Abstract
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with increased risk of stroke and death. Obesity is an independent risk factor for AF, but modifiers of this risk are not well known. We studied the roles of obesity, physical activity, and their interaction in conferring risk of incident AF.
Methods and Results
The Women's Health Initiative (WHI) Observational Study was a prospective observational study of 93 676 postmenopausal women followed for an average of 11.5 years. Incident AF was identified using WHI‐ascertained hospitalization records and diagnostic codes from Medicare claims. A multivariate Cox's hazard regression model adjusted for demographic and clinical risk factors was used to evaluate the interaction between obesity and physical activity and its association with incident AF. After exclusion of women with prevalent AF, incomplete data, or underweight body mass index (BMI), 9792 of the remaining 81 317 women developed AF. Women were, on average, 63.4 years old, 7.8% were African American, and 3.6% were Hispanic. Increased BMI (hazard ratio [HR], 1.12 per 5‐kg/m2 increase; 95% confidence interval [CI], 1.10 to 1.14) and reduced physical activity (>9 vs. 0 metabolic equivalent task hours per week; HR, 0.90; 95% CI, 0.85 to 0.96) were independently associated with higher rates of AF after multivariate adjustment. Higher levels of physical activity reduced the AF risk conferred by obesity (interaction P=0.033).
Conclusions
Greater physical activity is associated with lower rates of incident AF and modifies the association between obesity and incident AF.
Keywords: atrial fibrillation, electrophysiology, epidemiology, exercise, obesity
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in women; 1.1 million U.S. women are affected, and the prevalence is expected to rise 2.5‐fold in the next 50 years.1 Women comprise 60% of individuals with AF 75 years of age and older and have increased rates of stroke and death, compared to men with AF.2–3 Despite its rising prevalence and significant clinical consequences, the current understanding of AF pathophysiology is inadequate to guide preventative strategies. Research aimed at identifying AF risk factors and their modifiers is imperative to elucidate its pathophysiology and provide novel targets for preventative interventions.
Obesity has recently been established as an important independent risk factor for AF,4–6 accounting for 12% to 18% of the population‐attributable risk in women.7–8 Although obesity is increasing in prevalence, modifiers of its association with AF have not been well studied. Physical activity reduces the incidence of several cardiovascular (CV) risk (CVR) factors associated with obesity, as well as the risks of CV events and mortality in obese individuals.9–10 It may therefore modify the association between obesity and incident AF. However, the independent effects of physical activity on AF risk in the general population remain controversial. Several studies have reported an association between moderate levels of physical activity and decreased11–12 or unchanged13 AF risk. Others have shown an association with increased AF risk, particularly in individuals engaging in strenuous physical activity.14–18 There have been limited studies examining whether physical activity modifies the association between obesity and AF.19 If physical activity reduces the risk of AF conferred by obesity in older women, it may have important implications for the primary prevention of AF in this vulnerable population.
The aims of this study were to investigate the independent links between physical activity, obesity, and AF and determine whether physical activity modifies the association between obesity and incident AF.
Methods
Study Population
The Women's Health Initiative (WHI) Observational Study (WHI‐OS) is a prospective cohort study of postmenopausal women between the ages of 50 and 79 at enrollment. Details of the study design and methods have been previously published.20–21 From 1994 to 1998, a total of 93 676 participants were enrolled at 40 U.S. clinical centers for the purpose of assessing the relationship of self‐reported demographics, medical history, and other measurements on the risk of key health outcomes over time. Participants were recruited primarily through mass mailings to age‐eligible women. Women were eligible to participate if they were postmenopausal, with ages between 50 and 79 years at time of enrollment. Women were ineligible if they were unlikely to survive or remain in the vicinity of a WHI clinic for 3 years or had alcoholism, drug dependency, or dementia. In this study, we also excluded women with baseline AF, missing data, or underweight body mass index (BMI) in order to restrict analysis to World Health Organization (WHO) BMI categories.22
Study Procedures
At time of study enrollment, women completed self‐ or interviewer‐administered questionnaires. Baseline characteristics were obtained, including demographic, reproductive, personal, and family histories. Participants underwent assessment of anthropometric and blood pressure measurements as well as complete physical examination. They updated their medical and lifestyle information on annual questionnaires. The study was reviewed and approved by the institutional review boards at each clinical center, and all participants provided written informed consent.
