Abstract
Background
Pulmonary artery pressure (PAP) is an important marker in cardiovascular disorders, being closely associated with morbidity and mortality. Noninvasive assessment by Doppler echocardiography is recommended by current guidelines. So far, the reliability of this method has been assessed only in small studies with contradictory results. Therefore, the aim of this study was to analyze the reliability of noninvasive PAP assessment by Doppler echocardiography compared to invasive measurements in a large patient population.
Methods and Results
We retrospectively analyzed data from a large tertiary cardiology department over 6 years in order to compare invasively measured PAP to estimated PAP from echocardiography examinations. N=15 516 patients fulfilled inclusion criteria and n=1695 patients with timely matched examinations (within 5 days) were analyzed. In n=1221 (72%) patients, pulmonary hypertension (PH) was diagnosed invasively (postcapillary PH: n=1122 [66%]; precapillary PH: n=99 [6%]). Systolic pulmonary artery pressure (sPAP) was 45.3±15.5 mm Hg by Doppler echocardiography and 47.4±16.4 mm Hg by right heart catheterization. Pearson's correlation coefficient was r=0.87 (P<0.0001). Mean right atrial pressure (RAP) was 12.0±5.7 mm Hg by right heart catheterization and was estimated to be 12.1±6.6 mm Hg by echocardiography (r=0.82, P<0.0001). Bland–Altman analysis showed a bias of −2.0 mm Hg for sPAP (95% limits of agreement −18.1 to +14.1 mm Hg) and +1.0 mm Hg for RAP (95% limits of agreement +0.1 to +1.9 mm Hg). Noninvasive diagnosis of pulmonary hypertension with Doppler echocardiography had a good sensitivity (87%) and specificity (79%), positive and negative predictive values (91% and 70%), as well as accuracy (85%) for a sPAP cut‐off value of 36 mm Hg (AUC 0.91, P<0.001, CI 0.90 to 0.93).
Conclusions
In this study, Doppler echocardiography proved to be a reliable method for the assessment of sPAP, being well suited to establish the noninvasive diagnosis of pulmonary hypertension in patients with cardiac diseases.
Keywords: Doppler echocardiography, pulmonary artery pressure, pulmonary hypertension, right heart catheterization
Introduction
Numerous cardiac and pulmonary pathologies are associated with an increase in pulmonary artery pressures (PAP), and multiple studies evidenced the prognostic relevance of pulmonary hypertension (PH). This condition is characterized by an increase in mean pulmonary arterial pressure and pulmonary vascular resistance, leading to right heart failure and death if left untreated.1–5 Furthermore, even slightly elevated PAP may have adverse prognostic implications in the general population.6 Therefore, the measurement of PAP has gained wide acceptance in the assessment and follow‐up of patients with cardiac or pulmonary disorders.
Direct pressure measurement with right heart catheterization is the reference method and “gold standard” for quantification of PAP; however, a noninvasive assessment of pulmonary artery systolic pressure (sPAP) by Doppler echocardiography is feasible, and represents a keystone examination in suspected PH according to current guidelines.7 Furthermore, echocardiography may represent an important step in the diagnosis work‐up in diseases in which prognosis may be largely affected by PH and may even differentiate the causes of PH.8–9 However, a reliable noninvasive quantification by Doppler echocardiography is an important prerequisite.
Assessment of sPAP by continuous‐wave Doppler was first reported by Yock and Popp 30 years ago.10 Right ventricular systolic pressure (RVSP) is calculated from the maximal flow velocity of the tricuspid valve regurgitation using the modified Bernoulli equation ΔP=4×Vmax2, and adding an estimated RAP.10 First publications of noninvasive sPAP measurements by Doppler echocardiography were very encouraging.10–13 Although assessment of RAP was simplified, a study by Chan et al14 in 1987 suggested that this method of noninvasive PAP assessment is superior compared to other noninvasive approaches.14 For many years, noninvasive PAP measurement by addition of RVSP and estimated RAP is advised and used in standard echocardiography routinely.7,9,15 However, until now only studies with small and selected patient populations were conducted to assess correlation between sPAP measurements by right heart catheterization and Doppler echocardiography. Furthermore, some recent studies questioned the accuracy of noninvasively measured sPAP.16–20
Thus, in this study we aimed to analyze the diagnostic accuracy and reliability of noninvasive PAP assessment compared to invasive measurements in a large patient population of a high‐turnover tertiary cardiac care center.
