GLP‐1 and BNP had no effect on net norepinephrine release.
Conclusions
These data suggest that GLP‐1 does not act as a direct vasodilator in humans and does not contribute to sympathetic activation. Sitagliptin does not regulate vascular function in healthy humans by affecting the degradation of GLP‐1 and BNP.
Clinical Trial Registration
URL: www.clinicaltrials.gov/ Unique identifier: NCT01413542.
Keywords: diabetes mellitus, dipeptidyl‐peptidase 4, glucagon‐like peptide‐1, natriuretic peptide, vasodilation
Introduction
Dipeptidyl‐peptidase 4 (DPP4) is a ubiquitously expressed cell surface protease that cleaves dipeptides from the amino terminus of peptides containing a penultimate alanine or proline; soluble DPP4 resulting from proteolytic cleavage of the membrane form is also present in the circulation.1–3 The first selective DPP4 inhibitor, sitagliptin, was approved by the US Food and Drug Administration in 2006 for the management of hyperglycemia in patients with type 2 diabetes mellitus. DPP4 inhibition decreases the degradation of endogenous incretin hormones, including glucagon‐like peptide‐1 (GLP‐1) and thereby augments nutrient‐stimulated insulin release, suppresses glucagon secretion, and slows gastric emptying.4–5 The widespread expression of DPP4 within the vasculature and its numerous vasoactive hormone substrates also raise the possibility that DPP4 could affect vascular function.6
GLP‐1 and brain natriuretic peptide (BNP) represent 2 vasoactive peptide hormone substrates of DPP4. In rodent models, GLP‐1 activates the GLP‐1 receptor to produce mild vasodilation, inotropic action, and ischemic preconditioning.7–8 At the same time, central and peripheral administration of GLP‐1 receptor agonists in a rat model increases blood pressure and heart rate by activating autonomic regulatory neurons.9 DPP4 cleaves GLP‐1 to its metabolite GLP‐1(9‐36), which promotes endothelium‐dependent vasodilation independent of the GLP‐1 receptor.7 Consequently, inhibition of DPP4 may potentiate the effects of GLP‐1 but also could also decrease the favorable effects of its metabolite GLP‐1(9‐36).
BNP(1‐32) is produced by the ventricular cardiomyocytes in response to increased filling pressures and promotes vasodilation and natriuresis. BNP(1‐32) is rapidly cleaved to BNP(3‐32) by DPP4 in human plasma, and this is prevented by addition of the DPP4 inhibitor vildagliptin.10 The metabolite BNP(3‐32) causes less vasodilation and natriuresis than its intact precursor in a dog model.11
Adults with diabetes are nearly two times more likely to die from heart disease than adults without diabetes, and over two‐thirds of adults with diabetes have hypertension or are prescribed antihypertensive therapies.12 Two recent placebo‐controlled clinical trials demonstrated no beneficial effect of the DPP4 inhibitors alogliptin or saxagliptin on cardiovascular events, although saxagliptin use was associated with an increased risk of hospitalization for heart failure.13–14 Consequently, it is important to understand the cardiovascular effects of this class of antidiabetic medications at a mechanistic level. This study tested the hypothesis that DPP4 inhibition potentiates the vasodilator responses to GLP‐1 and BNP in the human forearm vasculature.
Methods
Study Protocol
Seventeen healthy, nonobese (body mass index <30 kg/m2), nonsmoking adults participated in a double‐blind, randomized, placebo‐controlled, crossover study (Figure 1; subject characteristics are shown in Table 1). The study adhered to the principles of the Declaration of Helsinki and Title 45, US Code of Federal Regulations, Part 46, Protection of Human Subjects, following approval by the Vanderbilt University institutional review board, and all subjects provided written informed consent prior to initiation of study procedures. Patients with a history of chronic illness, including diabetes, hypertension, cardiovascular disease, and chronic renal or hepatic insufficiency, were excluded from participation. Medication use other than a multivitamin was prohibited at the time of study. Pregnancy was excluded among women of child‐bearing age.
Figure 1.