Assessment of Baseline Variables and Endpoints
Study questionnaires, physical measurements, and quality assurance have been previously described.20–21 Race/ethnicity, age, income, education, and history of hypertension (HTN), diabetes, hyperlipidemia, coronary heart disease (CHD), stroke, congestive heart failure (CHF), peripheral artery disease (PAD), smoking, and alcohol use were ascertained by self‐report on baseline questionnaires. Participants with measured resting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at the initial clinic visit were also classified as hypertensive. BMI was calculated as weight (kg) divided by the square of measured height (m2), using baseline weight and height measurements. Participants were divided into BMI categories as defined by the WHO.22
Physical activity was assessed using self‐reported questionnaires completed at study enrollment. Participants were asked how frequently they walked outside the home for more than 10 minutes without stopping and engaged in recreational physical activity at levels that increased heart rate and produced sweating. Strenuous activity was assessed on baseline questionnaires. Participants were asked how frequently they engaged in strenuous or very hard exercise resulting in sweating and fast heart beat, such as aerobics, aerobic dancing, jogging, tennis, and swimming laps. The frequency, intensity, duration, and type of physical activity were evaluated. The validity and reproducibility of the WHI physical activity assessment methods have been previously established.21,23–24 Determination of total weekly physical activity for each participant has been previously described.25 An estimated metabolic equivalent task (MET) level was assigned to different speeds of walking and intensities of recreational physical activity using a compendium of physical activity. The midpoint values for ranges of frequency and duration were imputed and multiplied to create an hours per week (h/week) variable. MET levels for each activity were multiplied by h/week to compute total weekly physical activity (MET‐h/week) for each participant. Women were divided into 4 categories of physical activity: 0 MET‐h/week; >0 to 3 MET‐h/week; >3 to 9 MET‐h/week; and >9 MET‐h/week. Categories of physical activity were chosen for consistency with previously published studies,25 established benefit in CV disease outcomes,26 and recommendations from federal guidelines.27 Participants in the sedentary category (0 MET‐h/week) were those who did not walk outside the home for more than 10 minutes without stopping at least once‐weekly and who did not engage in recreational physical activity at sufficient intensity to produce increased heart rate or sweating. A sensitivity analysis was also performed using physical activity divided into quintiles of MET‐h/week.
Ascertainment of AF in the WHI has been previously described.8,28 Women with baseline AF identified by self‐report on baseline questionnaire or by the presence of AF on 12‐lead electrocardiogram during the baseline clinic visit were excluded from analysis. Participants completed medical history update questionnaires at years 3 to 8, which specifically inquired about new diagnoses and intervening hospitalizations. If participants had been hospitalized, medical records were obtained and the International Classification of Disease, version 9 (ICD‐9), code for AF (427.31) was extracted over the time period since the previous questionnaire. WHI data were also linked with their Centers for Medicare and Medicaid Services (CMS) data using Social Security numbers, birth dates, and death dates, with 97% of Medicare‐eligible participants successfully linked. Among those with Medicare coverage, incident AF was identified by the first occurrence of ICD‐9 code 427.31 in any diagnosis position in the inpatient (MEDPAR), outpatient, and carrier files during years 1994–2011. Because CMS data were available for some participants, but not others, at different time periods over WHI follow‐up, a time‐dependent indicator variable of Medicare coverage was added to the Cox's hazard models described below to adjust for possible ascertainment bias related to differential exposure to CMS. Medicare time eligible for analysis included those intervals where participants were enrolled in fee‐for‐service Medicare and not simultaneously enrolled in a Medicare‐managed care plan. Women with any single ICD‐9 code of 427.31 from review of Medicare claims or hospital records were classified as having new‐onset AF.
Statistical Analysis
Baseline characteristics of participants were compared across BMI and AF categories using ANOVA for continuous variables as well as the chi‐square (χ2) test for categorical variables. Associations between covariates and incident AF were assessed using multivariate Cox's hazard regression analyses. Multivariate analyses were performed using a primary model containing baseline covariates with known or suspected association with AF: age, race/ethnicity, education, BMI, HTN, diabetes, hyperlipidemia, coronary artery disease (CAD), CHF, PAD, smoking, and physical activity.29–30 All Cox's hazard regression models were adjusted for time‐dependent exposure to Medicare coverage.
