Abstract
Background
Red cell distribution width (RDW), a measure of the variability in size of circulating erythrocytes, is associated with mortality and adverse outcome in selected populations with cardiovascular disease. It is scarcely known whether RDW is associated with incident myocardial infarction (MI). We aimed to investigate whether RDW was associated with risk of first‐ever MI in a large cohort study with participants recruited from a general population.
Methods and Results
Baseline characteristics, including RDW, were collected for 25 612 participants in the Tromsø Study in 1994–1995. Incident MI during follow‐up was registered from inclusion through December 31, 2010. Cox regression models were used to calculate hazard ratios with 95% confidence intervals for MI, adjusted for age, sex, body mass index, smoking, hemoglobin, white blood cells, platelets, and other traditional cardiovascular risk factors. A total of 1779 participants experienced a first‐ever MI during a median follow‐up time of 15.8 years. There was a linear association between RDW and risk of MI, for which a 1% increment in RDW was associated with a 13% increased risk (hazard ratio 1.13; 95% CI, 1.07 to 1.19). Participants with RDW above the 95th percentile had 71% higher risk of MI compared with those with RDW in the lowest quintile (hazard ratio 1.71; 95% CI, 1.34 to 2.20). All effect estimates were essentially similar after exclusion of participants with anemia (n=1297) from the analyses.
Conclusion
RDW is associated with incident MI in a general population independent of anemia and cardiovascular risk factors.
Keywords: blood cells, cardiovascular disease, epidemiology, risk factors
Introduction
Red cell distribution width (RDW), a measure of the variability in size of circulating erythrocytes,1 is calculated by automated blood cell counters as part of the routine blood cell count analysis. Traditionally, RDW and mean corpuscular volume (MCV) are used in the differential diagnosis of anemia, particularly anemias that are microcytic (caused by iron deficiency) or macrocytic (due to vitamin B12 or folate deficiency). An increased RDW can also result from conditions that modify the shape of red blood cells due to the premature release of immature cells into the bloodstream, to hemoglobinopathies, or to other hematological diseases.2
Myocardial infarction (MI) remains a major cause of morbidity and mortality worldwide.3–4 Consequently, identification of novel risk factors is of great potential value to improve risk stratification and facilitate targeted prevention of MI. In a study from the National Health and Nutrition Examination Survey (NHANES), a representative US population, participants with RDW values above the 75th percentile were aggregated in the highest 10‐year Framingham risk category for coronary heart disease.5 Other observational studies in selected cohorts of patients with heart disease have reported that RDW predicts all‐cause mortality,6–7 cardiac morbidity and mortality,8–9 and adverse outcome in patients with heart failure.10–12 Furthermore, RDW predicted all‐cause mortality and cardiovascular (CV) mortality in 2 population‐based studies from NHANES.13–14
The association between RDW and MI has previously been reported mainly in patients with known CV disease or heart failure.8–12 Although growing evidence supports the hypothesis that high RDW is associated with an unfavorable CV risk profile and higher total and CV mortality in various populations, the impact of RDW on the risk of incident MI in a general population is unclear. Consequently, we aimed to investigate whether RDW was associated with risk of first‐ever event of MI in a large prospective study with participants recruited from a general population.
Material and Methods
Participants
Participants were recruited from the fourth survey of the Tromsø Study, conducted in 1994–1995. The Tromsø Study is a single‐center, population‐based, prospective study of total birth cohorts and population samples, with repeated health surveys of the inhabitants in Tromsø, Norway. Tromsø is the largest city in the northern part of Norway. The population is predominately urban, middle‐ class whites of Norwegian origin. A detailed description of the study design and population has been published earlier.15
All inhabitants aged 25 years and older were invited to the survey. In total, 27 158 (77%) of the invited population participated. We excluded 202 persons without written consent, 688 persons with a previous history of MI, 43 persons not officially registered as inhabitants of Tromsø, and 613 persons without RDW measurement. Overall, 25 612 persons (13 659 women and 11 953 men) were eligible for the present study.
The study was conducted by the University of Tromsø in cooperation with the National Health Screening Service. The Regional Committee for Medical and Health Research Ethics, the Data Inspectorate, and the Directorate of Health and Social Affairs approved the study. Each participant gave written informed consent prior to participation.
Baseline Measurements
Baseline information was collected by physical examinations, blood samples, and self‐administered questionnaires.
Height and weight were measured with participants wearing light clothing and no shoes and using electronic scales. Body mass index was calculated as kg/m2. Blood pressure was recorded automatically in seated participants by trained personnel using the Dinamap Vital Signs Monitor. Three recordings were taken, and the mean of the last 2 readings was used in this analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self‐reported use of antihypertensive medication.
Blood samples were collected from an antecubital vein. For RDW, hemoglobin, white blood cells, platelets and MCV, a 5‐mL blood sample was drawn into vacutainer tubes containing EDTA as the anticoagulant (K3‐EDTA 40 μL, 0.37 mol/L per tube). These samples were analyzed within 12 hours in an automated blood cell counter (Coulter Counter; Coulter Electronics). RDW was calculated as the SD of MCV divided by MCV×100%. The analytical variation coefficient of RDW was less than 3%.
Serum and citrated plasma were prepared by centrifugation after 1 hour respite at room temperature. Nonfasting serum total cholesterol and triglycerides were analyzed by enzymatic colorimetric methods with commercial kits, as described elsewhere.16 Serum high‐density lipoprotein cholesterol was measured after precipitation of lower density lipoproteins with heparin and manganese chloride. The Department of Clinical Chemistry, University Hospital of North Norway, analyzed all blood samples.
