Abstract
Background
No previous studies have examined the interplay among socioeconomic status, sex, and race with the risk of atrial fibrillation (AF).
Methods and Results
We prospectively followed 14 352 persons (25% black, 75% white, 55% women, mean age 54 years) who were free of AF and participating in the Atherosclerosis Risk in Communities (ARIC) study. Socioeconomic status was assessed at baseline (1987–1989) through educational level and total family income. Incident AF through 2009 was ascertained from electrocardiograms, hospitalizations, and death certificates. Cox regression was used to estimate hazard ratios and 95% CIs of AF for education and family income. Interactions were tested between socioeconomic status and age, race, or sex. Over a median follow‐up of 20.6 years, 1794 AF cases occurred. Lower family income was associated with higher AF risk (hazard ratio 1.45, 95% CI 1.27 to 1.67 in those with income less than $25 000 per year compared with those with $50 000 or more per year). The association between education and AF risk varied by sex (P=0.01), with the lowest education group associated with higher AF risk in women (hazard ratio 1.88, 95% CI 1.55 to 2.28) but not in men (hazard ratio 1.15, 95% CI 0.97 to 1.36) compared with the highest education group. Adjustment for cardiovascular risk factors attenuated the associations. There were no interactions with race or age. Blacks had lower AF risk than whites in all income and education groups.
Conclusions
Lower family income was associated with a higher AF risk overall, whereas the impact of education on AF risk was present only in women. Differences in socioeconomic status do not explain the lower risk of AF in blacks compared with whites.
Keywords: atrial fibrillation, education, sex, income, race/ethnicity, socioeconomic status
Introduction
Atrial fibrillation (AF) persists as a growing health concern in the United States. More than 2 million persons are estimated to have AF, and its prevalence is anticipated to double by 2050.1 AF, the most frequent arrhythmia in clinical practice, is associated with increased risk of cardiovascular disease (CVD), heart failure (HF), stroke, and overall mortality.2–4 Previous research has assessed AF risk factors to gain a better understanding of its causation and prediction, but 44% of the attributable risk of AF remains unexplained after accounting for several established risk factors.5
Markers of socioeconomic status (SES), including education and total family income, have been inversely associated with CVD.6 Sex differences have also been reported in the association between education and CVD, for which lower education is associated with higher CVD risk in women but not men.7–13 A previous Swedish study concluded that neighborhood deprivation and socioeconomic disparities in Sweden were not independent risk factors for AF, although a positive association was identified for women, with minimal adjustment.14 However, studies examining the association between individual measures of SES and incident AF in the US population are limited, especially those examining sex and racial differences. The incidence of AF is lower in blacks1,15 even though they have higher stroke rates and a higher prevalence of risk factors for AF and stroke.16 Underdiagnosis of AF cases in blacks due to lower individual SES and poorer access to quality health care could help explain the so‐called AF paradox in blacks16; therefore, exploring the interplay among race, SES, and AF incidence could deepen the understanding of the observed racial differences in the risk of AF.
In order to research this association, data from the Atherosclerosis Risk in Communities (ARIC) study, a community‐based cohort including middle‐aged, mostly white and black persons, was used to determine whether individual SES was associated with the risk of incident AF. We hypothesized that persons with lower SES would have a higher risk for AF independent of other cardiovascular risk factors, that the association of SES with AF risk would be of a similar magnitude between both races and sexes, and that blacks would have lower risk of AF than whites across all levels of SES.
Methods
Study Design and Population
The ARIC study is a prospective cohort study designed to investigate risk factors of atherosclerosis and CVD.17 From 1987 to 1989, participants aged 45 to 64 years had a baseline examination including a home interview and clinical visit in 4 US communities: Forsyth County, NC; Jackson, MS (blacks only); the northwest suburbs of Minneapolis, MN; and Washington County, MD. A total of 15 792 participants initially enrolled in the study; 3 further examinations occurred over an ≈3‐year interval between examinations through 1998. Response rates for the follow‐up triennial examinations were 93%, 86%, and 80% of those alive at the time of the examination, respectively. Participants are interviewed annually by telephone to obtain follow‐up information related to hospital admissions and to determine vital status, with an average response rate of more than 90%. Surveillance of death certificates and hospital discharges within prespecified catchment areas is also continuous. The institutional review board of each participating university approved the ARIC study protocol, and all enrolled participants gave their written informed consent at each visit.