Interaction between obesity and physical activity was assessed by adding a BMI*PA interaction term to the primary multivariate Cox's hazard regression model. To examine the risk of incident AF across strata of both BMI and physical activity, participants were divided into 9 categories defined by BMI (normal=18.5 to 24.9 kg/m2, overweight=25 to 29.9 kg/m2, and obese ≥30 kg/m2) and physical activity (0 to 3 MET‐h/week, >3 to 9 MET‐h/week, and >9 MET‐h/week). Each BMI*PA (physical activity) category was added to the primary multivariate Cox's hazard regression model, and risk of incident AF was evaluated using the normal weight and >9 MET‐h/week category as the reference group.
We sought to determine whether the AF risk conferred by obesity differs across other baseline characteristics. Therefore, we performed a subgroup analysis across predetermined categories of baseline characteristics: age, race/ethnicity, physical activity, HTN, diabetes, CHD, CHF, smoking, and alcohol use. Interactions between obesity and subgroup variables were assessed by adding interaction terms to the primary multivariate Cox's hazard regression model.
A secondary analysis was performed to evaluate the association of a high level of strenuous physical activity, defined as >15 MET‐h/week of strenuous activity, with risk of incident AF. This threshold was chosen to capture the approximate top 10% of participants performing the most strenuous physical activity. The association between a high level of strenuous physical activity and incident AF was assessed using multivariate Cox's hazard regression analysis, including all covariates in the primary model.
Associations were reported as hazard ratios (HRs) with 95% confidence intervals (CIs). The proportional hazards assumption was verified by visual inspection of the log‐likelihood plots of developing AF over time. Analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
Of the 93 676 participants in the WHI‐OS, 12 359 were excluded from analysis: 4397 with baseline AF; 7833 with missing data; and 129 with underweight BMI (<18.5 kg/m2). A total of 81 317 women remained for analysis. The average time of follow‐up was 11.5 years. Baseline characteristics of the study group by BMI category and AF status are presented in Table 1. On average, participants were 63.4 years old, 25.1% were obese, 34.5% overweight, 3.9% diabetic, and 42.6% had a history of HTN. With regard to physical activity, 13.4% of participants were sedentary, 11.4% performed >0 to 3 MET‐h/week, 22.8% performed >3 to 9 MET‐h/week, and 52.4% performed >9 MET‐h/week. As expected, obesity was associated with an increased prevalence of multiple CV comorbidities, compared to normal weight participants, including HTN (38.8% vs. 15.6%), diabetes (8.8% vs. 1.4%), hyperlipidemia (17.3% vs. 11.1%), CAD (4.2% vs. 2.1%), stroke (1.8% vs. 1.0%), CHF (1.2% vs. 0.4%), and PAD (3.0 vs. 1.4%; P<0.001 for all). Over an average of 11.5 years of follow‐up, 9792 participants developed new‐onset AF with an annual cumulative incidence of 1.1%. Of the 9792 incident AF events, 2772 were first identified by WHI hospitalization records and 7020 were first identified through CMS.
Table 1.
Baseline Characteristics According to BMI Categories