Methods
Study Protocol
The study was conducted retrospectively and is based on digitized data from right heart catheterizations and Doppler echocardiography examinations carried out at the Cardiology Department of the University Hospital of Heidelberg over 6 years (Figure 1). This analysis was carried out fulfilling the standards of the Ethics Committee of the University of Heidelberg and in concordance with the Declaration of Helsinki.
Figure 1.
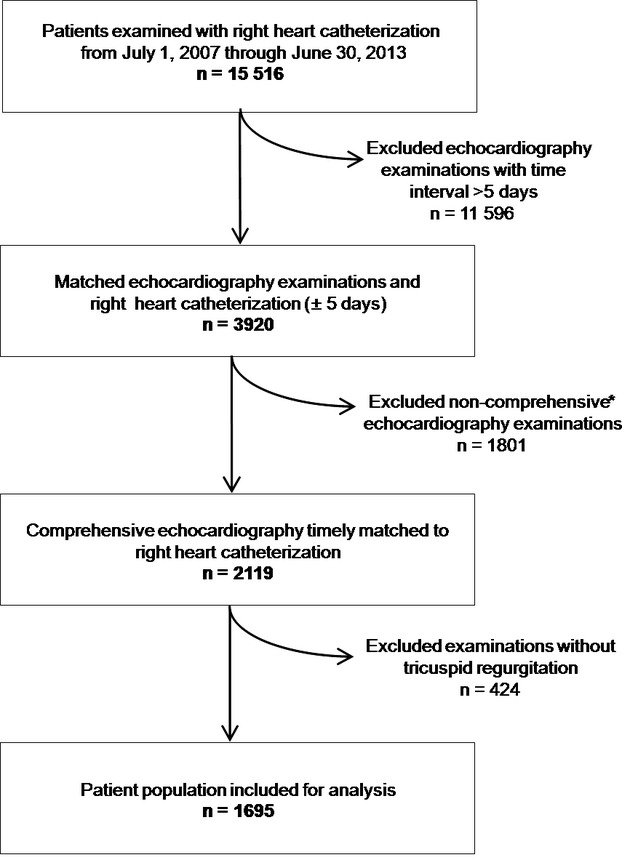
Flow chart depicting study protocol for analysis of systolic pulmonary artery pressures measurements by right heart catheterization and Doppler echocardiography over 6 years. *Noncomprehensive examinations (eg, transoesophageal echocardiography to rule out intracardiac thrombus prior to cardioversion of atrial fibrillation, examination to rule out pericardial effusion, focused examination to rule out left ventricular thrombus, and stress echocardiography).
All patients included in the study had appropriate clinical indications for echocardiography. Indications for right heart catheterization are listed in Table 1. We identified 15 516 consecutive patients with right heart catheterization and echocardiography examinations from July 1, 2007 through June 30, 2013 (Figure 1). In 3920 patients, a cardiac ultrasound examination was performed within 5 days before or after invasive examination. A number of the 2543 echocardiographic examinations had to be excluded because PAP measurements were not explicitly documented (eg, tricuspid regurgitation was absent, transesophageal and stress echocardiography, as well as selective examinations to rule out pericardial effusion or intracardiac thrombi). There were no further reasons for exclusion from the statistical analysis (eg, atrial fibrillation or severe tricuspid insufficiency). Thus, 1695 timely matched examinations were included in the statistical analysis.
Table 1.
Characteristics of the Study Population
| Characteristics | |
|---|---|
| N | 1695 |
| Age, y | 63±15 |
| Gender, % males | 67 |
| Height, cm | 172±9 |
| Weight, kg | 78±16 |
| BMI, kg/m² | 26±5 |
| BSA, m² | 1.9±0.2 |
| SBP, mm Hg | 128±19 |
| DBP, mm Hg | 68±13 |
| Functional classification, n (%) | |
| NYHA I | 85 (5) |
| NYHA II | 458 (27) |
| NYHA III | 864 (51) |
| NYHA IV | 288 (17) |
| Indications for heart catheterization, n (%) | |
| Known or suspected CMP | 542 (32) |
| Evaluation valve disease | |
| Aortic valve disease | 322 (19) |
| Mitral valve disease | 104 (6) |
| Tricuspid valve disease | 34 (2) |
| Known or suspected ICM | 459 (27) |
| Known or suspected PH | 99 (6) |
| Other miscellaneous indications | 135 (8) |
Values are given as mean±SD or numbers (percentage) as required. BMI indicates body mass index; BSA, body surface area; CMP, cardiomyopathy; DBP, diastolic blood; ICM, ischemic cardiomyopathy; NYHA, New York Heart Association; PH, pulmonary artery hypertension; SBP, systolic blood pressure.