Of the initial 15 792 ARIC participants, the following were excluded: those with self‐reported races other than white or black, due to small sample sizes (n=48); blacks in either Minneapolis or Washington County, due to small sample sizes at these ARIC sites (n=55); those with prevalent AF or atrial flutter diagnosed by the baseline ECG (n=37); those with missing or unreadable baseline ECG (n=309); those who were missing baseline educational level or total family income (n=910); and those who were missing any information on other covariates (n=81). Overall, 14 352 participants remained for analysis (Figure 1).
Figure 1.
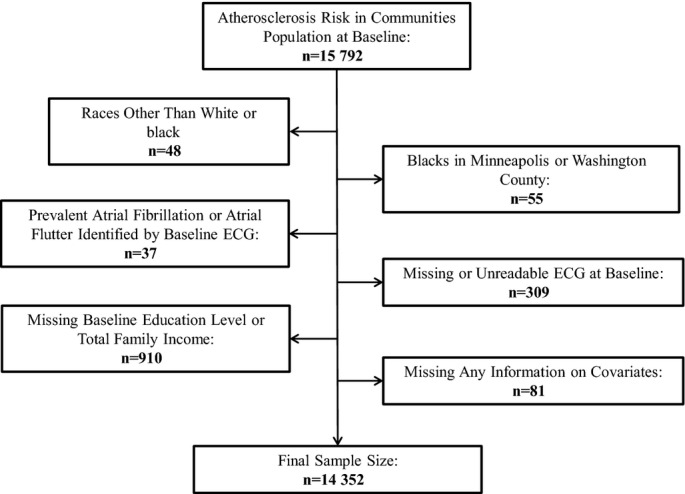
Flow chart of participants excluded at baseline in the Atherosclerosis Risk in Communities study, 1987–1989.
Assessment of SES and Covariates
Education level and total family income were collected at baseline through questionnaires. Education level was categorized into 3 groups: some high school or less, high school graduate or vocational school, and some college or graduate school. Total family income was grouped into 3 categories for this analysis: less than $25 000 per year, $25 000 to $49 999 per year, and $50 000 or more per year.
Information on other CVD risk factors was obtained at baseline, as described previously.17 Questionnaires were used to assess smoking status and drinking status. All medications used in the preceding 2 weeks before each clinic visit were recorded from prescription bottles brought by the participant. Blood pressure was measured using a random‐zero sphygmomanometer after 5 minutes of rest and while sitting. Systolic blood pressure was defined as the average of the last 2 of 3 consecutive measurements. Height and weight were measured while the participant was wearing a scrub suit and no shoes. Body mass index was defined as weight in kilograms divided by height in meters squared. Prevalent diabetes mellitus at baseline was characterized by fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self‐report of a physician diagnosis, or current treatment for diabetes. Prevalent myocardial infarction (MI) at baseline was characterized by a previously reported MI event or from ECGs, whereas prevalent HF was defined by a Gothenburg criterion value of 3 or the intake of HF medications. Prevalent stroke was designated by a self‐reported history of physician‐diagnosed stroke. Incident cases of HF, MI, or stroke were identified through annual telephone interviews, triennial field center examinations, and the ongoing surveillance of ARIC community hospitals for any hospitalizations or deaths of any cohort participants.18
Incident AF Events
Incident AF cases were ascertained from 3 sources: ECGs from study examinations, hospital discharge codes, and death certificates. ECGs during study visits were performed with MAC PC Personal Cardiographs (Marquette Electronics Inc). A standard supine 12‐lead resting ECG was transmitted to the ARIC ECG Reading Center for coding, interpretation, and storage. The ECGs automatically coded as AF were visually rechecked by a trained cardiologist to confirm the diagnosis.19
Using the International Classification of Diseases, ninth revision, clinical modification (ICD‐9‐CM) codes, hospitalization AF events were identified when 427.31 or 427.32 was listed as a discharge diagnosis code. Any AF event identified during a hospitalization for cardiac surgery was excluded. In a physician review of discharge summaries from 125 possible AF cases, ≈90% of the cases were confirmed.20 Last, AF cases were identified as a cause of death if ICD‐9 code 427.3 or ICD‐10 code I48 was listed on the death certificates. More than 98% of incident AF cases were identified from hospital discharge codes, and less than 1% of AF cases were identified from death certificates.
AF incidence date was defined as the date of the first ECG showing AF, the first hospital discharge date for an AF or atrial flutter diagnosis, or the date when death occurred due to AF, whichever occurred first.