| Normal Weight | Overweight | Obese | P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF (n=3613) | No AF (n=29 212) | AF (n=3403) | No AF (n=24 681) | AF (n=2776) | No AF (n=17 632) | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| Demographics | |||||||||||||
| Age, mean (SD) | 68 | 6.2 | 63 | 7.4 | 68 | 6.1 | 63 | 7.3 | 67 | 6.3 | 62 | 7 | <0.001 |
| Race/ethnicity | <0.001 | ||||||||||||
| White | 3396 | 94 | 25 449 | 87.1 | 3118 | 91.6 | 20 421 | 82.7 | 2459 | 88.6 | 13 454 | 76.3 | |
| African American | 75 | 2.1 | 1108 | 3.8 | 150 | 4.4 | 2052 | 8.3 | 218 | 7.9 | 2733 | 15.5 | |
| Hispanic | 29 | 0.8 | 833 | 2.9 | 47 | 1.4 | 1086 | 4.4 | 48 | 1.7 | 893 | 5.1 | |
| American Indian | 11 | 0.3 | 85 | 0.3 | 7 | 0.2 | 91 | 0.4 | 13 | 0.5 | 124 | 0.7 | |
| Asian/Pacific Islander | 75 | 2.1 | 1349 | 4.6 | 36 | 1.1 | 666 | 2.7 | 8 | 0.3 | 181 | 1.0 | |
| Other/unknown | 27 | 0.7 | 388 | 1.3 | 45 | 1.3 | 365 | 1.5 | 30 | 1.1 | 247 | 1.4 | |
| Income <$20 000 | 434 | 12 | 2991 | 10.2 | 519 | 15.3 | 3455 | 14 | 662 | 23.8 | 3440 | 19.5 | <0.001 |
| College degree or higher | 1676 | 46.4 | 14 571 | 49.9 | 1371 | 40.3 | 10 234 | 41.5 | 876 | 31.6 | 5885 | 33.4 | <0.001 |
| Medical history | |||||||||||||
| Hypertension | 913 | 25.3 | 4209 | 14.4 | 1242 | 36.5 | 5935 | 24 | 1413 | 50.9 | 6507 | 36.9 | <0.001 |
| Diabetes mellitus | 77 | 2.1 | 388 | 1.3 | 155 | 4.6 | 795 | 3.2 | 367 | 13.2 | 1429 | 8.1 | <0.001 |
| Hyperlipidemia | 535 | 14.8 | 3107 | 10.6 | 685 | 20.1 | 4069 | 16.5 | 566 | 20.4 | 2973 | 16.9 | <0.001 |
| Coronary artery disease | 195 | 5.4 | 509 | 1.7 | 238 | 7 | 686 | 2.8 | 238 | 8.6 | 626 | 3.6 | <0.001 |
| MI | 122 | 3.4 | 342 | 1.2 | 157 | 4.6 | 452 | 1.8 | 178 | 6.4 | 470 | 2.7 | <0.001 |
| CABG/PTCA | 125 | 3.5 | 284 | 1 | 145 | 4.3 | 414 | 1.7 | 139 | 5 | 326 | 1.8 | <0.001 |
| Stroke | 65 | 1.8 | 268 | 0.9 | 63 | 1.9 | 305 | 1.2 | 74 | 2.7 | 299 | 1.7 | <0.001 |
| Heart failure | 41 | 1.1 | 78 | 0.3 | 47 | 1.4 | 119 | 0.5 | 73 | 2.6 | 163 | 0.9 | <0.001 |
| Peripheral arterial disease | 106 | 2.9 | 350 | 1.2 | 134 | 3.9 | 395 | 1.6 | 139 | 5 | 478 | 2.7 | <0.001 |
| Habits | |||||||||||||
| Physical activity (MET‐h/week), mean (SD) | 15.9 | 14.7 | 16.9 | 15.6 | 13 | 13.3 | 13.6 | 13.9 | 8.7 | 11.3 | 9.5 | 11.9 | <0.001 |
| Smoking | 246 | 6.8 | 1969 | 6.7 | 182 | 5.3 | 1506 | 6.1 | 110 | 4 | 972 | 5.5 | <0.001 |
| Alcohol use | 2707 | 74.9 | 22 010 | 75.3 | 2426 | 71.3 | 17 570 | 71.2 | 1637 | 59 | 10 873 | 61.7 | <0.001 |
All values represent the total and percentage of participants, unless otherwise indicated. P value is for the comparison of all subjects across 3 different BMI categories using ANOVA. BMI indicates body mass index; CABG, coronary artery bypass graft; MET, metabolic equivalent task; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Obesity, Physical Activity, and Risk of Incident AF
The age‐ and race/ethnicity‐adjusted as well as the multivariate‐adjusted HRs for each of the covariates in the Cox's regression models of incident AF are displayed in Table 2. After adjustment for several risk predictors, including physical activity, higher BMI was independently associated with an increased risk of AF (HR, 1.12 per 5‐kg/m2 increase; 95% CI, 1.10 to 1.14).8
Table 2.