Echocardiography
Echocardiographic examinations were performed on commercially available ultrasound systems (Vivid S5, Vivid i, Vivid 7, and Vivid E9 GE Healthcare Vingmed, Trondheim, Norway and ie33, Philips, Eindhoven, the Netherlands) according to the guidelines of the American Society of Echocardiography.21 Images were obtained in left lateral decubitus for parasternal and apical views and supine position for subxyphoidal views using 1.5 to 4.0 MHz phased‐array transducers. The comprehensive examination included standard 2D echocardiography for anatomic imaging and Doppler echocardiography for assessment of velocities. Doppler measurements were carried out over 3 heart cycles during passive expiration. All examinations were digitally stored in a Picture Archiving and Communication System (PACS) with accessibility for offline analysis on workstations (Centricity, GE Healthcare Vingmed, Trondheim, Norway).
Noninvasive assessment of pulmonary artery systolic pressures (sPAP) was achieved by measurement of right ventricular systolic pressure (RVSP) and adding RAP. RVSP was derived from the peak systolic velocity of the tricuspid regurgitation obtained with continuous‐wave (CW) Doppler using the modified Bernoulli equation: ΔP=4×Vmax2. RAP was estimated by the diameter of the inferior vena cava and its variability during inspiration as described before.9,21–22
Offline reassessment of CW Doppler spectral envelopes, as well as inferior vena cava diameter and respiratory behavior, was conducted in n=258 examination for clarification of misdiagnosis of PH by 2 independent, experienced examiners blinded to invasive data.
Right Heart Catheterization
A femoral or jugular venous approach was used for right heart catheterization. Cardiac output and cardiac index were calculated by saturation measurement according to Fick's method. PAP, pulmonary capillary wedge pressure, and right ventricular and right atrial pressures were measured during breath hold in baseline over at least 3 heart cycles. Mean pulmonary artery pressure was calculated by integration of the pressure curve by Metek software (Metek GmbH, Roetgen, Germany). Pulmonary vascular resistance was derived from pulmonary vascular resistance = (mean pulmonary artery pressure − pulmonary capillary wedge pressure)/cardiac output.
Statistical Analysis
Statistical analyses were performed by SPSS version 18.0 (IBM Corporation, Armonk, NY) and GraphPad Prism v5.0 (GraphPad Software Inc., La Jolla, CA) was used for plotting. Normal distribution was assessed by the Kolmogorov–Smirnov test. Results are expressed as mean±SD. A P<0.05 was considered statistically significant. For comparison of invasive and noninvasive measurements of sPAP and RAP, linear regression analyses were conducted and Pearson's correlation coefficient was calculated. Bland–Altman analyses were carried for sPAP and RAP measurements to show systematic deviations. For discrimination of sPAP threshold, receiver operating characteristics analysis was conducted. Sensitivity, specificity, positive and negative predictive values, as well as accuracy were analyzed for different cut‐off values.
Results
Clinical characteristics of the study population and indications for heart catheterization are presented in Table 1. A group of 1695 individuals who had time‐matched (±5 days) echocardiography examinations and right heart catheterization was included for analysis (Figure 1). A preponderance of male individuals could be found in this unselected patient population (67%). The majority of patients were found to be in New York Heart Association functional classification II (27%) and III (51%) at the time of examinations.