Statistical Analysis
Baseline characteristics depicting percentages for categorical variables and means with SDs for continuous variables were stratified by race and SES level. Comparisons of overall percentages and means by race were examined using chi‐square and Student t tests, respectively. Cox proportional hazards regression was used to calculate adjusted hazard ratios (HRs) and 95% CIs for the association of SES with incident AF. Education level and total family income were examined in separate analyses. Follow‐up time was defined as the time between the baseline visit and the date of AF incidence, death, loss to follow‐up, or December 31, 2009, whichever came first. Death and loss to follow‐up were considered censoring events. The initial model was adjusted for age, race, sex, and ARIC field center site at baseline. The second model was further adjusted for baseline cardiovascular risk factors including body mass index (kg/m2), diabetes (yes, no), drinking status (current, former, never), smoking status (current, former, never), systolic blood pressure (mm Hg), and use of antihypertensive medications (yes, no). The third model was additionally adjusted for prevalent MI, HF, or stroke at baseline. The fourth model was adjusted for incident MI, HF, and stroke as time‐dependent covariates and potential mediators of the association between SES and AF risk. Each model tested for a linear trend using the education level or total family income number as an ordinal, continuous variable.
Effect‐measure modification was evaluated by age‐, sex‐, and race‐stratified analyses and including multiplicative terms in the full model between the effect modifier and SES measures. An interaction term between each SES variable and follow‐up time, along with the inspection of log‐negative log survival curves, indicated that the proportional hazards assumption was met in both sets of Cox regressions. In a sensitivity analysis, participants who had an incident AF event within the first 2 years of follow‐up from baseline were excluded to avoid including potentially undetected prevalent AF cases as incident cases. All statistical analyses were performed using SAS version 9.2 (SAS Inc).
Results
At baseline, 7870 women and 6482 men aged 45 to 64 years and free of AF met the inclusion criteria. Table 1 shows the characteristics of the ARIC participants at baseline by education level and race. Lower education level was associated with higher prevalence of most CVD risk factors in both races. In most instances, blacks had a more adverse cardiovascular profile than whites, and these results were significantly different when comparing the overall means and percentages by race. Similar results were seen when assessing the baseline characteristics by total family income and race (Table 2).
Table 1.
Baseline Characteristics by Education Level and Race in the Atherosclerosis Risk in Communities Study, 1987–1989
| White Education Level | Black Education Level | P Value* | |||||
|---|---|---|---|---|---|---|---|
| Some High School or Less (n=1834) | High School Graduate/Vocational School (n=4874) | Some College/Graduate School (n=4033) | Some High School or Less (n=1505) | High School Graduate/Vocational School (n=1024) | Some College/Graduate School (n=1082) | ||
| Age, y | 56.4 (5.4) | 54.1 (5.6) | 53.7 (5.8) | 55.2 (5.7) | 52.8 (5.7) | 52.0 (5.5) | <0.0001 |
| Women, % | 49.8 | 58.7 | 45.8 | 60.0 | 65.2 | 62.6 | <0.0001 |
| Body mass index, kg/m2 | 27.9 (5.2) | 27.1 (5.0) | 26.6 (4.5) | 29.9 (6.6) | 29.7 (6.0) | 29.0 (5.6) | <0.0001 |
| Current drinker, % | 43.0 | 63.4 | 76.4 | 28.1 | 31.5 | 37.0 | <0.0001 |
| Current smoker, % | 34.9 | 25.7 | 19.3 | 34.0 | 29.4 | 24.6 | <0.0001 |
| Diabetes, % | 14.6 | 9.1 | 6.7 | 24.2 | 18.5 | 14.1 | <0.0001 |
| Systolic BP, mm Hg | 121.7 (17.6) | 118.9 (17.1) | 116.5 (16.3) | 132.0 (22.1) | 128.2 (22.5) | 126.0 (19.3) | <0.0001 |
| Use of antihypertensive medications, % | 33.6 | 26.5 | 21.3 | 49.5 | 40.7 | 39.5 | <0.0001 |
| Total family income, % | <0.0001 | ||||||
| Under $25 000 | 55.7 | 27.8 | 12.0 | 90.1 | 76.3 | 39.5 | |
| $25 000 to $49 999 | 37.0 | 49.2 | 36.6 | 8.8 | 20.5 | 42.0 | |
| $50 000 or higher | 7.3 | 23.0 | 51.4 | 1.1 | 3.2 | 18.5 | |
| Prevalent heart failure, % | 5.5 | 3.9 | 2.9 | 9.9 | 6.6 | 4.1 | <0.0001 |
| Prevalent myocardial infarction, % | 8.0 | 3.7 | 3.0 | 4.7 | 3.5 | 2.3 | 0.17 |
| Prevalent stroke, % | 2.5 | 1.3 | 1.8 | 2.3 | 2.7 | 1.2 | 0.10 |
Values are percentage or mean (SD). BP indicates blood pressure.
