Introduction
Atrial fibrillation (AF) is a global health problem. The condition brings an increased risk of stroke, systemic embolism, and heart failure (HF) and is associated with impaired quality of life, frequent hospitalizations, and mortality.1 Observational studies have been the main source of information for many years and have defined the clinical presentation, clinical course, and prognosis of AF. Based on key issues identified by observational studies, management of patients with AF has been informed by randomized, controlled trials (RCTs) that provide the main support for guideline recommendations regarding management of patients with AF and prevention of thromboembolic complications. Nevertheless, important questions regarding the clinical course, risks, and management of AF in clinical practice remain unanswered.1
Although RCTs provide high‐level evidence on the efficacy and safety of therapeutic interventions, they generally involve well‐defined study populations that exclude complex patients and have standardized protocols for management with closer monitoring and stricter follow‐up than is typical of routine clinical practice. Thus, their results are not always directly applicable to the general population or routine practice.
Traditional observational studies, often limited to small patient populations and performed at a single institution, are giving way to multicenter and national registries, supported by the transfer of information to large databases. Structured data collection can inform the generation of new hypotheses and help to test established ones. Registries are also subject to limitations as well as potential confounding factors related to the population selected, number, and scope of tracked variables and prevailing concepts of the disease under investigation.2 These difficulties have been highlighted by expert groups that elaborated guidelines for reliable reporting of observational studies.3
Study designs vary depending on whether the objectives involve disease definition, diagnostic methodology, occurrence, etiology, prevention, prognosis, and treatment. The information obtained from observational and interventional studies provide different approaches that require integration of a wide array of data to derive a complete perspective on a disease or condition.
This review provides an overview of available registry data on patients with AF and focuses on 3 areas at the heart of AF management: (1) stroke prevention; (2) pharmacological rhythm and rate control; and (3) catheter‐based ablation. In addition to cataloging the types of registry data available, we consider how these data contribute to understanding and management of patients with AF and speculate on the future directions of observational research.
The Search Strategy
Registries or databases reporting on AF management, identified from sources that included CINAHL, Medline, EMBASE, and the Cochrane Database of Systematic Reviews from 2000 to 2012, were selected for closer review, and publications from these registries were tracked. We identified 34 large international or national registries of AF patients, including 17 reporting on thromboprophylaxis and stroke prevention, 8 focused on antiarrhythmic drug (AAD) therapy and cardioversion, 7 studying AF ablation, and 2 detailing left atrial appendage closure (LAAC) registries.
Cataloging AF Registries
Tables 1 through 4 provide overviews of the registries identified, which have been grouped into the following broad categories: (1) those focused on thromboprophylaxis and stroke prevention (Table 1); (2) those on cardioversion and AAD therapy (Table 2); (3) those on ablation (Table 3); and LAAC (Table 4). Several registries address multiple aspects of AF diagnosis and management and could be allocated to more than 1 category. To enable some key findings to be visualized more clearly, registries that provide information on medical treatments for AF (including use of agents for rhythm and rate control, warfarin, aspirin, and new oral anticoagulants [NOACs]) are also shown separately in Table 5.
Table 3.
Registries Providing Information on AF Ablation
| Registry | Country | Years | Setting | Study Aim | Patient Characteristics | No. of AF Patients | Female (%) | Mean Age (±SD) | Paroxysmal AF (%) | CHADS2 (CHA2DS2VASC) |
|---|---|---|---|---|---|---|---|---|---|---|
| AF ablation pilot study of European Heart Rhythm Association31 | 10 countries Europe | Oct 2010–May 2011 | Inpatients | Clinical epidemiology and therapy of European ablation patients | Undergoing AF ablation | 1410 | 28 | 60 | 62 | 0 to 5; ≥1 in 57.8% (0 to 7; ≥1 in 78.9%) |
| AF ablation Long‐term Registry of EURObservational Research Programme32 | 54 countries invited (29 participating so far) | Ongoing | Inpatients | Long‐term study of the above | Undergoing AF ablation | 875 so far | 41.02% | 71.16±11.3 | 19.04 | — |
| AF‐Intermountain hospitals33 | USA | Ongoing | Inpatients | Long‐term impact of ablation | Undergoing AF ablation | 21 060 (4212 underwent AF ablation) | 39.2 | 66±13.3 | — | — |
| CARAF21 | Canada | 1990–1996 | Inpatients | Gender‐related differences in AF | AF | 1097 | 38 | Women 65.4±0.7 Men 60.5±0.6 | 100 | — |
| National Multicenter Registry on Procedural Safety of Catheter Ablation for AF34 | Italy | Jan–Dec 2011 | Inpatients | Safety of catheter ablation for AF | Patients who underwent AF catheter ablation | 2323 | 28 | 60 | 54 | 1 |
| Prospective registry35 | USA | Jan 2010–July 2011 | Ablation in high‐volume centers | Feasibility and safety of dabigatran in ablation | Undergoing AF ablation | 290 | 21 | 60 | 57 | 30% ≥2 (1.6) |
| SAFARI36 | USA | Planned | Inpatients | Safety and efficacy of catheter ablation | Undergoing AF ablation | 5000 (aim) | — | — | — | — |
AF indicates atrial fibrillation.