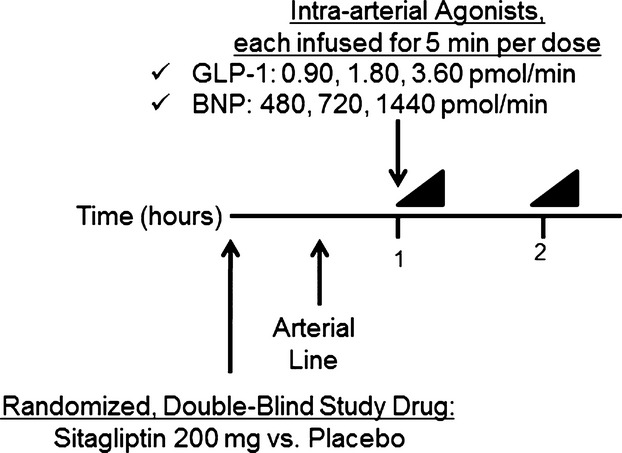
Study protocol. Intra‐arterial GLP‐1 was first infused in 3 graded doses, lasting 5 minutes each, followed by BNP. A 30‐minute washout separated the 2 peptide infusions. Forearm blood flow measurement, followed by arterial and venous sampling, was performed at baseline and at completion of each dose of peptide. A minimum of 7 days separated each study day. BNP indicates brain natriuretic peptide; GLP‐1, glucagon‐like peptide‐1.
Table 1.
Subject Characteristics
| Parameter | N=17 |
|---|---|
| Age, y | 35.4±10.0 |
| Race | |
| White | 12 (71%) |
| Black | 5 (29%) |
| Sex | |
| Female | 8 (47%) |
| Male | 9 (53%) |
| Weight, kg | 75.8±15.2 |
| Body mass index, kg/m2 | 25.4±3.2 |
| Menopause status | |
| Premenopausal | 7 (88%) |
| Menopausal | 1 (12%) |
Results are presented as mean±SD, unless otherwise noted.
Each subject was studied on 2 days at least 1 week apart. Subjects were assigned to treatment order (sitagliptin or matching placebo) using a block randomization algorithm provided by the study biostatistician. Randomization was stratified by race and sex. On each study day, subjects reported to the Vanderbilt Clinical Research Center in the morning after an overnight fast. All subjects were studied in the supine position in a temperature‐controlled room. Subjects were given an oral study drug (sitagliptin 200 mg or matching placebo), and an arterial line was placed in the brachial artery of the nondominant arm with an adjacent peripheral intravenous line.
Baseline forearm blood flow (FBF) measurements and blood sampling were obtained 60 minutes following ingestion of the study drug and at least 30 minutes after insertion of the arterial catheter. Subjects then received sequential intra‐arterial infusions of GLP‐1 followed by BNP; a 30‐minute washout separated each infusion. We observed a carryover effect on FBF when BNP was infused prior to GLP‐1 in the first subject, despite the intervening washout. The affected data were excluded from the analyses, and GLP‐1 was infused first on all subsequent study days. Each peptide was infused in 3 graded doses of 5 minutes each. FBF was assessed during the last 2 minutes of each dose, and arterial and venous blood samples were then drawn simultaneously. On the second study day, the protocol was repeated using the opposite study drug (sitagliptin or matching placebo). Blood pressure and heart rate were continuously monitored throughout each study day.
Insertion of Arterial Line and Intra‐arterial Administration of Study Drugs
After subdermal administration of 1% lidocaine, a size 3F catheter (Cook Inc) was inserted into the brachial artery of the nondominant arm for direct intra‐arterial administration of peptides and arterial blood sampling. Arterial catheter patency was maintained by infusion of intravenous fluid (0.9% sodium chloride solution) at a rate of 1 mL/min.
GLP‐1 (Clinalfa Basic) was infused at 0.45, 0.90, and 1.80 pmol/min in the first 3 randomized subjects; however, we did not see any effect of GLP‐1 on vasodilation at these doses. GLP‐1 infusions were increased to 0.90, 1.80, and 3.60 pmol/min in 11 additional subjects. After we still saw no effect of GLP‐1, we discontinued GLP‐1 in the remaining 3 subjects. BNP (nesiritide; Scios Inc) was infused at 480, 720, and 1440 pmol/min. We chose the doses of BNP based on previously published vasodilator responses.15–16 All 17 subjects received BNP infusions. Drug concentrations in the infusate were adjusted to maintain an infusion volume of 1 mL/min throughout the study.