P value compares differences in overall means and percentages between whites and blacks.
Table 2.
Baseline Characteristics by Total Family Income Level and Race in the Atherosclerosis Risk in Communities Study, 1987–1989
| White Total Family Income Level | Black Total Family Income Level | P Value* | |||||
|---|---|---|---|---|---|---|---|
| <$25 000/y (n=2859) | $25 000 to $49 999/y (n=4554) | ≥$50 000/y (n=3328) | <$25 000/y (n=2565) | $25 000 to $49 999/y (n=797) | ≥$50 000/y (n=249) | ||
| Age, y | 56.6 (5.6) | 54.2 (5.6) | 52.7 (5.3) | 54.4 (5.8) | 51.8 (5.4) | 50.8 (4.5) | <0.0001 |
| Women, % | 62.2 | 50.6 | 46.2 | 66.6 | 53.3 | 45.8 | <0.0001 |
| Body mass index, kg/m2 | 27.6 (5.5) | 27.1 (4.8) | 26.5 (4.3) | 29.9 (6.5) | 28.9 (5.4) | 28.1 (4.5) | <0.0001 |
| Current drinker, % | 48.3 | 64.6 | 79.2 | 27.9 | 39.4 | 47.0 | <0.0001 |
| Current smoker, % | 30.3 | 24.8 | 20.3 | 31.7 | 26.0 | 24.1 | <0.0001 |
| Diabetes, % | 13.9 | 8.6 | 5.8 | 22.3 | 13.8 | 9.6 | <0.0001 |
| Systolic BP, mm Hg | 121.5 (17.8) | 118.7 (17.1) | 115.7 (15.7) | 130.8 (22.4) | 125.5 (19.0) | 122.8 (17.7) | <0.0001 |
| Use of antihypertensive medications, % | 32.6 | 25.7 | 20.0 | 46.8 | 38.8 | 31.7 | <0.0001 |
| Education level, % | <0.0001 | ||||||
| Some high school or less | 35.7 | 14.9 | 4.1 | 52.9 | 16.7 | 6.4 | |
| High school graduate/ vocational school | 47.4 | 52.7 | 33.6 | 30.4 | 26.3 | 13.3 | |
| Some college/graduate school | 16.9 | 32.4 | 62.3 | 16.7 | 57.0 | 80.3 | |
| Prevalent heart failure, % | 6.0 | 3.4 | 2.4 | 8.9 | 3.5 | 2.4 | <0.0001 |
| Prevalent myocardial infarction, % | 5.9 | 4.2 | 2.7 | 4.3 | 1.9 | 2.4 | 0.17 |
| Prevalent stroke, % | 2.3 | 1.3 | 1.6 | 2.7 | 0.8 | 0.4 | 0.10 |
Values are percentage or mean (SD). BP indicates blood pressure.
P value compares differences in overall means and percentages between whites and blacks.
Total Family Income and AF Risk
Over a median follow‐up time of 20.6 years, 1794 incident AF cases were identified through December 31, 2009. The risk of AF was 45% higher (95% CI 1.27 to 1.67) for participants in the low‐income group and 16% higher (95% CI 1.02 to 1.32) for those in the middle‐income group compared with those in the high‐income group after adjustment for age, race, sex, and field center (Table 3, model 1). Additional adjustment for multiple covariates including a history of CVD (Table 3, model 3) attenuated this association in both groups. Using whites who earned $50 000 or more per year as a reference group, blacks had slightly lower HRs for each total income group than whites (Figure 2). No significant interactions were identified between total income and age, race, or sex (P>0.05).
Table 3.