Table 4.
Registries Reporting on LAAC
| Registry | Country | Years | Setting | Study Aim | Patient Characteristics | No. of AF Patients | Female (%) | Mean Age (±SD) | Paroxysmal AF (%) | CHADS2 (CHA2DS2VASC) |
|---|---|---|---|---|---|---|---|---|---|---|
| ACP37 | Europe | May 2010–June 2017 | Patients with AF at a high risk of stroke | Initial experience with ACP in European patients | On warfarin or dabigatran therapy, CHADS2 score ≥2 | 143 | — | — | — | — |
| ASAP38 | Europe | Jan 2009–Dec 2013 | Patients contraindicated for long‐term warfarin therapy | Safety and efficacy of LAAC in patients | AF contraindicated for warfarin | 150 | — | 73 (±7.4) | — | 2.8 (4.4) |
ACP indicates Amplatzer cardiac plug; AF, atrial fibrillation; ASA, acetylsalicylic acid; LAAC, left atrial appendage closure.
Table 1.
Registries With a Focus on Thromboprophylaxis and Stroke Prevention
| Registry (Sponsor) | Country | Years | Setting | Study Aim | Patient Characteristics | No. of AF Patients | Female (%) | Mean Age (±SD) | Paroxysmal AF (%) | CHADS2 (CHA2DS2VASC) |
|---|---|---|---|---|---|---|---|---|---|---|
| AFNET1 (German Competence Network) | Germany | 2010 | Inpatients/outpatients | Personalized management | AF | 9558 | — | — | — | 1.6 to 1.9 |
| AVAIL4 | USA | July 2006–Sept 2009 | Inpatients | Long‐term use of antithrombotic therapies | Patients with ischemic stroke/TIA and AF post discharge | 291 | 51.6 | 76 | — | 2 to 6 |
| CAPTURE5 | USA (Illinois) | Nov 2002–March 2003 | Inpatients | Comparison of quality indicators | Stroke/TIA | 1953 | 53.2/57.1 | 66.7/67.5 | — | — |
| Euro Heart Survey6 | 35 countries Europe | Sep 2003–Jul 2004 | Inpatients/outpatients | Management against European guidelines | AF confirmed by ECG or Holter recording | 3890 | 43.5 | 66.4±12.2 | 30.1 | 0 to 6 |
| GARFIELD (Bayer)7 | 50 countries worldwide | Ongoing (2009–2015) | Inpatients/outpatients | Evaluate management and outcomes | Newly diagnosed nonvalvular AF with additional risk factor for stroke | Aiming for 55 000 | — | — | — | — |
| GLORIA‐AF (Boehringer Ingelheim)8 | Global (62 centers) | May 2011–Jan 2013 | Inpatients | Characteristics influencing choice of therapy | Newly diagnosed nonvalvular AF patients at risk for stroke | Aiming for 55 000 | — | — | — | — |
| J‐RHYTHM9 | Japan | Jan 2009–July 2009 | Outpatients | Regional use of anticoagulation therapy | AF | 7937 | 31.1 | 69.7±9.9 | 37.1 | 1.7±1.2 |
| National Cardiovascular Data Registry's ACTION Registry–Get With the Guidelines10 | USA | July 2008–Sept 2009 | Outcomes of patients with acute myocardial infarction and AF | Myocardial infarction with AF | 4947 | 42.4 | Median 78 | — | 0: 3.7% 1: 12.9% ≥2: 80.9% |
|
| Nationwide Danish study11 | Denmark | Patient registry data from 1997–2008 | Inpatients/outpatients | Net clinical benefit of OACs | Non‐valvular AF | 141 500 | 47.2 | 72.6 ±12.9 | — | 0 to 6 (0 to 9) |
| ORBIT‐AF12 | USA | June 2010–Nov 2014 | Outpatients | Characterize treatment and outcomes | Incident or prevalent AF | 10 126 | 43 | 75 | 46 | 75% ≥2 (85% ≥2) |
| PINNACLE‐AF (http://www.ncdr.com/webncdr/pinnacle) (National Cardiovascular Data Registry) | USA | Ongoing | Outpatients | Monitoring practise pattern changes over time | AF | 100 000+ | – | — | — | — |