Forearm Blood Flow Measurements
FBF was measured using mercury‐in‐silastic strain gauge plethysmography. The wrist was supported in a sling to raise the level of the forearm above the level of the atrium, and a strain gauge was placed around the widest part of the forearm of the nondominant hand. The strain gauge was connected to a plethysmograph (model EC‐6; D.E. Hokanson), which was connected to a chart recorder to record flow measurements. For each measurement, a cuff placed around the upper arm was inflated to 45 mm Hg with a rapid cuff inflator (model E‐20 rapid cuff inflator and AG 101 cuff inflator air source; D.E. Hokanson) to occlude venous outflow from the extremity. The hand was excluded from the measurement of blood flow by inflation of a pediatric sphygmomanometer cuff to 200 mm Hg around the wrist. Flow measurements were recorded for ≈7 seconds, and a minimum of 6 readings were analyzed using a noninvasive vascular software program (D.E. Hokanson NIVP3 version 5.40) to obtain each mean. Forearm vascular resistance was calculated as mean arterial pressure (MAP) divided by FBF.
Arteriovenous concentration gradients were calculated by subtracting the plasma level measured in simultaneously collected venous and arterial blood. Forearm plasma flow was calculated from the FBF, and hematocrit was corrected for 1% trapped plasma. Net release was calculated at each time point:
Laboratory Analyses
Simultaneous arterial and venous samples were obtained from the infused arm at baseline and at completion of each dose of infused peptide. All samples were obtained after the first 3 mL of blood were discarded. Blood samples were collected on ice and centrifuged immediately, and plasma was stored at −80°C in prespecified aliquots until time of assay. Venous DPP4 antigen concentration was determined by ELISA (eBioscience). Venous DPP4 activity was assayed by incubating sera with a colorimetric substrate, l‐glycyl‐l‐prolyl p‐nitroanilide, hydrochloride (Sigma), at 37°C.17 Blood for measurement of venous GLP‐1 levels was collected in tubes containing EDTA and protease inhibitor (aprotinin; Roche Diagnostics). GLP‐1 levels were determined using a multiplex magnetic bead assay (Milliplex MAP Human Metabolic Hormone Magnetic Bead Panel; EMD Millipore) that detects active GLP‐1(7‐36) in the range of 4 to 3033 pmol/L with no cross‐reactivity for GLP‐1(9‐36). Arterial and venous blood for catecholamine measurement was collected in prechilled tubes containing sodium heparin. Samples were measured by high‐performance liquid chromatography with electrochemical detection.
Statistical Analysis
Data are presented as mean±SD unless otherwise noted. Potential carryover and period effects were tested for by comparing the measures of FBF obtained prior to each infusion. Wilcoxon signed rank test was used to compare baseline variables as well as venous GLP‐1 levels. Mixed‐effect models were used to analyze the data with a random subject effect and with fixed effects of treatment (placebo or sitagliptin), peptide dose, and their interaction. The mixed‐effect model with a random subject effect allowed the inclusion of subjects with missing data at some time points. For inferences of interest, a 2‐sided P<0.05 was considered significant. Statistical analyses were performed using IBM SPSS software v. 21.0, GraphPad Prism 5, and R 2.15.0 (www.r-project.org).
Results
Effect of Treatment on DPP4 Activity and Initial Hemodynamic Parameters
DPP4 inhibition with sitagliptin decreased DPP4 activity by 64% (P=0.002), whereas DPP4 antigen was unchanged (Table 2). DPP4 inhibition increased systolic blood pressure by 5% (P=0.03) but had no effect on diastolic blood pressure (P=0.70), MAP (P=0.21), or heart rate (P=0.92) prior to intra‐arterial peptide infusions. DPP4 inhibition increased FBF by 30% (P=0.02) and decreased forearm vascular resistance by 18% (P=0.03) prior to intra‐arterial peptide infusions.
Table 2.