Multivariate‐Adjusted Hazard Ratios of Incident AF
| Characteristic | Age/Race Adjusted | Multivariate Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.65 (1.62, 1.67) | <0.001 | 1.47 (1.44, 1.49) | <0.001 |
| Race/ethnicity | <0.001 | <0.001 | ||
| White | 1.00 (ref) | 1.00 (ref) | ||
| African American | 0.74 (0.67, 0.81) | 0.61 (0.55, 0.67) | ||
| Hispanic | 0.52 (0.44, 0.62) | 0.61 (0.51, 0.72) | ||
| Other/unknown* | 0.57 (0.51, 0.65) | 0.65 (0.58, 0.74) | ||
| Education | <0.001 | 0.039 | ||
| ≤High school/GED | 1.00 (ref) | 1.00 (ref) | ||
| Some college | 0.93 (0.88, 0.98) | 0.97 (0.92, 1.03) | ||
| ≥College degree | 0.83 (0.79, 0.87) | 0.94 (0.89, 0.99) | ||
| BMI (5‐kg/m2 increase) | 1.18 (1.16, 1.20) | <0.001 | 1.12 (1.10, 1.14) | <0.001 |
| Hypertension | 1.57 (1.50, 1.63) | <0.001 | 1.41 (1.35, 1.47) | <0.001 |
| Diabetes | 2.02 (1.86, 2.20) | <0.001 | 1.50 (1.38, 1.64) | <0.001 |
| Hyperlipidemia | 1.16 (1.10, 1.22) | <0.001 | 0.96 (0.91, 1.02) | 0.188 |
| Coronary artery disease | 2.27 (2.10, 2.45) | <0.001 | 1.79 (1.65, 1.95) | <0.001 |
| Heart failure | 3.48 (2.98, 4.07) | <0.001 | 2.12 (1.80, 2.49) | <0.001 |
| Peripheral artery disease | 2.03 (1.83, 2.25) | <0.001 | 1.54 (1.39, 1.71) | <0.001 |
| Current smoking | 1.25 (1.14, 1.36) | <0.001 | 1.25 (1.15, 1.37) | <0.001 |
| Physical activity | <0.001 | 0.003 | ||
| No activity | 1.00 (ref) | 1.00 (ref) | ||
| >0 to 3 MET‐h/week | 0.96 (0.89, 1.04) | 0.98 (0.91, 1.06) | ||
| >3 to 9 MET‐h/week | 0.87 (0.81, 0.93) | 0.94 (0.88, 1.01) | ||
| >9 MET‐h/week | 0.78 (0.73, 0.83) | 0.90 (0.85, 0.96) | ||
The multivariate model included as covariates all demographic and clinical characteristics listed in the first column. AF indicates atrial fibrillation; BMI, body mass index; CI, confidence interval; GED, general educational development; HR, hazard ratio; MET, metabolic equivalent task.
Includes American Indian and Asian/Pacific Islander.
Compared to sedentary women, participants in the highest stratum of physical activity (>9 MET‐h/week) had a significantly lower multivariable‐adjusted risk of incident AF (HR, 0.90; 95% CI, 0.85 to 0.96). Each increase in strata of physical activity was associated with a lower hazard of incident AF (P<0.001 for linear trend). Sensitivity analyses using physical activity divided into quintiles of MET‐h/week demonstrated similar findings.
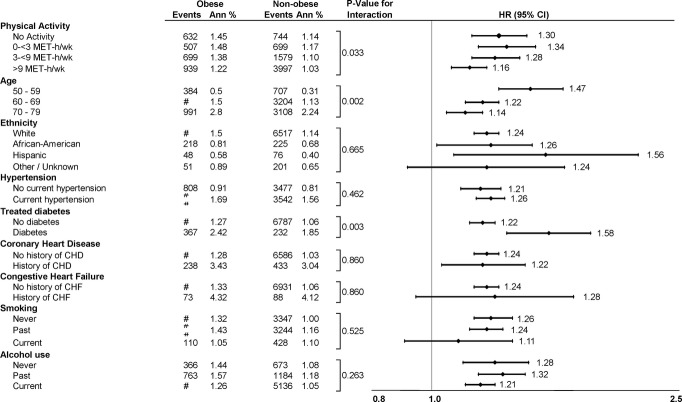
When we examined the association between obesity and AF stratified by physical activity (Figure 1), the risk of incident AF conferred by obesity was greater in the sedentary group (HR, 1.30; 95% CI, 1.17 to 1.45) than in the physically active group (HR, 1.16; 95% CI, 1.08 to 1.24). The interaction between obesity and physical activity was significantly associated with incident AF (P=0.033). Secondary analyses also demonstrated significant interactions between obesity and age (P=0.002) and diabetes (P=0.003) in AF risk (Figure 1). Obesity conferred the highest AF risk in younger and diabetic participants.
Figure 1.
Hazard ratios of incident atrial fibrillation by subgroup of body mass index (obese vs. overweight plus normal weight participants). Hazard ratios are multivariate adjusted for all covariates in the primary model. P values for continuous variables (physical activity, age) are done with separate interaction models of obese (yes/no) versus linear trend across level. CHD indicates coronary heart disease; CHF, congestive heart failure; CI, confidence interval; HR, hazard ratio; MET‐h/wk, metabolic equivalent task hours per week.