| PREFER‐AF (Daiichi‐Sankyo)13 | 7 countries Europe | 2012 | Inpatients/outpatients | Management of patients under 2010 guidelines | History of AF | 7243 | 40 | 71.5±11 | 30 | (3.4) |
| REACH14 | 44 countries | Outpatients | Implications of AF | PAD patients at risk of AF | 6814 | 35.6 | 72.8±9.2 | — | 2.8±1.3 | |
| RE‐LY15 | 47 countries | Nov 2005–April 2009 | Inpatients/outpatients | Comparison of warfarin and dabigatran | AF with an additional risk factor for stroke | 13 507 | 45.4 | 66.2 | — | — |
| REVERSE16 (Sociedad Española de Cardiolgía) | Spain | Feb–June 2004 | Inpatients (cardioversion) | Comparison of treatment and clinical characteristics | Persistent AF referred for cardioversion | 1515 | 37 | 63±11 | 0 | — |
| Swedish AF Cohort17 | Sweden | July 2005–Dec 2008 | Inpatients/outpatients | Investigate risk factors in AF | AF | 182 678 | 47% | 76.2 | — | 0 to 6 |
ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; AF, atrial fibrillation; OAC, oral anticoagulation; PAD, peripheral artery disease; TIA, transient ischemic attack.
Table 2.
Registries Reporting on Antiarrhythmic Drug Therapy and Cardioversion in AF
| Registry | Country | Years | Setting | Study Aim | Patient Characteristics | No. of AF Patients | Female (%) | Mean Age (±SD) | Paroxysmal AF (%) | CHADS2 (CHA2DS2VASC) |
|---|---|---|---|---|---|---|---|---|---|---|
| AFFECTS18–19 | USA | Mar 2005–Dec 2007 | Outpatients | Clinical practice patterns in management | AF with increased risk of stroke | 1461 | 46.3 | 66.2±13.3 | 79.7 | — |
| ATRIUM20 | Germany | 2009 | Outpatients | Management of AF in Germany | All stages of AF | 3667 | 42.0 | 72.0±9.0 | 27.1 | 2.2±1.3 (3.8±1.7) |
| CARAF I and II21 | Canada | 1991–2007 | Outpatients | Use of AADs in Canada from 1991 to 2007 | New‐onset paroxysmal AF | 1400 | — | — | 100 | — |
| GULF‐SAFE22 (Gulf Heart Association) | 6 Middle Eastern countries | Oct 2009–July 2010 | Emergency department | AF management in the Gulf | AF | 2043 | 48.0 | 57.0±16.0 | 17.0 | 2.1±0.5 |
| MADRE23 | Germany | 2010–2012 | Outpatients | Efficacy and tolerance of dronedarone in clinical practice | Paroxysmal or persistent AF | 191 | 44.5 | 63±9.9 | 62.5 | — |
| REALISE‐AF24 | 26 countries worldwide | Nov 2009–June 2010 | Outpatients | World‐wide management | At least 1 AF episode in the last 12 months | 10 523 | 44 | 66.6±12.2 | 24.8 | — |
| RECORDAF25–27 | 21 countries worldwide | May 2007–Apr 2008 | Inpatients/outpatients | World‐wide management | Recent‐onset AF | 5604 | 42.8 | 66±11.9 | 45.6 | — |
| RECORDAF‐Asia Pacific28 | 8 countries Asia/Pacific | Apr 2009–July 2010 | Outpatients | Management across Asia‐Pacific | Recently diagnosed AF | 2629 | 40 | 64±13 | 49 | — |
| RHYTHM‐AF29–30 | 10 countries worldwide | May 2010–Feb 2013 | Inpatients (cardioversion) | Antithrombotic therapy in relation to stroke risk and AF duration | AF patients suitable for cardioversion | 3940 | 38 | 66±12 | — | 3±2* |
AADs indicates antiarrhythmic drugs; ACTION, Acute Coronary Treatment and Intervention Outcomes Network; AF, atrial fibrillation.