Initial Hemodynamic Parameters
| Variable | Placebo (n=16) | DPP4 Inhibition (n=14) |
|---|---|---|
| DPP4 activity, U/L | 27.3±4.5 | 9.8±5.7* |
| DPP4 antigen, ng/mL | 417.7±90.0 | 386.3±91.3 |
| Systolic blood pressure, mm Hg | 109.3±8.3 | 115.1±8.7* |
| Diastolic blood pressure, mm Hg | 67.5±7.0 | 68.9±8.3 |
| Mean arterial pressure, mm Hg | 84.1±5.9 | 86.7±6.5 |
| Heart rate, bpm | 56.8±9.2 | 57.4±8.5 |
| FVR, mm Hg/mL per minute per 100 mL | 36.3±18.1 | 29.8±12.7* |
| FBF, mL/min per 100 mL | 2.7±0.9 | 3.5±1.5* |
Results are presented as mean±SD. bpm indicates beats per minute; DPP4, dipeptidyl peptidase 4; FBF, forearm blood flow; FVR, forearm vascular resistance.
P<0.05 vs placebo.
Effect of DPP4 Inhibition on GLP‐1 Concentrations and Forearm Blood Flow
Intra‐arterial infusion of GLP‐1 significantly increased venous GLP‐1 concentrations from baseline during both placebo (P=0.01) and sitagliptin (P=0.01). Venous GLP‐1 levels were significantly higher during sitagliptin (P=0.04 versus placebo at highest dose of GLP‐1) and exceeded postprandial levels normally found in healthy adults (Figure 2).18
Figure 2.
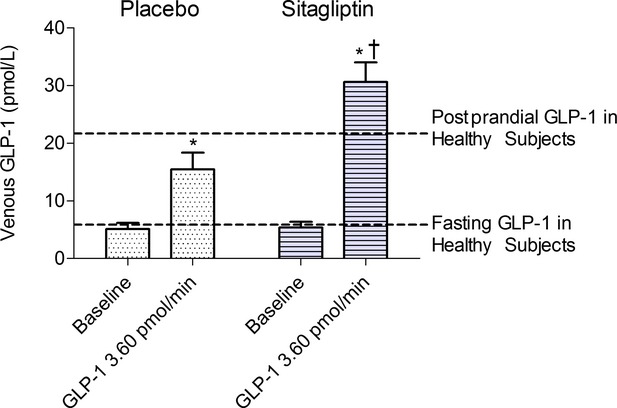
Effect of DPP4 inhibition on venous GLP‐1 levels at baseline and during the maximum dose of GLP‐1. Data presented as mean±SEM (14 subjects). P values obtained from Wilcoxon signed rank. *P<0.05 vs baseline during same treatment. †P<0.05 vs placebo at same peptide dose. Dotted lines indicate references for fasting and postprandial GLP‐1 levels in healthy subjects.18 DPP4 indicates dipeptidyl‐peptidase 4; GLP‐1, glucagon‐like peptide‐1.
Vasodilator response is presented as FBF, because local intra‐arterial infusion of peptide did not significantly affect MAP in either treatment group, and as a percent change, given that baseline FBF differed between treatment with sitagliptin and placebo (Figure 3). Intra‐arterial infusion of GLP‐1 did not increase FBF, even when its concentrations were increased by sitagliptin.
Figure 3.

Effect of DPP4 inhibition on forearm blood flow response to intra‐arterial GLP‐1 (14 subjects) and to BNP (17 subjects). As noted in the methods, the first 3 subjects received lower doses of GLP‐1. Data presented as mean±SEM. P values from mixed‐effect models are presented in the text. BNP indicates brain natriuretic peptide; DPP4, dipeptidyl‐peptidase 4; GLP‐1, glucagon‐like peptide‐1.
Intra‐arterial infusion of BNP increased FBF in a dose‐dependent manner (P<0.001 effect of dose); however, treatment with sitagliptin did not affect this vasodilator response.
Effect of DPP4 Inhibition on Mean Arterial Pressure, Heart Rate, and Norepinephrine Levels
Intra‐arterial infusion of GLP‐1 did not significantly affect heart rate, MAP, norepinephrine levels, or net vascular norepinephrine release during placebo or sitagliptin treatment (data not shown).