Information on smoking habits and self‐reported diabetes was obtained from self‐administered questionnaires. The question on smoking read, “Do you smoke: Cigarettes or cigars/cigarillos or pipe daily?” (“yes” means yes to any of these 3 questions, “no” means no to all of these 3 questions). The question on diabetes read, “Do you have or have you had diabetes?” (yes or no).
Outcome Measurement of Myocardial Infarction
The unique Norwegian national 11‐digit identification number allowed linkage to national and local diagnosis registries. All first‐time events of MI were identified by linkage to the diagnosis registries at University Hospital of North Norway (outpatient diagnoses included) and the National Causes of Death Registry at Statistics Norway. Cases of possible incident nonfatal and fatal MI were identified by a broad search for the International Classification of Diseases, 9th revision codes 410 to 414, 430 to 438, and 798 to 799 in the period 1994–1998 and thereafter for the International Classification of Diseases, 10th revision codes I20 to I25, I60 to I69, and R96, R98, and R99. University Hospital of North Norway is the only hospital serving this community, with the next hospital being located ≈250 km away by road (148 km by air). The Causes of Death Registry covers participants registered as living in Norway at the time of their death, regardless of whether the death took place in Norway or abroad.
All possible events of MI were validated by an independent end point committee. The hospital medical records were retrieved for case validation. Information from the National Causes of Death Registry and from death certificates was used to collect relevant information of the event from additional sources such as autopsy reports and records from nursing homes, ambulance services, and general practitioners. We performed manual and/or electronic text searches in paper versions (used until 2001) and digital versions of hospital records for notes on MI in all participants with 1 diagnosis or more of those mentioned above. A systematic text search for MI was also performed in participants with one of the diagnoses other than MI.
We included all incident events classified as definite, probable, or possible MI, based on a classification algorithm that included clinical symptoms and signs, findings in electrocardiograms, values of cardiac biomarkers, and autopsy reports, when applicable (Table 1).17
Table 1.
Classification Algorithm for Myocardial Infarction. The Tromsø Study
| Definite MI | Definite MI was defined by one of the following sets of conditions:
|
| Probable MI | Probable MI was defined by one of the following sets of conditions:
|
| Possible MI | An event that can be dated and for which secondary data of typical history in combination with ECG findings and/or echocardiography and/or autopsy are consistent with MI but for which no primary data source is available |
| Unstable angina | Angina at rest or minimal exertion and ST‐depression or negative T‐wave in ECG |
| Unclassifiable | Increase in troponins or enzymes in relation to cardiac revascularization procedures (percutaneous coronary intervention or coronary artery bypass grafting) or otherwise unclassifiable |
| Silent MI | In the absence of clinical symptoms that can be dated:
|
| No MI | The conclusion after the validation procedure is that the event does not fulfill the criteria for an acute coronary event |
CABG indicates coronary artery bypass graft surgery; ECG, electrocardiography; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Follow‐up time was assigned from the date of examination (1994–1995) to the date of first‐ever MI, date of death (n=2845), date of migration from Tromsø (n=3811), or through December 31, 2010, whichever came first.
Statistics
The RDW values were divided into quintiles (quintile 1: 10.7% to 12.2%; quintile 2: 12.3% to 12.5%; quintile 3: 12.6% to 12.8%; quintile 4: 12.9% to 13.2%; quintile 5: 13.3% to 30.5%). The 95th percentile cut‐off value of RDW was 14.3%. Age‐adjusted values of the different baseline variables across quintiles of RDW were estimated using linear regression. Tests for baseline differences of potential confounders between those without and with incident MI were made using 2‐sample t tests (continuous variables) or the chi‐square test (binary variables).
Crude incidence rates were calculated as the total number of events divided by the total person‐time and expressed as events per 1000 person‐years. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs of incident MI across quintiles of RDW or RDW above the 95th percentile. Age was used as a time scale in the analyses, and the lowest quintile of RDW was used as the reference. Model 1 estimated the univariable associations. In model 2, we added other factors that could confound the association (sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelet count). In model 3, we also included the following CV risk factors: hypertension, total cholesterol, triglycerides, diabetes, and red blood cell count.
Finally, we analyzed RDW as a continuous variable and estimated HR of MI per 1% increase in RDW. Multivariable adjusted associations between RDW and MI were visualized by an additive Cox regression plot. In this plot, RDW was modeled with a smoothing spline with 4 degrees of freedom fit in a Cox proportional hazards model including the same variables as in model 2.
The proportional hazards assumption was tested using Schoenfeld residuals. Statistical interactions were tested by including cross‐product terms of sex and RDW or age and RDW into the final model (model 2). There were no sex interactions. The number of participants included in the different adjustment models varied slightly due to missing data for covariates (in total 1.3% missing). P values <0.05 were regarded as statistically significant. The statistical packages R (version 2.15.1 for Windows; R Foundation), SPSS (version 21 for Mac; IBM Corp) and STATA 12.1SE (Stata Corp) were used.
Results
A total of 1779 participants (1080 men and 699 women) experienced a first‐ever MI during a median follow‐up time of 15.8 years. The crude incidence rate was 5.09 (95% CI, 4.85 to 5.33) per 1000 person‐years.
Age at baseline and age‐adjusted baseline characteristics of the study participants across quintiles of RDW are shown in Table 2. Mean age, body mass index, systolic and diastolic blood pressure, serum total cholesterol, prevalence of daily smokers, white blood cells, and platelets increased across increasing RDW quintiles, whereas hemoglobin decreased.