Hazard Ratio and 95% CI of Atrial Fibrillation by Total Family Income in the Atherosclerosis Risk in Communities Study, 1987–2009
| Total Family Income | P for Trend | |||
|---|---|---|---|---|
| <$25 000/y (n=5424) | $25 000 to $49 999/y (n=5351) | ≥$50 000/y (n=3577) | ||
| AF events | 754 | 672 | 368 | |
| Person‐years | 92 276 | 99 708 | 68 470 | |
| Crude incidence rate (per 1000 person‐years) | 8.2 | 6.7 | 5.4 | |
| Model 1 HR (95% CI) | 1.45 (1.27 to 1.67) | 1.16 (1.02 to 1.32) | 1.00 (Reference) | <0.0001 |
| Model 2 HR (95% CI) | 1.18 (1.02 to 1.36) | 1.04 (0.92 to 1.19) | 1.00 (Reference) | 0.02 |
| Model 3 HR (95% CI) | 1.15 (1.00 to 1.33) | 1.04 (0.91 to 1.18) | 1.00 (Reference) | 0.04 |
| Model 4 HR (95% CI) | 1.02 (0.88 to 1.17) | 1.02 (0.90 to 1.17) | 1.00 (Reference) | 0.86 |
Model 1: Cox proportional hazards model adjusted for age, sex, race, and study site. Model 2: Model 1 with additional adjustment for body mass index, diabetes, drinking status, smoking status, systolic blood pressure, and antihypertensive medication. Model 3: Model 2 with additional adjustment for prevalent heart failure, myocardial infarction, or stroke at baseline. Model 4: Model 3 with additional adjustment for incident heart failure, myocardial infarction, or stroke as time‐varying covariates. AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio.
Figure 2.
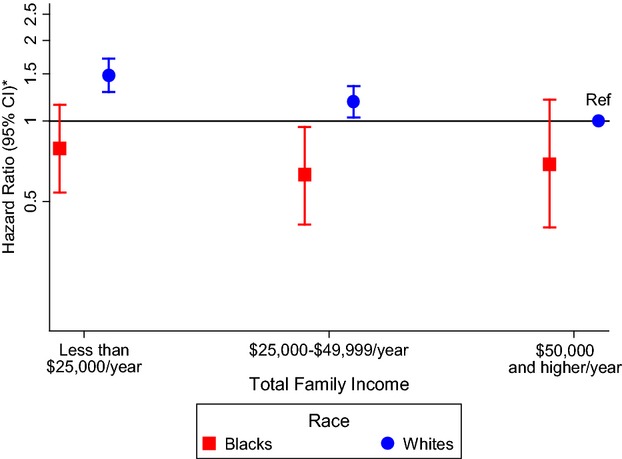
Association between total family income and atrial fibrillation stratified by race in the Atherosclerosis Risk in Communities study, 1987–2009. The reference group is whites with a total family income of $50 000 or more per year. *Cox proportional hazards model adjusted for age, sex, and study site. CI indicates confidence interval; Ref, reference group.
Education and AF Risk
Due to a significant education–sex interaction (P=0.01), sex‐specific results are presented in Table 4. Women with less than a high school degree had an 88% higher risk of AF compared with those with at least some college (HR 1.88, 95% CI 1.55 to 2.28) after adjustment for age, race, and field center (Table 4, model 1). Additional adjustment for other CVD risk factors attenuated this association, which remained statistically significant (HR 1.38, 95% CI 1.13 to 1.69]) (Table 4, model 3). No association of education with AF was seen in men. Using whites in the highest education group as the reference group in sex‐specific analyses, blacks had lower HRs for AF than whites with the same education level (Figures 3 and 4). There were no significant interactions between education and race or age (P>0.05).
Table 4.