Mean±SD.
Table 5.
Registries Providing Information on Medical Treatment of AF
| Registry | Rate‐Control Treatment (%) | Rhythm‐Control Treatment (%) | Warfarin (%) | NOACs (%) | Aspirin (%) |
|---|---|---|---|---|---|
| AVAIL4 | — | — | 49.1 | — | 5.5 |
| CAPTURE5 | — | — | 4.73 | — | — |
| J‐RHYTHM9 | — | — | 87.3 | — | 22.3 |
| Nationwide Danish study11 | — | — | 17.6 | — | — |
| ORBIT‐AF12 | — | — | — | — | 35 |
| PREFER AF (Daiichi‐Sankyo)13 | — | 59.8 | 34.1 | 6.1 | 19.8 |
| REACH14 | — | — | 36.2 | — | 42.6 |
| RE‐LY15 | — | — | 32 | — | — |
| Swedish AF Cohort17 | — | — | 40 | — | — |
| Euro Heart Survey6 | — | — | 60.9 | — | 30.5 |
| National Cardiovascular Data Registry's ACTION Registry–Get With the Guidelines10 | — | — | 32.5 on admission (41 at discharge) |
— | 51.5 on admission (95 at discharge) |
| AFFECTS18–19 | 36 | 64 | 58.7 at enrollment (63.7 at follow‐up) |
— | 27.2 at enrollment (31.8 at follow‐up) |
| ATRIUM20 | 75 | 16 | 93.0 | — | — |
| MADRE23 | — | 100 | — | — | 71 |
| REALISE‐AF24 | 32.7 | 57.5 | 42 | — | — |
| RECORDAF25–27 | 45.1 | 54.9 | — | — | 43 |
| Prospective registry35 | — | — | 50 | 50 (dabigatran) | 44 |
ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; AF, atrial fibrillation; NOAC, new oral anticoagulant.
Thromboprophylaxis and Stroke Prevention Registries
A number of registries provide data on management and prevention of thromboembolism in hospitalized and ambulatory patients with AF, offering insights into the effectiveness of oral anticoagulation (OAC) therapy (Table 1). Several large‐scale registries on OAC use have been initiated by the pharmaceutical industry (Table 1) and focus on NOACs. For example, the Global Anticoagulant Registry in the Field (GARFIELD)7 and Global Registry on Long‐term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA‐AF),8 which are observational, global, large‐scale prospective registries, assess patterns and outcomes of thromboprophylaxis.
A number of national and regional registries address medication preference by patients. The Adherence eValuation After Ischemic Stroke Longitudinal (AVAIL) registry, for example, expanded on the hospital‐based Get with the Guidelines‐Stroke quality improvement program and Carotid RX ACCULINK/RX ACCUNET Post‐Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE), a prototype registry from the Paul Coverdale National Acute Stroke Registry.4–5 These evaluate whether patient management accords with practice guidelines and evidence‐based research. The Nationwide Danish study,11 Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF)12 and Prevention of thromboembolic events–European Registry in Atrial Fibrillation (PREFER‐AF)13 registries address this in European and U.S. patient cohorts. Data have been collected from each of these with the exception of CAPTURE, GARFIELD, and ORBIT‐AF registries, which are ongoing.
Registries Reporting on AAD Therapy and Cardioversion
Pharmacological and direct‐current cardioversion of AF has been charted by a number of registries to provide information on global treatment patterns (Table 2). The global Registry on Cardiac rhythm disorders assessing the control of Atrial Fibrillation (RECORD‐AF)25 was one of the first world‐wide observational surveys of the management of patients with newly diagnosed paroxysmal or persistent AF, and the Real Life Global Survey Evaluating Patients with Atrial Fibrillation (REALISE‐AF)24 cross‐sectional study captured data in 26 participating countries on AF management and cardiovascular (CV) risk profiles. RECORD‐AF has also been extended to cover the Asia‐Pacific region (RECORD‐AF Asia Pacific).28 In addition, RHYTHM‐AF is an international prospective study aimed at determining regional variability, outcomes, and cost‐effectiveness among patients with recent‐onset AF considered for cardioversion in 10 countries of antiarrhythmic and antithrombotic treatment.29 As shown in Table 2, other smaller, national registries assessing AAD use and cardioversion include US Atrial Fibrillation Focus on Effective Clinical Treatment Strategies (AFFECTS),18 the German Outpatient Registry Upon Morbidity of Atrial Fibrillation (ATRIUM) registry,20 and the Canadian Registry of Atrial Fibrillation (CARAF).21
Registries Reporting on AF Ablation and LAAC
Table 3 details registries assessing ablation therapy for AF. The Atrial Fibrillation Ablation Pilot Study, a prospective, multinational registry conducted by the European Heart Rhythm Association of the European Society of Cardiology,31 was undertaken preceding a long‐term Atrial Fibrillation Ablation pan‐European Registry by the Euro‐observational Research Programme, which opened in 2012 and invited participation from clinical sites in 54 countries. To date, some 875 patients have been enrolled from 61 or 137 registered centers in 19 or 29 registered nations. Table 3 describes other registries on AF ablation, such as the Safety of Atrial Fibrillation Ablation Registry Initiative (SAFARI),36organized by the US Cardiac Safety Research Consortium (CSRC),39 and the CARAF program in Canada.21 In addition to AF ablation, LAAC is under investigation as a potential alternative to anticoagulation for patients at high risk of stroke in the Amplatzer cardiac plug (ACP)37 and ASA Plavix Feasibility Study With Watchman LAAC (ASAP)38 registries (Table 4).