Table 2.
Baseline Characteristics by Quintiles of Red Cell Distribution Width. The Tromsø Study
| Red cell distribution width | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|---|---|---|---|---|---|
| N | 5081 | 4702 | 4886 | 4900 | 6043 |
| Median, % (range) | 12.0 (10.7 to 12.2) | 12.4 (12.3 to 12.5) | 12.7 (12.6 to 12.8) | 13.0 (12.9 to 13.2) | 13.7 (13.3 to 30.5) |
| Age, y | 40.2 (12.0) | 43.4 (13.2) | 45.4 (13.9) | 48.8 (14.7) | 52.8 (15.8) |
| Systolic blood pressure, mm Hg | 130 (17) | 133 (19) | 134 (20) | 136 (21) | 139 (23) |
| Diastolic blood pressure, mm Hg | 75 (11) | 77 (12) | 78 (12) | 79 (12) | 80 (14) |
| Hypertension, %* | 32.2 (1306) | 33.3 (1469) | 31.9 (1614) | 31.2 (1843) | 32.7 (2719) |
| Body mass index, kg/m2 | 24.6 (3.4) | 25.0 (3.7) | 25.2 (3.7) | 25.3 (3.9) | 25.5 (4.3) |
| Total cholesterol, mmol/L | 5.7 (1.2) | 5.9 (1.3) | 6.0 (1.3) | 6.2 (1.3) | 6.3 (1.3) |
| High‐density lipoprotein cholesterol, mmol/L | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.6 (0.4) |
| Triglycerides, mmol/L | 1.5 (1.1) | 1.5 (1.0) | 1.6 (1.1) | 1.5 (1.0) | 1.5 (1.0) |
| Daily smoking, % | 26.8 | 31.8 | 36.2 | 41.8 | 47 |
| Self‐reported diabetes, % | 1.9 | 1.8 | 2.1 | 1.3 | 1.1 |
| Hemoglobin (total), g/dL | 14.1 (1.1) | 14.1 (1.1) | 14.1 (1.1) | 14.1 (1.1) | 13.7 (1.4) |
| Hemoglobin (women), g/dL | 13.4 (0.8) | 13.4 (0.8) | 13.4 (0.9) | 13.4 (0.9) | 13.0 (1.2) |
| Hemoglobin (men), g/dL | 15.0 (0.8) | 14.9 (0.8) | 14.9 (0.9) | 14.8 (0.9) | 14.5 (1.1) |
| Mean corpuscular volume, fL | 89 (3.3) | 89 (3.5) | 89 (3.6) | 89 (3.8) | 88 (5.8) |
| Red blood cells, ×1012/L | 4.6 (0.4) | 4.7 (0.4) | 4.7 (0.4) | 4.6 (0.4) | 4.6 (0.4) |
| White blood cells, ×109/L | 6.9 (1.8) | 7.0 (1.9) | 7.1 (2.1) | 7.1 (2.0) | 7.3 (2.2) |
| Platelets, ×109/L | 251 (51) | 250 (52) | 250 (53) | 252 (55) | 260 (66) |
Age at baseline and age‐adjusted baseline characteristics by quintiles of red cell distribution width, expressed as means (with SD in parentheses) for continuous variables and percentages for dichotomous variables.
Defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self‐reported use of antihypertensive medication.
Baseline characteristics of participants without and with incident MI are shown in Table 3. Participants with MI were older, had higher systolic and diastolic blood pressure, body mass index, total cholesterol, triglycerides, hemoglobin, red and white blood cell counts, and a higher proportion of daily smokers and self‐reported diabetes at baseline. High‐density lipoprotein cholesterol concentrations and platelet counts were lower in the MI group. P values were <0.001 for differences between groups for all variables.
Table 3.
Baseline Characteristics by Development of Myocardial Infarction. The Tromsø Study
| Myocardial Infarction | Without | With | P Value |
|---|---|---|---|
| N | 23 833 | 1779 | |
| Mean (SD) | Mean (SD) | ||
| Age, y | 45.2 (14.2) | 62.4 (13.2) | <0.001 |
| Sex (female), % | 54.4 | 39.3 | <0.001 |
| Systolic blood pressure, mm Hg | 133 (19) | 153 (24) | <0.001 |
| Diastolic blood pressure, mm Hg | 77 (12) | 87 (14) | <0.001 |
| Hypertension, %* | 32.3 (7689) | 70.9 (1262) | <0.001 |
| Body mass index, kg/m2 | 25.0 (3.8) | 26.6 (4.1) | <0.001 |
| Total cholesterol, mmol/L | 6.0 (1.3) | 6.9 (1.3) | <0.001 |
| High‐density lipoprotein cholesterol, mmol/l | 1.5 (0.4) | 1.4 (0.4) | <0.001 |
| Triglycerides, mmol/L | 1.5 (1.0) | 2.0 (1.2) | <0.001 |
| Daily smoking, % | 36.9 (8767) | 41.2 (731) | <0.001 |
| Self‐reported diabetes, % | 1.3 (298) | 6.5 (116) | <0.001 |
| Hemoglobin (total), g/dL | 14.0 (1.2) | 14.4 (1.1) | <0.001 |
| Mean corpuscular volume, fL | 89 (4) | 89 (4) | <0.001 |
| Red blood cells, ×1012/L | 4.6 (0.4) | 4.7 (0.4) | <0.001 |
| White blood cells, ×109/L | 7.1 (2.0) | 7.5 (2.2) | <0.001 |
| Platelets, ×109/L | 254 (56) | 247 (57) | <0.001 |
Baseline characteristics of the population who did not and did develop myocardial infarction, expressed as means (with SD in parentheses) for continuous variables and percentages (with numbers in parentheses) for dichotomous variables.
Defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self‐reported use of antihypertensive medication.
When RDW was modeled as a continuous variable, a clear linear dose‐response relationship between RDW and risk of MI was found (Figure). A 1% increment in RDW was associated with a 16% increased risk of MI (HR 1.16; 95% CI, 1.11 to 1.21) after adjustment for age, sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelets (model 2). Further adjustment for hypertension, total cholesterol, triglycerides, diabetes, and red blood cell count (model 3) did not change the risk estimate (HR 1.13; 95% CI 1.07 to 1.19).
Figure 1.
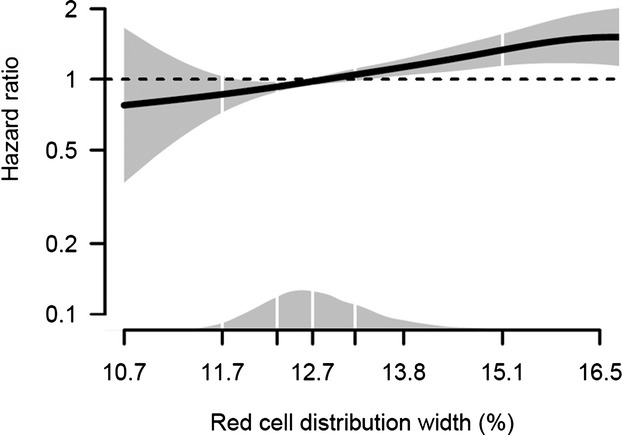
Dose–response relationship between red cell distribution width and risk of myocardial infarction obtained by additive Cox regression plot. The regression model is adjusted for sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelets. The solid line shows the hazard ratio, and the shaded area shows the 95% confidence interval. The density plot shows the distribution of red cell distribution width, and white vertical lines indicate 2.5th, 25th, 50th, 75th and 97.5th percentiles.
Crude incidence rates and adjusted HRs across quintiles of RDW and above the 95th percentile of RDW are shown in Table 4. The risk of MI was 69% higher in the highest quintile (quintile 5) compared with the lowest quintile (quintile 1, model 1). Adjustment for sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelets (model 2) attenuated the risk estimate by 15% (HR 1.44; 95% CI, 1.20 to 1.72). Further adjustments for hypertension, total cholesterol, triglycerides, diabetes, and red blood cell count (model 3) did not substantially affect the risk estimates (quintile 5: HR 1.34; 95% CI, 1.11 to 1.60). Participants with RDW above the 95th percentile had 94% higher risk of MI compared with those within the lowest quintile of RDW (model 2: HR 1.94; 95% CI, 1.53 to 2.45). Further adjustments for hypertension, total cholesterol, triglycerides, diabetes, and red blood cell count (model 3) did not substantially alter the risk estimate (HR 1.71; 95% CI, 1.34 to 2.20).
Table 4.
Adjusted Hazard Ratios for Myocardial Infarction. The Tromsø Study 1994–2010
| RDW | N | Median RDW, % (range) | Person‐Years | Event No. | IR (95% CI) | Model 1* | Model 2* | Model 3* |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Quintile 1 | 5081 | 12.0 (10.7 to 12.2) | 68 561 | 158 | 2.3 (2.0 to 2.7) | Reference | Reference | Reference |
| Quintile 2 | 4702 | 12.4 (12.3 to 12.5) | 63 661 | 226 | 3.6 (3.1 to 4.0) | 1.15 (0.94 to 1.41) | 1.11 (0.90 to 1.36) | 1.09 (0.89 to 1.34) |
| Quintile 3 | 4886 | 12.7 (12.6 to 12.8) | 65 588 | 291 | 4.4 (3.9 to 5.0) | 1.23 (1.02 to 1.50) | 1.11 (0.91 to 1.35) | 1.05 (0.86 to 1.28) |
| Quintile 4 | 4900 | 13.0 (12.9 to 13.2) | 64 523 | 414 | 6.4 (5.8 to 7.1) | 1.43 (1.19 to 1.72) | 1.26 (1.05 to 1.52) | 1.21 (1.00 to 1.46) |
| Quintile 5 | 6043 | 13.7 (13.3 to 30.5) | 74 705 | 690 | 9.2 (8.6 to 9.9) | 1.69 (1.41 to 2.01) | 1.44 (1.20 to 1.72) | 1.34 (1.11 to 1.60) |
| >95th percentile | 1262 | 15.1 (14.4 to 30.5) | 14 824 | 151 | 10.2 (8.7 to 11.9) | 1.98 (1.58 to 2.48) | 1.94 (1.53 to 2.45) | 1.71 (1.34 to 2.20) |
Crude IR per 1000 person‐years and adjusted HR with 95% CI for myocardial infarction across quintiles and values above the 95th percentile (>14.3%) for RDW. IR indicates incidence rate; HR, hazard ratio; RDW, red cell distribution width.
Model 1: Age was used as time scale.
Model 2: Model 1 plus sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelets.
Model 3: Model 2 plus hypertension, total cholesterol, triglycerides, self‐reported diabetes, and red blood cell count.