Sex‐Specific Hazard Ratio and 95% CI of Atrial Fibrillation by Education Level in the Atherosclerosis Risk in Communities Study, 1987–2009
| Education Level | P for Trend | |||
|---|---|---|---|---|
| Some High School or Less | High School Graduate/Vocational School | Some College/Graduate School | ||
| Women | (n=1817) | (n=3529) | (n=2524) | |
| AF events | 273 | 337 | 194 | |
| Person‐years | 31 606 | 67 149 | 48 977 | |
| Crude incidence rate (per 1000 person‐years) | 8.6 | 5.0 | 4.0 | |
| Model 1 HR (95% CI) | 1.88 (1.55 to 2.28) | 1.16 (0.97 to 1.39) | 1.00 (Reference) | <0.0001 |
| Model 2 HR (95% CI) | 1.39 (1.14 to 1.69) | 1.02 (0.86 to 1.22) | 1.00 (Reference) | 0.001 |
| Model 3 HR (95% CI) | 1.38 (1.13 to 1.69) | 1.02 (0.86 to 1.22) | 1.00 (Reference) | 0.002 |
| Model 4 HR (95% CI) | 1.29 (1.06 to 1.58) | 1.03 (0.86 to 1.23) | 1.00 (Reference) | 0.01 |
| Men | (n=1522) | (n=2369) | (n=2591) | |
| AF events | 249 | 371 | 370 | |
| Person‐years | 24 231 | 41 159 | 47 333 | |
| Crude incidence rate (per 1000 person‐years) | 10.3 | 9.0 | 7.8 | |
| Model 1 HR (95% CI) | 1.15 (0.97 to 1.36) | 1.14 (0.99 to 1.32) | 1.00 (Reference) | 0.08 |
| Model 2 HR (95% CI) | 0.98 (0.82 to 1.17) | 1.04 (0.90 to 1.20) | 1.00 (Reference) | 0.89 |
| Model 3 HR (95% CI) | 0.95 (0.80 to 1.14) | 1.03 (0.89 to 1.19) | 1.00 (Reference) | 0.66 |
| Model 4 HR (95% CI) | 0.88 (0.74 to 1.05) | 0.99 (0.86 to 1.15) | 1.00 (Reference) | 0.19 |
Model 1: Cox proportional hazards model adjusted for age, race, and study site. Model 2: Model 1 with additional adjustment for body mass index, diabetes, drinking status, smoking status, systolic blood pressure, and antihypertensive medication. Model 3: Model 2 with additional adjustment for prevalent heart failure, myocardial infarction, or stroke at baseline. Model 4: Model 3 with additional adjustment for incident heart failure, myocardial infarction, or stroke as time‐varying covariates. AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio.
Figure 3.
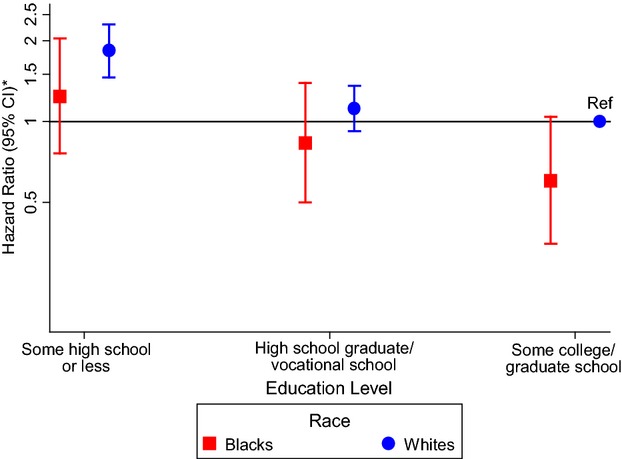
Association between education level and atrial fibrillation in women stratified by race in the Atherosclerosis Risk in Communities study, 1987–2009. The reference group is white women who attended some college or graduate school. *Cox proportional hazards model adjusted for age and study site. CI indicates confidence interval; Ref, reference group.
Figure 4.
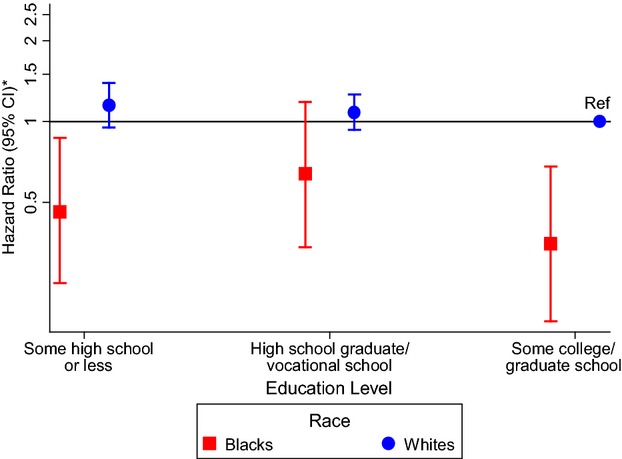
Association between education level and atrial fibrillation in men stratified by race in the Atherosclerosis Risk in Communities study, 1987–2009. The reference group is white men who attended some college or graduate school. *Cox proportional hazards model adjusted for age and study site. CI indicates confidence interval; Ref, reference group.