Intra‐arterial infusion of BNP infusion increased heart rate in a dose‐dependent manner (P=0.01 effect of dose); treatment with sitagliptin did not affect this response. Intra‐arterial infusion of BNP increased arterial norepinephrine levels only during sitagliptin (P<0.001 effect of dose). There was no effect of intra‐arterial infusion of BNP on MAP, venous norepinephrine levels, or net norepinephrine release.
Safety
Seventeen subjects participated in study procedures. Three subjects did not complete the second study day due to inability to obtain adequate arterial access. One subject experienced a syncopal episode ≈1 hour after completion of his first study visit. He was found to be orthostatic, was given intravenous fluids, and was withdrawn from the study. The data from these 4 subjects is included in the analyses. The remaining 13 subjects completed both study days. Other adverse events included transient lightheadedness and nausea, which resolved with increased oral fluid intake and rest (3 subjects), and neuropraxia in the instrumented arm, which resolved over a period of 2 weeks without therapy (1 subject). There were no instances of hypoglycemia.
Discussion
This study tested the hypothesis that DPP4 inhibition potentiates the vasodilator responses to GLP‐1 and BNP in the human forearm. We found that GLP‐1 does not cause vasodilation in the forearm vasculature of healthy humans, even when its degradation is inhibited by sitagliptin and high concentrations are achieved. We also found that sitagliptin does not potentiate the vasodilator response to BNP. Neither intra‐arterial GLP‐1 nor BNP cause vascular release of norepinephrine.
Although several prior studies have examined the effect of intravenous GLP‐1 on endothelial function, our study is unique in examining the direct vascular effect of intra‐arterial GLP‐1 while blocking its degradation by DPP4. Specifically, 2 prior studies examining the effect of intravenous GLP‐1 on endothelial function during hyperglycemic clamp suggested that GLP‐1 improves endothelial function, as measured by flow‐mediated dilation during hyperglycemia in diabetic subjects but not during normoglycemia.19–20 In contrast, Basu et al reported that intravenous GLP‐1 enhanced the forearm vasodilator response to intra‐arterial acetylcholine but not to nitroprusside in healthy subjects.21 Because systemic administration of GLP‐1 increases insulin, we infused GLP‐1 directly in the brachial artery. Tesauro et al also assessed the effect of intra‐arterial GLP‐1 and reported that GLP‐1 enhanced the FBF response to acetylcholine and nitroprusside in patients with metabolic syndrome during coinfusion of insulin but not during saline.22 In contrast to our study, the investigators did not inhibit the degradation of GLP‐1 by DPP4 and did not achieve concentrations of GLP‐1 comparable to physiological concentrations achieved after a meal.
These data in humans conflict with data in rodent models, which indicate that GLP‐1 causes direct vasodilation.7,23 The lack of effect of DPP4 inhibition by sitagliptin on the vascular response to GLP‐1 is particularly important because Ban et al reported that both GLP‐1 and its DPP4 metabolite GLP‐1(9‐36) dilate preconstricted mesenteric arteries through a GLP‐1 receptor–independent and nitric oxide synthase–dependent mechanism.7 In contrast, Tesauro et al reported no effect of intra‐arterial GLP‐1(9‐36) in the human forearm.22 Likewise, if endogenous GLP‐1(9‐36) causes vasodilation in humans, we would have expected to observe an increase in forearm vascular resistance during DPP4 inhibition, but instead we observed a decrease in baseline forearm vascular resistance.
Activation of the GLP‐1 receptor in the brain has also been shown to modulate sympathetic activity in animal models and humans. Yamamoto et al demonstrated that systemic administration of GLP‐1 receptor agonist increased blood pressure and heart rate in a dose‐dependent fashion and activated autonomic neurons responsible for sympathetic outflow in rats.9 Bharucha et al found that intravenous infusion of GLP‐1 in healthy subjects increased skeletal muscle sympathetic nerve activity but did not affect cardiac sympathetic indices, as assessed by spectral analysis.24 Vasodilation in response to GLP‐1 may be compromised by increased sympathetic activity; however, we administered GLP‐1 intra‐arterially, thereby avoiding systemic counter‐regulatory responses, and did not see any effect of GLP‐1 on norepinephrine release.