To investigate whether smoking modified the association between RDW and MI, we stratified the analysis by smoking status. The relationship between RDW and incident MI was modified by smoking status, and the risk estimates were higher among smokers than among nonsmokers, although the differences in HRs were not statistically significant (Table 5). In smokers, the HR for quintile 5 versus quintile 1 was 1.43 (95% CI 1.05 to 1.95), and participants with RDW above the 95th percentile had a more than 2‐fold higher risk (model 3: HR 2.28; 95% CI 1.55 to 3.35). Nonsmokers with RDW within quintile 5 had a 34% higher risk of MI compared with quintile 1 (model 2: HR 1.34; 95% CI, 1.07 to 1.68). The association was further weakened after additional adjustment for hypertension, total cholesterol, triglycerides, diabetes, and red blood cell count (model 3: HR 1.21; 95% CI, 0.96 to 1.53) (Table 5).
Table 5.
Adjusted Hazard Ratios for Myocardial Infarction by Smoking. The Tromsø Study 1994–2010
| Person‐Years | Events | Model 1* | Model 2* | Model 3* | |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Nonsmokers | |||||
| Quintile 1 | 47 657 | 105 | Reference | Reference | Reference |
| Quintile 2 | 42 381 | 140 | 1.04 (0.81 to 1.35) | 1.00 (0.78 to 1.30) | 0.97 (0.75 to 1.26) |
| Quintile 3 | 41 602 | 166 | 1.02 (0.80 to 1.31) | 0.93 (0.72 to 1.19) | 0.87 (0.68 to 1.12) |
| Quintile 4 | 38 209 | 250 | 1.32 (1.04 to 1.66) | 1.22 (0.97 to 1.54) | 1.16 (0.91 to 1.46) |
| Quintile 5 | 41 483 | 383 | 1.47 (1.17 to 1.83) | 1.34 (1.07 to 1.68) | 1.21 (0.96 to 1.53) |
| >95th percentile | 8972 | 80 | 1.63 (1.21 to 2.18) | 1.55 (1.14 to 2.11) | 1.30 (0.94 to 1.80) |
| Smokers | |||||
| Quintile 1 | 20 793 | 53 | Reference | Reference | Reference |
| Quintile 2 | 21 168 | 86 | 1.24 (0.88 to 1.74) | 1.25 (0.89 to 1.77) | 1.25 (0.88 to 1.77) |
| Quintile 3 | 23 864 | 124 | 1.39 (1.01 to 1.93) | 1.37 (0.99 to 1.91) | 1.31 (0.94 to 1.82) |
| Quintile 4 | 26 127 | 161 | 1.26 (0.93 to 1.74) | 1.25 (0.91 to 1.72) | 1.22 (0.88 to 1.68) |
| Quintile 5 | 33 109 | 307 | 1.52 (1.14 to 2.06) | 1.52 (1.12 to 2.06) | 1.43 (1.05 to 1.95) |
| >95th percentile | 5800 | 71 | 2.01 (1.41 to 2.89) | 2.49 (1.71 to 3.63) | 2.28 (1.55 to 3.35) |
Adjusted HRs with 95% CI for myocardial infarction across quintiles and values above the 95‐percentile (>14.3%) for red cell distribution width among smokers and nonsmokers. HR indicates hazard ratio.
Model 1: Age was used as time scale.
Model 2: Model 1 plus sex, body mass index, hemoglobin, white blood cells, and platelets.
Model 3: Model 2 plus hypertension, total cholesterol, triglycerides, red blood cell count, and self‐reported diabetes.
A total of 1297 persons were anemic, according to the World Health Organization's definition of anemia.18 To investigate whether the association between RDW and risk of MI was modified by anemia, we excluded participants with anemia from the analysis. Participants in the highest quintile of RDW without anemia had a 43% higher risk of MI (model 2: HR 1.43; 95% CI, 1.19 to 1.72; model 3: HR 1.34; 95% CI, 1.12 to 1.62) than nonanemic participants with RDW in the lowest quintile (Table 6). Furthermore, the risk estimates for RDW above the 95th percentile in nonanemic participants did not differ from those of the total population (model 2: HR 1.85; 95% CI, 1.44 to 2.39; model 3: HR 1.72; 95% CI, 1.32 to 2.22).
Table 6.
Adjusted Hazard Ratios for Myocardial Infarction in Participants Without Anemia. The Tromsø Study 1994–2010
| RDW | N | Median RDW, % (range) | Person‐Years | Event No. | IR (95% CI) | Model 1* | Model 2* | Model 3* |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||
| Quintile 1 | 4963 | 12.0 (10.7 to 12.2) | 67 038 | 156 | 2.3 (2.0 to 2.7) | Reference | Reference | Reference |
| Quintile 2 | 4601 | 12.4 (12.3 to 12.5) | 62 311 | 222 | 3.6 (3.1 to 4.1) | 1.15 (0.94 to 1.41) | 1.11 (0.90 to 1.36) | 1.09 (0.88 to 1.34) |
| Quintile 3 | 4745 | 12.7 (12.6 to 12.8) | 63 785 | 288 | 4.5 (4.0 to 5.1) | 1.24 (1.02 to 1.51) | 1.11 (0.91 to 1.36) | 1.06 (0.87 to 1.29) |
| Quintile 4 | 4732 | 13.0 (12.9 to 13.2) | 62 365 | 405 | 6.4 (5.9 to 7.2) | 1.45 (1.21 to 1.75) | 1.27 (1.05 to 1.54) | 1.23 (1.02 to 1.49) |
| Quintile 5 | 5274 | 13.6 (13.3 to 25.4) | 65 287 | 631 | 9.7 (8.9 to 10.3) | 1.73 (1.43 to 2.07) | 1.43 (1.19 to 1.72) | 1.34 (1.12 to 1.62) |
| >95th percentile | 815 | 14.9 (14.4 to 25.4) | 9361 | 114 | 12.2 (10.1 to 14.6) | 2.13 (1.67 to 2.72) | 1.85 (1.44 to 2.39) | 1.72 (1.32 to 2.22) |
Crude IR per 1000 person years and adjusted HR with 95% CI for myocardial infarction across quintiles and values above the 95th percentile (>14.3%) for RDW in participants without anemia (n=24 315). RDW indicates red cell distribution width; IR, incidence rate; HR, hazard ratio.