Insights From Registries
Registries on Thromboprophylaxis and Stroke Prevention
Stroke epidemiology and risk factors
Patients with AF are at risk of stroke and twice as likely to die within 1 year as those without AF.40 The Euro Heart survey, in which patients with AF (new in 18%, paroxysmal in 28%, persistent in 22%, and permanent in 29%) had a mean age of 67 years and 26% older than 75 years, found that 86% were at high risk for stroke.6
Data from the Nationwide Danish study highlight that chronic kidney disease (CKD) in patients with AF is associated with even greater risk of stroke or systemic embolism. In this cohort, the risk of stroke or thromboembolism in patients with CKD (hazard ratio, 1.49) was significantly lower in patients treated with warfarin.41 Reports from this registry also note higher risk of bleeding among patients with CKD treated with warfarin, aspirin, or both.42 Bleeding risks associated with OACs are a potential disadvantage of therapy, but data from registries help identify patients most likely to encounter bleeding complications. The concept of “net clinical benefit” aims to balance the risks of ischemic stroke and bleeding on OAC therapy43 and was originally tested using U.S. registry data. This concept, used to compare NOACs and warfarin, have been facilitated by registry data.11,44 Banerjee et al. used “real‐world” data on net clinical benefit of warfarin from the Danish National Patient Registry and modeled the expected net clinical benefit for NOACs (dabigatran, rivaroxaban, and apixaban) on the basis of recent clinical trial outcomes.11 The findings indicated that when there is a high risk of both ischemic stroke and intracranial hemorrhage, all 3 of the new agents have a greater net clinical benefit, compared to warfarin (Figure).45–46
Figure 1.
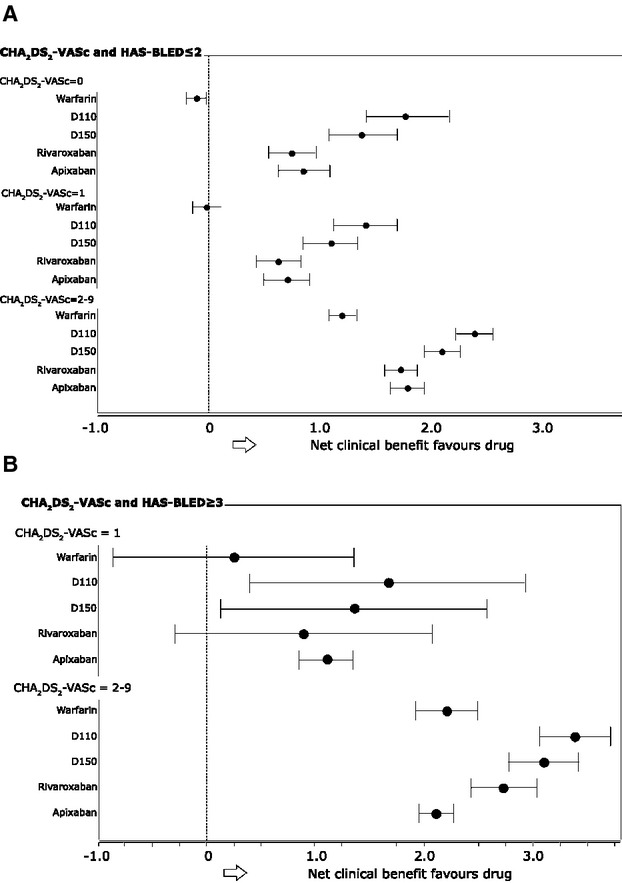
Net clinical benefit of warfarin and new oral anticoagulants dabigatran, apixaban, rivaroxaban by CHA2DS2‐VASC and HAS‐BLED scores. A, HAS‐BLED ≤2. B, HAS‐BLED ≤3. For HAS‐BLED ≥3, there were no data with CHA2DS2‐VASC score=0. D110, dabigatran 110 mg (twice‐daily; BID); D150, dabigatran 150 mg BID. Modeling was based on recent clinical trial outcome data for the new oral anticoagulants and “real‐world” data from the Danish National Patient Registry, collected from patients with nonvalvular AF between 1997 and 2008 to predict the net clinical benefit of new oral anticoagulants, compared to warfarin. Reproduced with permission from Banerjee et al.11 AF indicates atrial fibrillation.