To better illustrate the interaction between physical activity and obesity, participants were divided into 9 categories based on their BMI (18.5 to 24.9, 25 to 29.9, or ≥30kg/m2) and physical activity level (0 to 3, >3 to 9, and >9 MET‐h/week). The multivariate‐adjusted HRs of incident AF by BMI*PA category are displayed in Figure 2. Compared to the reference group of normal weight participants engaging in >9 MET‐h/week of physical activity, obese participants in the inactive (0 to 3 MET‐h/week) group had the highest risk of incident AF (HR, 1.44; 95% CI, 1.34 to 1.55). Obese participants in the highest category of physical activity had a lower AF risk than those in the inactive category (>9 MET‐h/week group: HR, 1.17; 95% CI, 1.09 to 1.27). The difference in AF risk among sedentary versus physically active women was greater in obese participants (HR, 1.44 vs. 1.17) than in overweight (1.11 vs. 1.03) or normal weight participants (1.07 vs. 1.0). However, higher physical activity levels did not eliminate the increased AF risk conferred by obesity.
Figure 2.
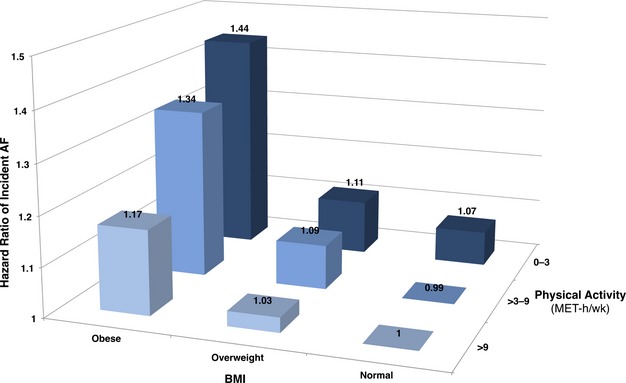
Hazard ratios of incident atrial fibrillation by category of body mass index and physical activity. The normal weight, >9 MET‐h/week category is used as the reference group. Hazard ratios are multivariate‐adjusted for all covariates in the primary model. AF indicates atrial fibrillation; BMI, body mass index; MET‐h/wk, metabolic equivalent task hours per week.
An analysis of the association between high levels of strenuous intensity physical activity and incident AF was performed. Of the 81 317 women included in this analysis, 8904 (11%) engaged in >15 MET‐h/week of strenuous physical activity. After multivariate adjustment for all covariates in the primary model, participants engaging in >15 MET‐h/week of strenuous physical activity demonstrated a significantly lower risk of incident AF, compared to participants engaging in 0 MET‐h/week of strenuous activity (HR, 0.91; 95% CI, 0.85 to 0.97).
Discussion
In this multiethnic cohort of 81 317 postmenopausal women, lower BMI and higher levels of physical activity were each independently protective against the development of incident AF. Physical activity modified the association between obesity and incident AF. To our knowledge, this is the largest study investigating the interaction between obesity and physical activity and its relationship to risk of incident AF.
Obesity is among the most important, potentially modifiable independent risk factors for AF. Increased adiposity may directly increase AF risk by generating systemic inflammation,31 local inflammation in pericardial fat deposits,32 changes in autonomic tone,33 and changes in cardiac size.34 Identifying modifiers of the AF risk conferred by obesity is imperative for the development of effective primary prevention strategies. We hypothesized that physical activity, a low‐cost intervention with myriad health benefits, would be an important modifier of the AF risk conferred by obesity.
The nature of the association between higher physical activity levels and AF risk is controversial. Several studies have reported that strenuous physical activity is associated with increased risk of AF.14–18 The participants in most of these studies were predominantly healthy young athletes or middle‐aged men with few CVR factors who later developed lone AF. Determining the association of more‐typical physical activity with AF risk in older, nonathletic individuals is of greater clinical relevance to the population at highest risk of AF. In a study of middle‐aged women, increasing levels of physical activity were associated with reduced rates of AF, though the association was nonsignificant after controlling for BMI.11 A recent meta‐analysis excluding athletes found that higher physical activity was not associated with a statistically significant difference in AF risk.13
In this study, we demonstrated that higher physical activity levels were associated with a modest, but significant, reduction in incident AF after multivariate adjustment including BMI. Compared to sedentary participants, women engaging in 3 to 9 and >9 MET‐h/week of physical activity had a 6% and 10% lower risk of AF, respectively. Participants engaging in >15 MET‐h/week of strenuous activity had a significant 9% reduction in AF risk. There are several reasons why our findings may differ from previous studies. First, the WHI cohort is particularly well characterized, enabling the inclusion of a more comprehensive list of possible confounders in multivariate regression analyses. Second, the relatively large number of incident AF outcomes provides significantly greater statistical power than most earlier studies. Third, there are likely diverse pathophysiological mechanisms underlying the development of AF in different patient populations. The WHI participants were older and at higher baseline AF risk than participants in many previous studies. What is considered strenuous physical activity in this population differs from that in young athletes.