Model 1: Age was used as time scale.
Model 2: Model 1 plus sex, body mass index, daily smoking, hemoglobin, white blood cells, and platelet.
Model 3: Model 2 plus hypertension, total cholesterol, triglycerides, red blood cell count, and self‐reported diabetes.
Discussion
Our study is, to the best of our knowledge, the first to demonstrate an association between RDW and risk of incident MI in a general population. The association was consistent when RDW was modeled both as continuous and categorical variables, and the risk of MI by RDW displayed a dose‐dependent pattern. The presence of anemia did not affect the risk estimates.
There are only a few previous reports on the relation between RDW and CV diseases from general populations. A strong association between higher RDW and CV mortality was found in NHANES.13–14 The risk of CV death increased by 22% for a 1‐SD increment of RDW (HR 1.22; 95% CI, 1.14 to 1.31)14 and was more than 2‐fold higher among participants in the highest quintile compared with the lowest.13 In a recent registry‐based study of 225 006 patients without previous CV events or cancer, the risk of CV events increased 39% among patients with RDW of 16% to 17% (HR 1.39; 95% CI, 1.24 to 1.57) compared with patients with RDW <13%.19 In contrast, RDW was not associated with MI (HR 1.05; 95% CI, 0.65 to 1.68) or myocardial mortality (HR 1.09; 95% CI, 0.96 to 1.23) in a community‐based cohort of 3226 Taiwanese participants without a history of CV disease at enrollment.20 Greater power to detect a significant association between RDW and risk of MI in our cohort may be the main reason for the apparent discrepant relationship between RDW and MI in the 2 cohorts. It is not possible to rule out the possibility that ethnicity or other residual confounders are unevenly distributed in the 2 cohorts.
The mechanism for the observed association between RDW and CV morbidity and mortality remain unsettled. Because RDW is a statistical concept, it can be assumed that RDW is a marker of other underlying biological mechanisms.
RDW is suggested to be a biomarker reflecting a proinflammatory condition. Oxidative stress and inflammation increase RDW by impairing iron metabolism, reducing red cell life span, and modulating the response to erythropoietin by the bone marrow.21–22 Smoking is associated with oxidative stress,23 and a small case‐control study found that RDW was higher in smokers than in nonsmokers24 The stronger association between RDW and MI among smokers in our study supports the suggestion that RDW reflects inflammation. Others have also speculated that the biological link between RDW and CV mortality may be mediated by systemic inflammation. Circulating C‐reactive protein and high‐sensitivity C‐reactive protein are strong predictors of future CV events.25–26 RDW was independently associated with high‐sensitivity C‐reactive protein in outpatients,27 and related to acute phase reactants such as interleukin‐6 and high‐sensitivity C‐reactive protein in patients with congestive heart failure.28 However, Perlstein et al found that the association between RDW and mortality was independent of inflammation defined by elevated C‐reactive protein.14
Iron deficiency in itself is also a common cause of high RDW. In 1981, Sullivan proposed that iron deficiency could protect against MI.29 Some later studies supported this hypothesis, whereas others did not find any relationship or even an inverse relationship between iron deficiency and MI.30 Iron deficiency without anemia has been shown to affect health issues31–33 and may specifically attenuate iron‐dependent scavenger functions of oxidative stress that may promote inflammation.34 Consequently, the impact of iron status on RDW and subsequent MI should be investigated in future studies.
It has been reported that increased CV mortality by RDW is confined to those with anemia.20 To explore the impact of anemia on the relationship between RDW and risk of MI in our study, we included hemoglobin in our multivariable model and performed analyses in which anemic participants were excluded. The risk estimates for MI by RDW in our study were not affected by adjustment for hemoglobin or by excluding participants with anemia. This demonstrates that anemia does not explain the strong association between RDW and MI. Furthermore, results from an elderly outpatient cohort implied that the association between extremely high RDW (>16.6%) and mortality was particularly strong in those with nonanemic macrocytosis (MCV >96 fL) or microcytosis (MCV <80 fL).35 We found no association between RDW and risk of MI in nonanemic participants with macrocytosis (893 participants with 85 MI events) or microcytosis (254 participants with 14 events).
Alternatively, RDW may exert a more direct effect in the pathway toward MI. The erythrocytes contain large amounts of free cholesterol,36 and pathological changes in the erythrocyte membrane may potentially lead to accumulation of erythrocytes within the atheromatous plaque. The erythrocyte membrane promotes atherosclerosis by deposition of free cholesterol to atherosclerotic plaques, thereby providing lipid rich membranes to foam cell formation, and by propagation of the inflammatory cascade.37–38 Accumulation of erythrocytes promotes plaque instability.37,39–40 The concept is supported by higher levels of total cholesterol in the erythrocyte membrane from patients presenting with acute coronary syndrome compared with patients with chronic stable angina41 and a positive association between cholesterol content of erythrocyte membranes and RDW values in patients with coronary disease.42 An association between the total cholesterol content of the erythrocyte membrane and the severity of coronary artery disease has also been reported.43 The mechanisms whereby RDW could influence development of atherosclerosis remain unsettled.