We also investigated the effect of sitagliptin on the vasodilatory response to BNP. Elevated BNP levels are characteristic of heart failure, and recombinant BNP reduces afterload and promotes natriuresis.25 DPP4 activity is increased in the setting of heart failure and thus may play a role in the pathophysiology of heart failure by increasing degradation of BNP to BNP(3‐32), which is a less potent vasodilator and natriuretic agent in dogs.11,26
We did not find that sitagliptin potentiated the vasodilatory response to BNP in the human forearm. We cannot exclude minimal potentiation of the effects of BNP because intra‐arterial infusion of BNP tended to reduce MAP from baseline during sitagliptin treatment only and did increase arterial norepinephrine levels. It is possible that there was mild systemic vasodilation and activation of the baroreflex following BNP infusion during DPP4 inhibition, but the lack of effect of sitagliptin on the heart rate response to BNP infusion does not support this. Chan et al reported that BNP promotes norepinephrine release in an ex vivo rodent model and proposed that this may account for the inability of recombinant BNP to reduce the rate of hospitalization and death from heart failure.27 We did not find an effect of intra‐arterial BNP on venous norepinephrine levels or net norepinephrine release in the presence or absence of sitagliptin.
We observed an increase in basal systolic blood pressure with an accompanying increase in FBF and decrease in forearm vascular resistance during sitagliptin, consistent with our previous observation in healthy subjects.3 Although we did not observe a significant increase in baseline heart rate or net norepinephrine release during sitagliptin in the present study, we have previously reported that DPP4 inhibition increases sympathetic activation by substance P.3 As noted, an effect of DPP4 inhibition on the degradation of GLP‐1 in the central nervous system could have contributed to this observed increase in systolic blood pressure.
Limitations
A few study design considerations warrant mention. We used an acute dose of 200 mg of sitagliptin because this dose inhibits DDP4 inhibition to the same extent as the clinically approved dose of 100 mg but within a shorter time period. We cannot exclude an effect of chronic DPP4 inhibition on vascular responses to GLP‐1 and BNP or on endothelial function. We studied a small sample size due to the rigorous nature of the protocol; this may have limited our power to detect an effect of sitagliptin on the vasodilator responses. We also studied healthy individuals in the fasting state to control for the fluctuations in other vasoactive hormones, including GLP‐1 and insulin, and to control for medications and diseases that may affect endothelial function. It is possible that our findings would have been different in diseased subjects, but prior studies do not support this, as discussed earlier. We focused on vasodilator responses and cannot exclude an effect of sitagliptin on the natriuretic response to BNP. We were unable to determine BNP levels due to limitations of commercially available assays.
Conclusions
Diabetes is associated with increased risk of heart attack and stroke. The recent Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial revealed an increased risk for hospitalization due to heart failure with the DPP4 inhibitor saxagliptin.13 DPP4 inhibitor therapies may influence cardiovascular risk by affecting the degradation of peptide substrates that influence vascular function and sympathetic activity. We previously reported that the DPP4 substrate substance P increases sympathetic activity when DPP4 inhibition is combined with angiotensin‐converting enzyme inhibition.3 In the present study, we investigated the impact of DPP4 inhibition on the vascular effects of GLP‐1 and BNP. Our data do not support the hypothesis that decreased degradation of GLP‐1 and BNP during acute DPP4 inhibition directly regulates vascular function or sympathetic activity in healthy humans, despite data to the contrary in animal models. Other mechanisms that may account for the increased risk of hospitalization for heart failure during chronic DPP4 inhibitor therapy should be explored.
Sources of Funding
This research was supported by NIH grants R01HL079184 and HL060906 (N.J.B.) and in part by Vanderbilt Clinical and Translational Science Awards (CTSA) grant UL1 TR000445‐06 from the NIH National Center for Advancing Translational Sciences. J.K.D. was supported by the Vanderbilt Physician Scientist Development Award and NIH grant 5P30GM092386. F.T.B. was supported by K23GM102676. The Vanderbilt Hormone Assay and Analytical Services Core is supported by NIH grants DK059637 and DK020593.
Disclosures
None.