Registry findings, such as those from Denmark and Sweden (Table 1), have helped develop and refine risk‐scoring systems for stroke and bleeding in AF patient cohorts.17,41 Scoring systems such as CHADS2 and CHA2DS2‐VASc have changed the approach to risk stratification of AF patients and guide judicious use of OAC in patients at greatest risk.47–48 Registry data have been crucial in deriving risk stratification schemes and validating the utility in clinical practice.48–50 Both the CHADS2 and CHA2D2‐VASC scores were found, by examination of registry data, to underestimate the risk of thromboembolism associated with previous ischemic events.51 Another scoring system, developed from the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study, identified risk stratification schemes for bleeding and thromboembolism to develop a simple method for quantifying the risk of warfarin‐associated hemorrhage based on 5 clinical variables with performance comparable to the CHADS2 index.52
Registry data show that the CHA2DS2VASc score performed better than CHADS2 in identifying patients at high or low risk of thromboembolism. In general, risk stratification systems should be reassessed, given that new data are developed from independent sources. A recent analysis of the ROCKET‐AF clinical trial and ATRIA cohort suggested that renal impairment could be added to the CHADS2 score to improve predictive value for identifying “high‐risk” patients at risk of stroke.53 Because all patients in these cohorts were at high risk of stroke and were treated with OACs, the full range and impact of renal function was not investigated. In another analysis of patients across a wide spectrum of renal impairment who were not anticoagulated, renal impairment added little to the predictive value of the CHADS2 and CHA2DS2‐VASc scores for stratification of thromboembolic risk.54–55 On the other hand, renal failure patients do have a higher risk of hemorrhagic complications,56–57 making the net risk‐benefit ratio difficult to establish. This is an area where further clinical trials are needed.
OACs and stroke prevention
Multiple clinical trials established the effectiveness of the vitamin K antagonists, compared to placebo, aspirin, other antiplatelet agents, or no antithrombotic therapy in patients with AF.58–61 Nevertheless, the 2006 Euro Heart Survey noted that AF management can be inadequate, with wide variations in physician adherence to evidence‐based recommendations for anticoagulation.6 In 2013, the EORP‐AF General Registry Pilot program showed improved uptake of OACs by European cardiologists, with OACs prescribed for 80% of patients with AF.32 Registry data have provided information regarding patterns of OACs across various health systems at the primary care,20,60 hospital,8 network,62 national,63–64 and international21,58 hospital,7 network,59 national,60–61 and international6,24 levels. Treatment trends over time can be used to project resource utilization and facilitate planning of health services and resources.
National prescribing registries suggest systematic underuse of warfarin,65 and data from national registries of AF patients, such as the Japanese J‐RHYTHM registry, indicate warfarin underutilization in patients at high stroke risk, as well as overuse of warfarin in patients at low risk.9 In contrast, the Euro Heart Survey found that OAC prescribing for AF was relatively high throughout all risk categories and noted that this placed a large proportion of patients with low risk of thromboembolism at higher risk of bleeding complications.6 Frequent OAC prescribing may relate to the relatively high proportion of academic and specialized centers participating in this study. Registry data from the National Cardiovascular Data Registry's Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry–Get with the Guidelines program also suggest that the patients at highest risk of ischemic stroke are least likely to take OAC medication,4 again highlighting the need for practice improvement and compliance to ensure the best outcomes among patients with AF. These data on utilization could be gleaned only from registries. Data from a number of registries providing information on the use of warfarin, NOACs, and aspirin are shown in Table 5.