Increased physical activity also significantly modified the association between obesity and AF risk. There are several potential pathophysiological mechanisms by which physical activity may modify the AF risk conferred by obesity, and the beneficial effects are likely multifactorial. Physical activity attenuates many of the CV consequences of obesity that are implicated in AF pathogenesis, including insulin resistance, HTN, dyslipidemia, and endothelial dysfunction.9 Systemic inflammation plays a prominent role in the pathogenesis of AF,35 and adipocytes cause chronic inflammation in obese individuals through the up‐regulated production of inflammatory adipocytokines.36 Several studies have shown that interventions to increase physical activity in overweight and obese participants reduce systemic inflammation independent of weight loss, with greatest benefit in those with higher levels of baseline inflammation.37 Therefore, the systemic anti‐inflammatory effects of exercise may reduce atrial arrhythmogenesis in the setting of obesity‐induced chronic inflammation. Additional studies are necessary to elucidate the mechanism of interaction between physical activity and obesity in conferring AF risk.
Although the protective effects of increased physical activity are modest, the lack of increased risk of AF in this older population is notable. The results of this analysis therefore challenge existing concerns against recommending physical activity to older individuals who have or are at risk of developing AF. Our findings suggest that interventions to increase physical activity may be considered as a primary prevention strategy to reduce the incidence of AF in aging women, with a particular emphasis in women with concurrent obesity. In this population, physical activity may serve a dual purpose to reduce body weight and mitigate the AF risk conferred by obesity.
A few limitations to this study should be acknowledged. The use of BMI as a surrogate for adiposity fails to distinguish fat from lean mass and does not capture the regional distribution of adiposity that may confer differential AF risk.32 Nonetheless, BMI is commonly used in clinical practice, easily measured, highly reproducible, and associated with CV outcomes and all‐cause mortality.38 The physical activity assessed in this study included walking and recreational activity; participants may have engaged in household or occupational physical activity that was not accounted for in our analysis. However, the WHI physical activity assessment methods have been validated, shown to be reproducible, and are consistent with the standard methods used in many previous studies. Finally, our study was conducted in older women; these findings should be validated in premenopausal women and in men.
Conclusions
In a large, multiethnic cohort of postmenopausal women, participants who were normal weight and physically active had a significantly lower risk of incident AF, compared to those who were obese and sedentary. Physical activity was particularly important in mitigating AF risk in obese women. Increasing physical activity levels may be an effective method for primary prevention of incident AF in older women, especially those who are obese.
Sources of Funding
The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Dr. Marco V. Perez is funded by an American Heart Association Fellow to Faculty grant (11FTF 7260019).
Disclosures
None.