Excluding participants who had an incident AF event within the first 2 years of follow‐up (n=51) showed HRs of a similar magnitude to those seen in the models including all of the incident AF cases for both education and income (results not shown).
Discussion
This prospective analysis of the ARIC cohort identified an inverse association between individual SES and incident AF, which is consistent with previous literature on the role of SES on CVD.6–7,6–22 There was a graded, inverse, dose‐response association between total family income and risk of AF after adjustment for demographic factors. A significant interaction between sex and education revealed that lower education was associated with an elevated AF risk in women but not in men. In models adjusting for CVD and its risk factors, the SES–AF association was greatly attenuated or disappeared, suggesting that the observed associations may be mediated through cardiovascular risk factors.
These results are consistent with a recent study in a Swedish population examining the association between neighborhood deprivation and AF.14 A positive association was identified for women, with those living in a highly deprived neighborhood having an increased risk of AF after adjustment for age and individual‐level SES characteristics. No association was identified for men. As in our analysis, adjustment for CVD risk factors and comorbidities attenuated all associations. The authors concluded that neighborhood deprivation and socioeconomic disparities are not direct independent risk factors for hospitalized AF. In addition, these socioeconomic factors may indirectly increase the risk of AF, particularly in women, through their effects on cardiovascular risk factors and other CVD that may mediate this association.
In the ARIC cohort, education attainment is a stronger predictor of AF among women than men. Prior research suggests that socioeconomic factors, particularly education, may be more clearly associated with a cardiovascular risk profile and a higher risk of CVD among women than men.7–13 Sex differences in the impact of low education might be explained by greater psychosocial adversity and poorer metabolic profile among poorly educated women compared with poorly educated men.7–11
In addition, racial differences in AF incidence by levels of SES were assessed. Previous studies have shown that incidence of AF is lower in blacks than whites.20,23 However, lower average SES among blacks and the subsequent reduced access to quality health care6 could partly explain this association if underdiagnosis of AF occurs more frequently among those with low SES.16 In this analysis, blacks had lower rates of AF than whites within the same SES group, and there was a similar trend in the association between lower SES and higher AF risk in both racial groups. These results suggest that lower SES in blacks compared with whites is not a major determinant of racial differences in AF incidence. Recently, an analysis of National Health and Nutrition Examination Survey (NHANES) data examining cardiovascular risk found that disparities were related more to SES than to race or ethnicity, further supporting these results.23 However, other explanations such as physician bias,24 with which blacks may be less likely to be diagnosed with AF than whites, or poorer access to health care for blacks compared with whites at the same SES level cannot be totally ruled out by these results.
An important limitation in this analysis is the ascertainment of AF. Most AF cases were identified through hospitalization discharges, which omit asymptomatic AF or AF cases managed in outpatient settings. Consequently, underascertainment of incident AF may have occurred. Despite this limitation, the ARIC study and other cohorts have shown acceptable validity for discharge code–based AF diagnosis.20,25 In addition, rates of AF in the ARIC cohort are similar to those from other population‐based studies, improving confidence in the validity of the AF case ascertainment.20 For the SES exposures, education is a more individualistic measure of SES than total family income; however, the number of years of education does not account for variation in educational quality across groups.26 In addition, only 1 measurement of total family income and education level at baseline was used in this analysis, and both may possibly change during follow‐up. The potential for selection bias cannot be totally rejected because low SES may be associated with loss to follow‐up, and it is unknown if there is a difference by AF outcome status. For this analysis, we assumed that attrition does not differ by AF outcome status based on the continuous surveillance of death certificates and community hospitals that the ARIC study performs, even when participants have been lost to follow‐up, which should reduce the probability of a difference by AF outcome status. Unmeasured or residual confounding could also partly explain the observed association. Despite these limitations, several strengths should be highlighted, including the large, biracial sample with an extensive follow‐up time; a large number of AF events; stable exposure measurements with education level and total family income; and comprehensive measurement of key cardiovascular covariates.
Conclusion
This study identified an association between lower total family income and higher AF risk regardless of race and sex, along with an association of lower education with incident AF risk for women. Cardiovascular risk factors and conditions may mediate these associations. These results do not support the hypothesis that lower SES in blacks compared with whites is responsible for the observed racial differences in AF risk. Future studies are needed to examine the specific mechanisms underlying this association, specifically the indirect effects of SES on cardiovascular risk factors and outcomes with the incidence of AF, and to determine the role of SES in shaping and targeting potential interventions for the prevention and diagnosis of AF in communities.