Findings during invasive examination and Doppler echocardiography are shown in Table 2. sPAP was 47.4±16.4 mm Hg by right heart catheterization and 45.3±15.5 mm Hg by Doppler echocardiography. Linear regression analysis showed a very good association, with a Pearson's correlation coefficient r of 0.87 (P<0.0001, SEE=8.1 mm Hg, Figure 2). Bland–Altman analysis showed a bias of −2.0 mm Hg (95% limits of agreement: −18.1 to +14.1 mm Hg, Table 3). Mean RAP was 12.0±5.7 mm Hg by right heart catheterization and 12.1±6.6 mm Hg by Doppler echocardiography. Pearson's correlation coefficient r was 0.82 (P<0.0001) and corresponding Bland–Altman analysis showed a bias of +1.0 (limits of agreement +0.1 to +1.9 mm Hg, Table 3). None of the patients presented a right ventricular outflow obstruction or a pulmonary valve stenosis at invasive examination. Details of the subgroup of patients with pulmonary arterial hypertension (n=99) are given in the supplemental analysis (Table 4). Analyses in regard to time latency were conducted on examinations that were carried out within 24 hours (n=932, 55%) and between 2 and 5 days (n=763, 45%), respectively. Near‐simultaneous examinations within 24 hours showed a closer correlation (r=0.89, P<0.0001), but an unchanged systematic underestimation of −2.3 mm Hg by Doppler echocardiography in the Bland–Altman analysis. Examinations with time latency of more than 24 hours show slightly lower correlation (r=0.81, P<0.0001) and a systematic underestimation of −2.4 mm Hg by Doppler echocardiography as well. Examinations that were carried out before right heart catheterization (n=1084, 64%) correlated better (r=0.89, P<0.0001) than examinations after right heart catheterization (n=611, 36%; r=0.84, P<0.0001) with systematic underestimation by Doppler echocardiography of −2.8 and −1.4 mm Hg, respectively.
Table 2.
Findings During Invasive Examination and Doppler Echocardiography
| Parameters | |
|---|---|
| Right heart catheterization | |
| CO, mL/min | 4.3±1.4 |
| CI, mL/min per m² | 2.3±0.7 |
| Hemoglobin, g/dL | 12.3±2.0 |
| PVR, dyn/(s∙cm−5) | 208±171 |
| sPAP, mm Hg | 47.4±16.4 |
| dPAP, mm Hg | 21.6±8.2 |
| mPAP, mm Hg | 31.6±10.9 |
| PCWP, mm Hg | 21.4±8.2 |
| mRAP, mm Hg | 12.0±5.7 |
| PH prevalence | |
| No PH, n (%) | 474 (28) |
| PH, n (%) | 1221 (72) |
| Doppler echocardiography | |
| sPAP, mm Hg | 45.3±15.5 |
| RAP, mm Hg | 12.1±6.6 |
Values are given as mean±SD. CO indicates cardiac output; CI, cardiac index; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension (no PH: mPAP<25 mm Hg; PH: mPAP≥25 mm Hg); PVR, pulmonary vascular resistance; RAP, right atrial pressure; sPAP, systolic pulmonary artery pressure.
Figure 2.
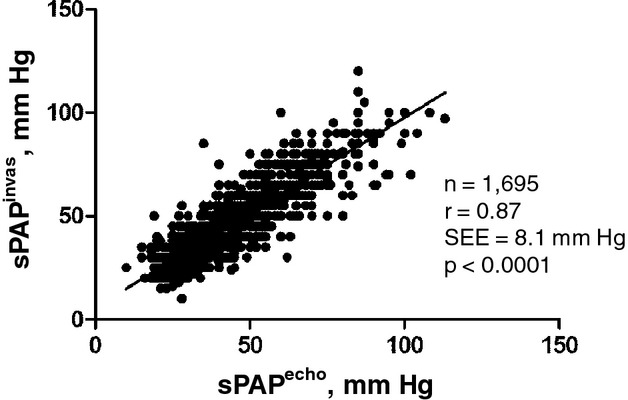
Linear regression analysis plot of invasive and noninvasive values of pulmonary artery systolic pressure. echo indicates noninvasive measurement by echocardiography; invas, invasive measurement by right heart catheterization; r, correlation coefficient (Pearson); sPAP, systolic pulmonary artery pressure.
Table 3.
Correlation and Bland–Altman Analysis of Pulmonary Artery Systolic Pressures and Right Atrial Pressures (Catheterization vs. Echocardiography)
| N | R | Bias | SD | 95% LOA | ||
|---|---|---|---|---|---|---|
| sPAP | 1695 | 0.87 | −2.0 | 8.2 | −18.1 | +14.1 |
| RAP | 1595 | 0.82 | +1.0 | 1.0 | +0.1 | +1.9 |
LOA, limits of agreement; RAP, right atrial pressure; sPAP, systolic pulmonary artery pressure.
Table 4.