The erythrocyte membrane stiffness and shape influence deformability and thereby influence blood flow in the microcirculation.44 The flow behavior of erythrocytes also affects oxygen supply from the erythrocyte to the tissue.44 RDW greater than 14% has been shown to decrease red blood cell deformability.45 Consequently, impaired flow properties and oxygen supply due to high RDW may promote MI under certain conditions. Recent studies showing that RDW is associated with increased risk of venous thromboembolism46–47 and stroke48 support the hypothesis that altered flow properties and mechanism of coagulation and thrombus formation may potentially explain the association between high RDW and CV diseases.
The main strength of our study is in the validated end points of MI in a general population with high participation and follow‐up rates. The study was conducted in a relatively homogenous population (≈99% white), which may limit the generalizability of the results. Only 1 hospital serves the entire Tromsø area, and this decreases the probability of missing end points. In addition, all blood samples were analyzed at the same laboratory. Because the RDW measure was not repeated, the chance of random measurement error remains, and the association between RDW and MI may have been somewhat underestimated due to regression dilution bias.49 Another limitation of the study is the self‐reported information on diabetes, which probably underestimates the true prevalence of diabetes. Moreover, we did not have information on kidney failure or inflammatory biomarkers, and this lack may have confounded the results.
In conclusion, we found that RDW was associated with risk of first‐time MI in a general population. The relationship was linear and independent of anemia and traditional CV risk factors. Whether RDW is a marker of an underlying pathophysiological condition that leads to risk of MI or merely represents an “innocent bystander” remains to be settled. Future basic and epidemiological studies should aim to reveal and understand the pathophysiological mechanisms behind the observed association between RDW and MI.
Acknowledgment
The authors thank participants of the Tromsø Study for their important contributions.
Sources of Funding
K.G. Jebsen Thrombosis Research and Expertise Center is supported by an independent grant from the K.G. Jebsen Foundation. Brækkan and Hansen have received research grants from the Northern Norway Regional Health Authority (URL: http://www.helse-nord.no/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures
None.
References
- 1.Buttarello M, Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol. 2008; 130:104-116. [DOI] [PubMed] [Google Scholar]
- 2.Fritsma GA, Rodak KDBF. Hematology. Clinical Principles and applications. 20073rd edPhiladelphia: Saunders Elsevier [Google Scholar]
- 3.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Hear J. 2013; 34:3028-3034. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The 10 leading causes of death in the world. 2011. Available from: http://who.int/mediacentre/factsheets/fs310/en/. Accessed May 31, 2014.
- 5.Zalawadiya SK, Veeranna V, Niraj A, Pradhan J, Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010; 106:988-993. [DOI] [PubMed] [Google Scholar]
- 6.Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010; 105:312-317. [DOI] [PubMed] [Google Scholar]
- 7.Azab B, Torbey E, Hatoum H, Singh J, Khoueiry G, Bachir R, McGinn JT, Jr, McCord D, Laffert J. Usefulness of red cell distribution width in predicting all‐cause long‐term mortality after non‐ST‐elevation myocardial infarction. Cardiology. 2011; 119:72-80. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Yang DH, Jang SY, Choi WS, Kim KH, Lee WK, Bae MH, Park HS, Cho Y, Chae SC. Incremental predictive value of red cell distribution width for 12‐month clinical outcome after acute myocardial infarction. Clin Cardiol. 2013; 36:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uyarel H, Ergelen M, Cicek G, Kaya MG, Ayhan E, Turkkan C, Yildirim E, Kirbas V, Onturk ET, Erer HB, Yesilcimen K, Gibson CM. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011; 22:138-144. [DOI] [PubMed] [Google Scholar]
- 10.Pascual‐Figal DA, Bonaque JC, Redondo B, Caro C, Manzano‐Fernandez S, Sanchez‐Mas J, Garrido IP, Valdes M. Red blood cell distribution width predicts long‐term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009; 11:840-846. [DOI] [PubMed] [Google Scholar]
- 11.Van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL., Jr Red blood cell distribution width and 1‐year mortality in acute heart failure. Eur J Heart Fail. 2010; 12:129-136. [DOI] [PubMed] [Google Scholar]
- 12.Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF., Jr Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010; 16:230-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle‐aged and older adults. Arch Intern Med. 2009; 169:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community‐based prospective cohort. Arch Intern Med. 2009; 169:588-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso Study. Int J Epidemiol. 2012; 41:961-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braekkan SK, Hald EM, Mathiesen EB, Njolstad I, Wilsgaard T, Rosendaal FR, Hansen JB. Competing risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: the Tromso study. Arterioscler Thromb Vasc Biol. 2012; 32:487-491. [DOI] [PubMed] [Google Scholar]
- 17.MORGAM Project. MORGAM manual. MORGAM Project ex2010publications. 2001. x2010; (1). URN:NBN:fix2010fe20041529. Available from URL: http://www.thl.fi/publications/morgam/manual/followup/form22.htm. Accessed July 18, 2014.