Acknowledgments
A full listing of Women's Health Initiative investigators can be found at http://whiscience.org/publications/WHI_investigators_longlist. The authors thank the Women's Health Initiative investigators, staff, and study participants for their outstanding dedication and commitment.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- 2.Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. 2004; 94:889-894. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995; 155:469-473. [PubMed] [Google Scholar]
- 4.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005; 118:489-495. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004; 292:2471-2477. [DOI] [PubMed] [Google Scholar]
- 6.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta‐analysis. Am Heart J. 2008; 155:310-315. [DOI] [PubMed] [Google Scholar]
- 7.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol. 2010; 55:2319-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil‐Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the women's health initiative observational study. Heart. 2013; 99:1173-1178. [DOI] [PubMed] [Google Scholar]
- 9.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005; 99:1193-1204. [DOI] [PubMed] [Google Scholar]
- 10.LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr Opin Clin Nutr Metab Care. 2006; 9:540-546. [DOI] [PubMed] [Google Scholar]
- 11.Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes. 2011; 4:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2008; 118:800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ofman P, Khawaja O, Rahilly‐Tierney CR, Peralta A, Hoffmeister P, Reynolds MR, Gaziano JM, Djousse L. Regular physical activity and risk of atrial fibrillation: a systematic review and meta‐analysis. Circ Arrhythm Electrophysiol. 2013; 6:252-256. [DOI] [PubMed] [Google Scholar]
- 14.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case‐control study. BMJ. 1998; 316:1784-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlanello F, Bertoldi A, Dallago M, Galassi A, Fernando F, Biffi A, Mazzone P, Pappone C, Chierchia S. Atrial fibrillation in elite athletes. J Cardiovasc Electrophysiol. 1998; 9:S63-S68. [PubMed] [Google Scholar]
- 16.Mont L, Tamborero D, Elosua R, Molina I, Coll‐Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A, Brugada JInvestigators GGIdReF‐lA. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle‐aged healthy individuals. Europace. 2008; 10:15-20. [DOI] [PubMed] [Google Scholar]
- 17.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009; 103:1572-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thelle DS, Selmer R, Gjesdal K, Sakshaug S, Jugessur A, Graff‐Iversen S, Tverdal A, Nystad W. Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309,540 men and women. Heart. 2013; 99:1755-1760. [DOI] [PubMed] [Google Scholar]
- 19.Grundvold I, Skretteberg PT, Liestol K, Gjesdal K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Importance of physical fitness on predictive effect of body mass index and weight gain on incident atrial fibrillation in healthy middle‐age men. Am J Cardiol. 2012; 110:425-432. [DOI] [PubMed] [Google Scholar]
- 20. Design of the women's health initiative clinical trial and observational study. The women's health initiative study group. Control Clin Trials. 1998; 19:61-109. [DOI] [PubMed] [Google Scholar]
- 21.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The women's health initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003; 13:S107-S121. [DOI] [PubMed] [Google Scholar]
- 22. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000; 894:1-253. [PubMed] [Google Scholar]
- 23.Johnson‐Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007; 31:193-202. [DOI] [PubMed] [Google Scholar]
- 24.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test‐retest reliability of the women's health initiative physical activity questionnaire. Med Sci Sports Exerc. 2009; 41:530-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, Wactawski‐Wende J, Craft L, Lane D, Martin LW, Chlebowski R. Physical activity and survival in postmenopausal women with breast cancer: results from the women's health initiative. Cancer Prev Res (Phila). 2011; 4:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002; 347:716-725. [DOI] [PubMed] [Google Scholar]
- 27.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995; 273:402-407. [DOI] [PubMed] [Google Scholar]
- 28.Perez MV, Wang PJ, Larson JC, Virnig BA, Cochrane B, Curb JD, Klein L, Manson JE, Martin LW, Robinson J, Wassertheil‐Smoller S, Stefanick ML. Effects of postmenopausal hormone therapy on incident atrial fibrillation: the women's health initiative randomized controlled trials. Circ Arrhythm Electrophysiol. 2012; 5:1108-1116. [DOI] [PubMed] [Google Scholar]
- 29.Menezes AR, Lavie CJ, Dinicolantonio JJ, O'Keefe J, Morin DP, Khatib S, Abi‐Samra FM, Messerli FH, Milani RV. Cardiometabolic risk factors and atrial fibrillation. Rev Cardiovasc Med. 2013a; 14:e73-e81. [DOI] [PubMed] [Google Scholar]
- 30.Menezes AR, Lavie CJ, DiNicolantonio JJ, O'Keefe J, Morin DP, Khatib S, Milani RV. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc. 2013b; 88:394-409. [DOI] [PubMed] [Google Scholar]
- 31.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001; 104:2886-2891. [DOI] [PubMed] [Google Scholar]
- 32.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010; 56:784-788. [DOI] [PubMed] [Google Scholar]
- 33.Pelat M, Verwaerde P, Merial C, Galitzky J, Berlan M, Montastruc JL, Senard JM. Impaired atrial M(2)‐cholinoceptor function in obesity‐related hypertension. Hypertension. 1999; 34:1066-1072. [DOI] [PubMed] [Google Scholar]
- 34.Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Döring A, Keil U, Hense HW, Schunkert HInvestigators MK. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009; 54:1982-1989. [DOI] [PubMed] [Google Scholar]
- 35.Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003; 108:3006-3010. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860-867. [DOI] [PubMed] [Google Scholar]
- 37.Milani RV, Lavie CJ, Mehra MR. Reduction in C‐reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004; 43:1056-1061. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995; 333:677-685. [DOI] [PubMed] [Google Scholar]