Supplemental Analysis of PAH Subgroup
| Characteristics of patients with PAH | |
|---|---|
| N | 99 |
| Age, y | 69±14 |
| Gender, % males | 61 |
| Height, cm | 171±10 |
| Weight, kg | 77±15 |
| BMI, kg/m² | 26±4 |
| BSA, m² | 1.9±0.2 |
| SBP, mm Hg | 117±27 |
| DBP, mm Hg | 65±13 |
| Right heart catheterization | |
| CO, mL/min | 4.4±1.3 |
| CI, mL/min per m² | 2.3±0.6 |
| Hemoglobin, g/dL | 12.7±2.0 |
| PVR, dyn/(s∙cm−5) | 400±234 |
| sPAP, mm Hg | 53.1±16.0 |
| dPAP, mm Hg | 21.4±7.7 |
| mPAP, mm Hg | 34.0±9.8 |
| PCWP, mm Hg | 12.8±2.3 |
| mRAP, mm Hg | 9.2±3.9 |
| Doppler echocardiography | |
| sPAP, mm Hg | 50.9±16.0 |
| RAP, mm Hg | 10.1±5.0 |
| Linear regression analysis (sPAP) | |
| r (Pearson) | 0.89 |
| SEE, mm Hg | 7.5 |
| P value | <0.0001 |
| Bland–Altman analysis (sPAP) | |
| Bias, mm Hg | −2.2 |
| SD, mm Hg | 7.6 |
| 95% LOA, mm Hg | −12.8 to 17.1 |
| Linear regression analysis (RAP) | |
| r (Pearson) | 0.73 |
| SEE, mm Hg | 3.5 |
| P value | <0.0001 |
| Bland–Altman analysis (RAP) | |
| Bias, mm Hg | +0.5 |
| SD, mm Hg | 3.5 |
| 95% LOA, mm Hg | −7.4 to 6.5 |
Values are given as mean±SD or numbers (percentage) as required. BMI indicates body mass index; BSA, body surface area; CI, cardiac index; CO, cardiac output; DBP, diastolic blood; dPAP, diastolic pulmonary artery pressure; LOA, limits of agreement; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure.
The diagnosis of pulmonary hypertension, as defined by invasively measured mean pulmonary artery pressure ≥25 mm Hg, was feasible by noninvasive sPAP assessment with an AUC of 0.91 in the receiver operating characteristics analysis (P<0.001, 95% CI=0.90 to 0.93, Figure 3). A cut‐off value of 36 mm Hg showed good sensitivity (87%) and specificity (79%), good positive predictive value (91%), negative predictive value (70%), and best accuracy (85%) (Table 5). A lower cut‐off value of 31 mm Hg with an accuracy of 84% missed only 66 of 1221 patients with PH, but yielded 199 false‐positive reports.
Figure 3.
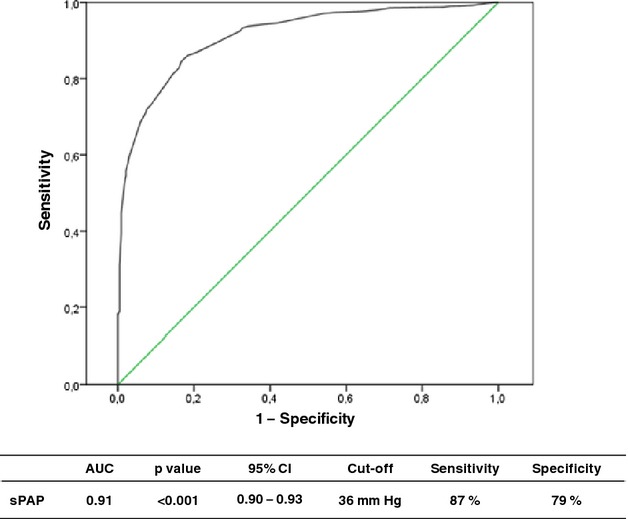
Receiver operating characteristics (ROC) analysis for noninvasive diagnosis of pulmonary hypertension as defined invasively (mean pulmonary artery pressure ≥25 mm Hg). AUC indicates area under the curve; sPAP, systolic pulmonary artery pressure.
Table 5.