The PREFER‐AF Registry found that OACs are now much more widely used than was reported in the German AFNET and the Euro Heart Survey registries on AF, and that NOACs are used by 6.1% of patients with AF.13
The increasing number of registries established in recent years to follow trends and outcomes linked to the NOACs—such as GARFIELD, GLORIA‐AF, PREFER‐AF, and Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY)—will continue to highlight practice variations in diverse healthcare settings and patient populations. For example, in the RE‐LY trial, dabigatran at 150 mg (but not 110 mg) twice‐daily was associated with lower rates of stroke, compared to warfarin.66 These variations may provide targets for improvement of OAC uptake and optimize available treatment options according to patient profiles. In addition to generating pharmacoepidemiological data on OAC use, these registries capture and assess treatment outcomes, specifically the incidence of bleeding and persistence of therapy (including discontinuation, interruption, and changes of therapy regimen).
Registries may offer insights into the off‐label use of OAC drugs. The safety and efficacy of periprocedural dabigatran in patients undergoing AF ablation was evaluated in a multicenter registry35 (Table 3), in which dabigatran was associated with a significant increase in the risk of bleeding and thromboembolism compared to uninterrupted warfarin therapy, although different lengths of time on treatment confounds interpretation of these observations. Two other systematic reviews showed no differences in bleeding or thromboembolism with periprocedural dabigatran versus warfarin in patients undergoing ablation.67–68 Given these contradictory results, more data are needed. Whereas registry data can point to potential new approaches to management, investigation in a controlled trial is the preferred way to compare therapeutic strategies and provide robust, reliable evidence with which to inform practice.
Registries on AAD Therapy and Cardioversion
Among the registries listed in Table 2, the RECORD‐AF registry involving 21 countries worldwide provides insight into the use of AADs, factors affecting progression from paroxysmal to persistent or permanent AF, and reveals the extent to which clinical practice follows practice guidelines.26–27,69 RECORD‐AF reported an overall success rate of 54% for antiarrhythmic therapy, 60% success for maintenance of sinus rhythm, and 47% for maintaining the heart rate at or below 80 beats per minute at 1 year.25 Key clinical outcomes reported for rate control and rhythm control strategies at 1 year are shown in Table 6. The data suggest that clinical outcomes of AF are driven by arrhythmia‐related hospitalization, rather than rate or rhythm management strategy,25 but in patients assigned to rate control, there was relatively rapid progression from paroxysmal to persistent AF. In RECORD‐AF, use of digoxin in patients without HF was uncommon, especially in North America, and amiodarone was used infrequently, except in patients with HF. One‐year findings from the related RECORD‐AF Asia Pacific registry showed that rhythm control strategies were often applied to patients with persistent AF and a history of HF or valvular heart disease (37% of patients), and rate‐control strategies to patients with recently identified, paroxysmal AF (63%).28 Patients in the rhythm‐control group were prescribed class Ic (39%) or III (49%) AADs, with lower use of β‐blockers in this group in the Asia Pacific registry (35%) compared with the global RECORD‐AF registry (51%).
Table 6.
Key Clinical Outcomes Reported in the RECORD‐AF Registry at 1 Year25
| Clinical Events | Rhythm Control (%) | Rate Control (%) | P Value |
|---|---|---|---|
| Any clinical event | 483/2809 (17.2) | 405/2225 (18.2) | 0.352 |
| Cardiovascular death | 24/2804 (0.9) | 61/2213 (2.8) | <0.001 |
| Myocardial infarction | 14/2785 (0.5) | 20/2175 (0.9) | 0.078 |
| Stroke or transient ischemic attack | 46/2784 (1.7) | 60/2179 (2.8) | 0.008 |
| Hospitalization for cardiovascular event | 465/2793 (16.6) | 366/2195 (16.7) | 0.891 |
| Hospitalization or increased duration of hospital stay | |||
| Due to arrythmia or proarrhythmia | 314/2790 (11.3) | 159/2179 (7.3) | <0.001 |
| Due to other cardiovascular events or interventions | 190/2791 (6.8) | 204/2182 (9.3) | 0.001 |
| Due to major complications of ablative procedure | 15/2786 (0.5) | 14/2171 (0.6) | 0.626 |
Occurrence of clinical outcomes was counted between baseline and 12 months in patients with AF or a history of AF, excluding permanent AF or transient/reversible causes of AF. Cardiovascular death recorded at 12 months includes any events reported until the end of the 15th month after baseline. AF indicates atrial fibrillation; RECORD‐AF, The global Registry on Cardiac rhythm disorders assessing the control of Atrial Fibrillation.