Sources of Funding
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. This study was additionally funded by grants 09SDG2280087 from the American Heart Association (AHA) in Dallas, Texas, and RC1‐HL099452 from the National Institutes of Health (NIH). Dr Everson‐Rose was supported in part by grant no. 1P60MD003422 from the National Institute for Minority Health and Health Disparities (NIMHD) and by the Applied Clinical Research Program and Program in Health Disparities Research at the University of Minnesota. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the views of the NIH, NHLBI, or NIMHD.
Disclosures
None.
Acknowledgments
We thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844. [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946-952. [DOI] [PubMed] [Google Scholar]
- 5.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011; 123:1501-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993; 88:1973-1998. [DOI] [PubMed] [Google Scholar]
- 7.Thurston RC, Kubzansky LD, Kawachi I, Berkman LF. Is the association between socioeconomic position and coronary heart disease stronger in women than in men? Am J Epidemiol. 2005; 162:57-65. [DOI] [PubMed] [Google Scholar]
- 8.Kavanagh A, Bentley RJ, Turrell G, Shaw J, Dunstan D, Subramanian SV. Socioeconomic position, gender, health behaviours and biomarkers of cardiovascular disease and diabetes. Soc Sci Med. 2010; 71:1150-1160. [DOI] [PubMed] [Google Scholar]
- 9.Chichlowska KL, Rose KM, Diez‐Roux AV, Golden SH, McNeill AM, Heiss G. Individual and neighborhood socioeconomic status characteristics and prevalence of metabolic syndrome: the atherosclerosis risk in communities (ARIC) study. Psychosom Med. 2008; 70:986-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos AC, Ebrahim S, Barros H. Gender, socio‐economic status and metabolic syndrome in middle‐aged and old adults. BMC Public Health. 2008; 8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veronesi G, Ferrario MM, Chambless LE, Sega R, Mancia G, Corrao G, Fornari C, Cesana G. Gender differences in the association between education and the incidence of cardiovascular events in Northern Italy. Eur J Pub Health. 2011; 21:762-767. [DOI] [PubMed] [Google Scholar]
- 12.Foraker RE, Rose KM, Kucharska‐Newton AM, Ni H, Suchindran CM, Whitsel EA. Variation in rates of fatal coronary heart disease by neighborhood socioeconomic status: the atherosclerosis risk in communities surveillance (1992–2002). Ann Epidemiol. 2011; 21:580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicki‐Deverts D, Cohen S, Matthews KA, Jacobs DR., Jr Sex differences in the association of childhood socioeconomic status with adult blood pressure change: the CARDIA study. Psychosom Med. 2012; 74:728-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoller B, Li X, Sundquist J, Sundquist K. Neighbourhood deprivation and hospitalization for atrial fibrillation in Sweden. Europace. 2013; 15:1119-1127. [DOI] [PubMed] [Google Scholar]
- 15.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the epidemiology, practice, outcomes, and costs of heart failure (EPOCH) study. J Am Coll Cardiol. 2004; 43:429-435. [DOI] [PubMed] [Google Scholar]
- 16.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009; 5:547-556. [DOI] [PubMed] [Google Scholar]
- 17. The atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989; 129:687-702. [PubMed] [Google Scholar]
- 18.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996; 49:223-233. [DOI] [PubMed] [Google Scholar]
- 19.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities (ARIC) study. Stroke. 2009; 40:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in Whites and African‐Americans: the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001; 345:99-106. [DOI] [PubMed] [Google Scholar]
- 22.Shah RU, Winkleby MA, Van Horn L, Phillips LS, Eaton CB, Martin LW, Rosal MC, Manson JE, Ning H, Lloyd‐Jones DM, Klein L. Education, income, and incident heart failure in post‐menopausal women: the Women's Health Initiative Hormone Therapy trials. J Am Coll Cardiol. 2011; 58:1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. 2010; 20:617-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fincher C, Williams JE, MacLean V, Allison JJ, Kiefe CI, Canto J. Racial disparities in coronary heart disease: a sociological view of the medical literature on physician bias. Ethn Dis. 2004; 14:360-371. [PubMed] [Google Scholar]
- 25.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997; 96:2455-2461. [DOI] [PubMed] [Google Scholar]
- 26.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005; 294:2879-2888. [DOI] [PubMed] [Google Scholar]