Sensitivity, Specificity, and Diagnostic Accuracy of Noninvasive Assessment of Pulmonary Hypertension by Doppler Echocardiography at Various Cut‐Off Levels
| Cut‐off sPAP, mm Hg | Sensitivity, % | Specificity, % | PPV, % | NPV, % | ACC, % |
|---|---|---|---|---|---|
| ≥26 | 98.5 | 28.7 | 78.1 | 88.3 | 79.0 |
| ≥31 | 94.6 | 58.0 | 85.3 | 80.6 | 84.4 |
| ≥36 | 87.0 | 79.1 | 91.5 | 70.2 | 84.8 |
| ≥41 | 73.1 | 91.4 | 95.6 | 56.8 | 78.2 |
| ≥46 | 59.5 | 97.0 | 98.1 | 48.2 | 70.0 |
ACC indicates accuracy; NPV, negative predictive value; PPV, positive predictive value; sPAP, systolic pulmonary arterial pressure.
Systolic PAP overestimation and underestimation leading to significant overdiagnosis or underdiagnosis of pulmonary hypertension are listed in Table 5. An incomplete CW Doppler spectral envelope was the predominant cause for underestimation, whereas overestimation was due to different causes. In one third of the cases, overestimation was due to false interpretation of the spectral envelope maximal velocity boundary in the presence of vertical linear Doppler artifacts (“fringes”), followed by incomplete spectral‐wave envelope and too‐soft signal due to low spectral Doppler gain setting. Severe tricuspid regurgitation by echocardiographic definition was present in n=79 of all patients (5%) and a sPAP was documented in the echo report in spite of the restrictions of the measurement in this setting. However, severe tricuspid regurgitation accounted for only 11 cases (7%) of underestimation and missed diagnosis of PH. Misinterpretation of the diameter and respiration dynamics of the inferior vena cava was also involved in both underestimation and overestimation, but in a lesser manner.
Discussion
To the best of our knowledge, this is the first study that analyzes accuracy of noninvasive assessment of sPAP by Doppler echocardiography in comparison to invasive pressure measurement in a large, unselected patient population. In our study, Doppler echocardiography has proven to be a reliable noninvasive method for the assessment of sPAP. Furthermore, pulmonary hypertension could be detected with high sensitivity and specificity.
Our findings confirm previous reports. Thirty years ago, a first publication by Yock and Popp10 found a high correlation between noninvasive and invasive data in 54 patients (r=0.93, SEE=8 mm Hg).10 Berger et al11 found an even higher correlation in a study population of 41, when invasive and noninvasive examinations were conducted simultaneously in almost all patients (r=0.97, SEE=4.9 mm Hg).11 Currie et al12 assessed accuracy of noninvasively measured RVSP simultaneously with right heart catheterization in 127 patients and described very good correlations (r=0.96, SEE=7 mm Hg).12 Vazquez de Prada et al13 conducted a similar study including 34 patients with adequate CW Doppler tracings, with a time interval of 24 to 48 hours between examinations; nonetheless, they showed high correlations as well (r=0.96).13
Although recommended in many guidelines7,9,15 and used routinely, noninvasive quantification of sPAP has never been tested sufficiently in unselected large patient populations undergoing routine echocardiography examination. This lack of information has sparked the discussion about reliability of this method. Few publications had mentioned doubts about is reliability. Arcasoy et al16 found in a cohort study of 374 patients with advanced lung disease that in 52% of the cases, pressure estimations were inaccurate, and that 48% of patients were false positive for pulmonary hypertension as determined by echocardiography. Noninvasive estimation of sPAP was not possible in 57% of the patients. The authors suggested that factors related to chronic pulmonary disease may have influenced the results, limiting accurate visualization and measurement of the tricuspid regurgitation jet.16
In our study, only 424 patients had to be excluded due to the absence of tricuspid regurgitation (20%). This ratio is much lower than described by Arcasoy et al.16 In 227 of these 424 patients, pulmonary hypertension with mean pulmonary artery pressure ≥25 mm Hg was documented invasively, confirming that absence of a measurable tricuspid regurgitation does not exclude PH.
Some years later, 3 smaller studies—Fisher et al17 (n=65), Rich et al18 (n=183), and D'Alto et al19 (n=152) —as well as the analysis of the REVEAL registry20 stated that Doppler echocardiography may frequently be inaccurate, causing frequent overestimation and underestimation of PAP in patients being evaluated for pulmonary hypertension. They suggested that this method should not be relied on to make individual diagnoses of pulmonary hypertension.