Acknowledgments
We thank Stacy Gilbert, RN; Delia Woods, RN; and Carol Meisch, RN, for their recruitment of volunteers and nursing assistance. We thank Anthony Dematteo, Zuofei Wang, the Vanderbilt Hormone Assay Core, and the Vanderbilt Clinical Research Center Core Laboratory for their technical assistance.
References
- 1.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk‐Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha RR. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide‐(1‐38). J Biol Chem. 2003; 278:22418-22423. [DOI] [PubMed] [Google Scholar]
- 2.Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, McCaughan GW, Gorrell MD. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010; 277:1126-1144. [DOI] [PubMed] [Google Scholar]
- 3.Devin JK, Pretorius M, Nian H, Yu C, Billings FT, Brown NJ. Substance P increases sympathetic activity during combined angiotensin‐converting enzyme and dipeptidyl peptidase‐4 inhibition. Hypertension. 2014; 63:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase‐4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther. 2007; 81:761-767. [DOI] [PubMed] [Google Scholar]
- 5.Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP. Once‐daily sitagliptin, a dipeptidyl peptidase‐4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007; 23:1329-1339. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Avogaro A. Cardiovascular effects of DPP‐4 inhibition: beyond GLP‐1. Vascul Pharmacol. 2011; 55:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Ban K, Noyan‐Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation. 2008; 117:2340-2350. [DOI] [PubMed] [Google Scholar]
- 8.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon‐like peptide‐1 relaxes rat conduit arteries via an endothelium‐independent mechanism. Regul Pept. 2005; 125:173-177. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon‐like peptide‐1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002; 110:43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl‐peptidase IV converts intact B‐type natriuretic peptide into its des‐SerPro form. Clin Chem. 2006; 52:82-87. [DOI] [PubMed] [Google Scholar]
- 11.Boerrigter G, Costello‐Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des‐serine‐proline brain natriuretic peptide 3‐32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007; 292:R897-R901. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, 2014. [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with Type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317-1326. [DOI] [PubMed] [Google Scholar]
- 14.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with Type 2 diabetes. N Engl J Med. 2013; 369:1327-1335. [DOI] [PubMed] [Google Scholar]
- 15.Komarek M, Bernheim A, Schindler R, Steden R, Kiowski W, Brunner‐La Rocca HP. Vascular effects of natriuretic peptides in healthy men. J Cardiovasc Pharmacol Ther. 2004; 9:263-270. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Arakawa N, Yoshida H, Makita S, Niinuma H, Hiramori K. Vasodilatory effects of B‐type natriuretic peptide are impaired in patients with chronic heart failure. Am Heart J. 1998; 135:414-420. [DOI] [PubMed] [Google Scholar]
- 17.Lefebvre J, Murphey LJ, Hartert TV, Jiao SR, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE‐inhibitor‐associated angioedema. Hypertension. 2002; 39:460-464. [DOI] [PubMed] [Google Scholar]
- 18.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003; 88:2706-2713. [DOI] [PubMed] [Google Scholar]
- 19.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004; 287:E1209-E1215. [DOI] [PubMed] [Google Scholar]
- 20.Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon‐like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon‐like peptide 1 in diabetes. Diabetes Care. 2011; 34:697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP‐1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007; 293:E1289-E1295. [DOI] [PubMed] [Google Scholar]
- 22.Tesauro M, Schinzari F, Adamo A, Rovella V, Martini F, Mores N, Barini A, Pitocco D, Ghirlanda G, Lauro D, Campia U, Cardillo C. Effects of GLP‐1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013; 36:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon‐like peptide 1‐dependent mechanism. Hypertension. 2012; 60:833-841. [DOI] [PubMed] [Google Scholar]
- 24.Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon‐like peptide‐1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R874-R880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munagala VK, Burnett JC, Jr, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004; 29:707-769. [DOI] [PubMed] [Google Scholar]
- 26.Pala L, Rotella CM. The role of DPP4 activity in cardiovascular districts: in vivo and in vitro evidence. J Diabetes Res. 2013; 2013:590456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan NY, Seyedi N, Takano K, Levi R. An unsuspected property of natriuretic peptides: promotion of calcium‐dependent catecholamine release via protein kinase G‐mediated phosphodiesterase type 3 inhibition. Circulation. 2012; 125:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]