- 18.World Health Organization. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968; 405:5-37. [PubMed] [Google Scholar]
- 19.Arbel Y, Weitzman D, Raz R, Steinvil A, Zeltser D, Berliner S, Chodick C, Shalev V. Red blood cell distribution width and the risk of cardiovascular morbidity and all‐cause mortality. A population‐based study. Thromb Haemost. 2014; 111:300-307. [DOI] [PubMed] [Google Scholar]
- 20.Chen PC, Sung FC, Chien KL, Hsu HC, Su TC, Lee YT. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol. 2010; 171:214-220. [DOI] [PubMed] [Google Scholar]
- 21.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005; 352:1011-1023. [DOI] [PubMed] [Google Scholar]
- 22.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008; 10:1923-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carnevali S, Petruzzelli S, Longoni B, Vanacore R, Barale R, Cipollini M, Scatena F, Paggiaro P, Celi A, Giuntini C. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003; 284:L955-L963. [DOI] [PubMed] [Google Scholar]
- 24.Kurtoğlu E, Aktürk E, Korkmaz H, Sincer I, Yilmaz M, Erdem K, Celik A, Ozdemir R. Elevated red blood cell distribution width in healthy smokers. Arch Turk Soc Cardiol. 2013; 43:199-206. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997; 336:973-979. [DOI] [PubMed] [Google Scholar]
- 26.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high‐sensitivity C‐reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984–1998. Clin Chem. 2008; 54:335-342. [DOI] [PubMed] [Google Scholar]
- 27.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009; 133:628-632. [DOI] [PubMed] [Google Scholar]
- 28.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009; 158:659-666. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981; 1:1293-1294. [DOI] [PubMed] [Google Scholar]
- 30.Munoz‐Bravo C, Gutiérrez‐Bedmar M, Gómez‐Aracena J, García‐Rodríguez A, Navajas JF. Iron: protector or risk factor for cardiovascular disease? Still controversial. Nutrients. 2013; 5:2384-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krayenbuehl P‐A, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011; 118:3222-3227. [DOI] [PubMed] [Google Scholar]
- 32.Patterson AJ, Brown WJ, Roberts DC. Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. J Am Coll Nutr. 2001; 20:337-342. [DOI] [PubMed] [Google Scholar]
- 33.Von Drygalski A, Adamson JW. Ironing out fatigue. Blood. 2011; 118:3191-3192. [DOI] [PubMed] [Google Scholar]
- 34.Koskenkorva‐Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013; 65:1174-1194. [DOI] [PubMed] [Google Scholar]
- 35.Lam AP, Gundabolu K, Sridharan A, Jain R, Msaouel P, Chrysofakis G, Yu Y, Friedman E, Price E, Schrier S, Verma AK. Multiplicative interaction between mean corpuscular volume and red cell distribution width in predicting mortality of elderly patients with and without anemia. Am J Hematol. 2013; 88:E245-E249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985; 822:267-287. [DOI] [PubMed] [Google Scholar]
- 37.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003; 349:2316-2325. [DOI] [PubMed] [Google Scholar]
- 38.Arbustini E, Morbini P, D'Armini AM, Repetto A, Minzioni G, Piovella F, Viganò M, Tavazzi L. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: the critical role of thrombotic material in pultaceous core formation. Heart. 2002; 88:177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolodgie FD, Burke AP, Nakazawa G, Cheng Q, Xu X, Virmani R. Free cholesterol in atherosclerotic plaques: where does it come from? Curr Opin Lipidol. 2007; 18:500-507. [DOI] [PubMed] [Google Scholar]
- 40.Tziakas DN, Chalikias GK, Stakos D, Tentes IK, Papazoglou D, Thomaidi A, Grapsa A, Gioka G, Kaski JC, Boudoulas H. Independent and additive predictive value of total cholesterol content of erythrocyte membranes with regard to coronary artery disease clinical presentation. Int J Cardiol. 2011; 150:22-27. [DOI] [PubMed] [Google Scholar]
- 41.Tziakas DN, Kaski JC, Chalikias GK, Romero C, Fredericks S, Tentes IK, Kortsaris AX, Hatseras DI, Holt DW. Total cholesterol content of erythrocyte membranes is increased in patients with acute coronary syndrome: a new marker of clinical instability? J Am Coll Cardiol. 2007; 49:2081-2089. [DOI] [PubMed] [Google Scholar]
- 42.Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012; 51:243-254. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Y, Tang H, Zeng Q, Wang X, Yi G, Meng K, Mao Y, Mao X. Total cholesterol content of erythrocyte membranes is associated with the severity of coronary artery disease and the therapeutic effect of rosuvastatin. Ups J Med Sci. 2012; 117:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda N. Erythrocyte rheology in microcirculation. Jpn J Physiol. 1996; 46:1-14. [DOI] [PubMed] [Google Scholar]
- 45.Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013; 765:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezende SM, Lijfering WM, Rosendaal FR, Cannegieter SC. Hematologic variables and venous thrombosis: red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica. 2014; 99:194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zöller B, Melander O, Svensson P, Engström G. Red cell distribution width and risk for venous thromboembolism: a population‐based cohort study. Thromb Res. 2014; 133:334-339. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez‐Moreno J, Gonzalez‐Gomez M, Ollero‐Ortiz A, Roa‐Montero A, Gomez‐Baquero M, Constantino‐Silva A. Relation between red blood cell distribution width and ischemic stroke: a case control study. Int J Stroke. 2013; 8:E36. [DOI] [PubMed] [Google Scholar]
- 49.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010; 340:c2289. [DOI] [PubMed] [Google Scholar]