Three recent meta‐analyses by Zhan et al,23 Janda et al24, and Taleb et al25 assessed this issue on the basis of n=736 (6 studies), n=1485 (29 studies), and n=522 (9 studies), respectively. The mean time interval between echocardiography and right heart catheterization of the included studies ranged from 2 hours to 90 days. The reported results are not completely consistent. Whereas accuracy calculated with the random effects model by Taleb et al25 was low (73%), sensitivity for diagnosis was similarly high in all 3 meta‐analyses (82%, 83%, and 88%, respectively). Specificity was found to show a higher variation (68%, 72%, and 56%, respectively).23–25 In our study, the receiver operating characteristics analysis demonstrates a high diagnostic accuracy for a sPAP cut‐off value of 36 mm Hg (AUC=0.91, sensitivity=87%, specificity=79%, accuracy=85%, Figure 3 and Table 5). Higher cut‐off levels show better specificity for diagnosis of PH, but on the basis of increasing numbers of patients with missed diagnoses. Even lower cut‐off levels may be appropriate in younger persons as sPAP values are age dependent as described in a comprehensive echocardiography study by Lam et al.6
Potential sources of error for the sPAP assessment with Doppler echocardiography were already discussed by Yock and Popp,10 and commented on in the subsequent studies.11–12,11–20 In our study, we could document a series of sources of error in an offline analysis of all cases, with significant overestimation or underestimation (Table 6). Contrary to former assertions, characteristics of the inferior vena cava were not the main reason for inaccuracy, but rather were due to the many pitfalls inherent to the Doppler method. Thus, optimal settings when assessing tricuspid regurgitation velocities should be taken into account. Further pitfalls should be kept in mind. Stenosis of the right ventricular outflow tract, pulmonary valve, or the pulmonary trunk may lead to systematic overestimation of right ventricular systolic pressure. Severe tricuspid insufficiency may lead to underestimation as pressure gradients decrease with increasing effective regurgitant orifice area. Furthermore, since the absence of tricuspid regurgitation does not rule out PH, the presence of indirect signs must be observed.
Table 6.
Sources of sPAP Overestimation or Underestimation Leading Correspondingly to Overdiagnosis or Underdiagnosis of Pulmonary Hypertension
| Overestimation n=99 | Underestimation n=159 | |
|---|---|---|
| CW Doppler, n (%) | ||
| Incomplete spectral wave envelope | 16 (16.2) | 110 (69.6) |
| Maximal velocity boundary artifacts (“fringes”) | 33 (33.3) | 2 (1.3) |
| Spectral gain set too soft | 12 (12.1) | 1 (0.6) |
| Velocity range set too high | 7 (7.1) | 1 (0.6) |
| Sweep velocity set to slow | 3 (3.0) | 1 (0.6) |
| Valve closure artifacts (“snaps”) | 2 (2.0) | 2 (1.3) |
| Atrial fibrillation | 7 (7.1) | 3 (1.9) |
| Severe tricuspid regurgitation | 1 (1.0) | 11 (7.0) |
| Inferior vena cava, n (%) | ||
| Respiration dynamics misinterpreted | 9 (9.1) | 20 (12.6) |
| Could not be depicted | 8 (8.1) | 7 (4.4) |
| Aorta mistaken as inferior vena cava | 1 (1.0) | 0 (0) |
Overestimation was defined when sPAP assessed with Doppler echocardiography was >35 mm Hg and mPAP measured invasively was <25 mm Hg, whereas underestimation was defined when sPAP assessed with Doppler echocardiography was ≤35 mm Hg and mPAP measured invasively was ≥25 mm Hg. CW indicates continuous‐wave Doppler; mPAP, pulmonary artery mean pressure; sPAP, systolic pulmonary artery pressure.
Based on our experience, the following aspects should be considered for best results: (1) since angle between ultrasound beam and direction of flow must be kept to a minimum, tricuspid regurgitation should first be detected with color Doppler from the best ultrasound view and multiple transducer positions may be necessary; (2) settings should be corrected, a colored spectral wave should be chosen, gain for optimal signal‐to‐noise ratio, velocity range allowing waves to fill at least two thirds of spectral depiction, and sweep velocity set to 100 to 200 mm/s should be attempted; (3) only signals extended for at least half of the systole should be measured, and incomplete or absent tricuspid regurgitation may be avoided by increasing blood pool volume with a strategy as simple as drinking a cup of water before examination; (4) maximal velocities in the presence of atrial fibrillation should be averaged; and (5) maximal velocities should be measured at the best spectral‐wave boundary, avoiding including Doppler artifacts (“fringes”).
Limitations
The interpretation of the study results is limited by its retrospective manner, as well as due to the fact that this analysis represents the data collected in only 1 cardiology center.
This study addressed all patients with right heart catheterization and PAP measurement by Doppler echocardiography within 5 days. The majority of patients with elevated PAP had postcapillary PH. Only a minority of n=99 patients had pulmonary arterial hypertension.
Doppler echocardiography and right heart catheterization were carried out nonsimultaneously, with time latency of 2 to 5 days for 45% of the study population. Fifty‐five percent of the examinations were carried out within 24 hours.
Conclusions
Our study validates the noninvasive quantification of sPAP by Doppler echocardiography in a large, unselected patient population. Values obtained noninvasively show reliable results and good correlation to invasive measurements. PH can be detected with high sensitivity and specificity. However, several pitfalls should be taken into account to achieve best results.
Sources of Funding
The authors had full access to the data and take responsibility for its integrity. All authors read and agreed to the article as written. All authors declare that they have no conflict of interest.
References
- 1.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann Intern Med. 1991; 115:343-349. [DOI] [PubMed] [Google Scholar]
- 2.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001; 37:183-188. [DOI] [PubMed] [Google Scholar]
- 3.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jöbsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002; 39:1214-1219. [DOI] [PubMed] [Google Scholar]
- 4.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006; 114:1883-1891. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013; 62:D42-D50. [DOI] [PubMed] [Google Scholar]
- 6.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age‐associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009; 119:2663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez‐Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau GESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009; 30:2493-2537. [DOI] [PubMed] [Google Scholar]
- 8.Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, Müller‐Ladner U, Pope JE, Vonk MC, Doelberg M, Chadha‐Boreham H, Heinzl H, Rosenberg DM, McLaughlin VV, Seibold JRon behalf of the DETECT study group. Evidence‐based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014; 73:1340-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23:685-713. [DOI] [PubMed] [Google Scholar]
- 10.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984; 70:657-662. [DOI] [PubMed] [Google Scholar]
- 11.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985; 6:359-365. [DOI] [PubMed] [Google Scholar]
- 12.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler‐catheterization study in 127 patients. J Am Coll Cardiol. 1985; 6:750-756. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez de Prada JA, Ruano J, Martin‐Duran R, Larman M, Zueco J, Ortiz de Murua JA, Torres A, Figueroa A. Noninvasive determination of pulmonary arterial systolic pressure by continuous wave Doppler. Int J Cardiol. 1987; 16:177-184. [DOI] [PubMed] [Google Scholar]
- 14.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987; 9:549-554. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009; 119:2250-2294. [DOI] [PubMed] [Google Scholar]
- 16.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003; 167:735-740. [DOI] [PubMed] [Google Scholar]
- 17.Fisher MR, Forfia PR, Chamera E, Housten‐Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009; 179:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011; 139:988-993. [DOI] [PubMed] [Google Scholar]
- 19.D'Alto M, Romeo E, Argiento P, D'Andrea A, Vanderpool R, Correra A, Bossone E, Sarubbi B, Calabrò R, Russo MG, Naeije R. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013; 168:4058-4062. [DOI] [PubMed] [Google Scholar]
- 20.Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011; 17:56-64. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJChamber Quantification Writing Group, American Society of Echocardiography's Guidelines and Standards Committee, European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 22.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990; 66:493-496. [DOI] [PubMed] [Google Scholar]
- 23.Zhang RF, Zhou L, Ma GF, Shao FC, Wu XH, Ying KJ. Diagnostic value of transthoracic Doppler echocardiography in pulmonary hypertension: a meta‐analysis. Am J Hypertens. 2010; 23:1261-1264. [DOI] [PubMed] [Google Scholar]
- 24.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta‐analysis. Heart. 2011; 97:612-622. [DOI] [PubMed] [Google Scholar]
- 25.Taleb M, Khuder S, Tinkel J, Khouri SJ. The diagnostic accuracy of Doppler echocardiography in assessment of pulmonary artery systolic pressure: a meta‐analysis. Echocardiography. 2013; 30:258-265. [DOI] [PubMed] [Google Scholar]