A number of registries provide insight into changing AF management practices that reflect the availability of new agents and updated practice guidelines. For example, CARAF charted the use of antiarrhythmic and atrioventricular‐nodal blocking drugs from 1991 to 2007 and showed a peak in AAD use in 1994 (42.5%), followed by a gradual decline, whereas use of rate‐controlling medications, particularly β‐blockers, increased (52.5% in 2007).21 Data from a number of registries providing information on the use of rhythm‐ and rate‐control treatments are shown in Table 5. The AFFECTS registry assessing patterns of care in the United States showed that most first‐line therapies were aligned with the 2006 American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology guidelines.18–19 In AFFECTS, rhythm‐control treatment was prescribed for 67% of patients with paroxysmal AF and 55% of patients with persistent AF and was more often the initial treatment strategy for older patients. The ATRIUM registry suggested that AF therapies used by primary care providers in Germany did not stabilize patients enough to avoid hospitalization.20 Rhythm control was used in approximately one third of patients, often in combination with rate control, and almost all patients received antithrombotic therapy. A comparison of the costs associated with rhythm‐ versus rate‐control strategies in Quebec, Canada, found no significant differences overall.70
The most commonly used AADs have limited efficacy to restore sinus rhythm and carry the risk of proarrhythmic toxicity, especially in elderly patients with structural heart disease. Registries provide information on outcomes associated with AAD use in clinical practice. The Nationwide Danish study examined the mortality rate associated with AAD therapy in patients with AF hospitalized between 1995 and 2004.71 In this unselected cohort, treatment with flecainide, propafenone, sotalol, or amiodarone was not associated with increased risk of death, compared to treatment without AADs, even during the first 30 days when risk is usually highest.
Registries assembled by regulatory agencies, such as the U.S. Food and Drug Administration (FDA) Mini‐Sentinel program and the U.S. Department of Defense database, reported lower all‐cause mortality with dronedarone than with other AADs.72 Population‐based administrative databases are able to provide information from a large general population of patients with AF, with longer follow‐up than is possible in most RCTs. The FDA Mini‐Sentinel program aims to detect and refine safety signals for marketed medical products and additionally identify algorithms used within administrative databases to detect and analyze health outcomes for patients with AF.73 This registry suggests that a substantial proportion of the variation in AF prevalence across studies may be the result of the choice of different criteria for confirming AF. A systematic literature review was performed to characterize the validity of algorithms to identify patients with AF from electronic health data.74 Use of nonrepresentative populations, a paucity of recent data, and a disproportionate focus on data from inpatients hindered conclusions about algorithm validity, and the researchers advised that additional research is needed in contemporary representative populations.
Follow‐up of patients in registries can track AF recurrence, although inferences require confirmatory evidence from clinical trials. In the MADRE registry, dronedarone was administered for rhythm control in a small cohort of patients followed for 300 days. AF recurrence was common (66.5%), suggesting that dronedarone may not be superior to other AADs in maintaining sinus rhythm.23 Outcomes among ambulatory patients, drawn from the cross‐sectional REALISE‐AF survey, showed that the target heart rate was not achieved in 71% of the cases in which a rate‐control strategy was chosen, whereas 26% of those selected for a rhythm‐control strategy found that AADs failed to maintain sinus rhythm.6 Overall, 44% of patients with controlled AF were asymptomatic. Results from REALISE‐AF highlight that guidelines for use of AADs are not closely followed, and alternative therapies are needed to improve CV outcomes in patients with AF.
Table 2 highlights country‐to‐country differences in electrical versus pharmacological cardioversion practice, with RHYTHM‐AF showing a preference for direct‐current cardioversion at academic centers in Germany, where there is ease of access and a generally high primary success rate.30 Electrical cardioversion is also a common choice in Sweden, whereas pharmacological cardioversion is more often chosen in Spain.
Registries on AF Ablation
Catheter‐based pulmonary vein isolation for AF ablation, introduced more than 2 decades ago, is viewed today as a reasonable therapeutic option to prevent symptomatic AF in patients refractory to, or intolerant of, antiarrhythmic medication.26 Although several small randomized trials of AF ablation of AF showed significantly higher rates of freedom from AF with ablation, compared to AADs, this reflects outcomes in highly selected patients performed by experienced operators. Some were single‐center studies, follow‐up was generally limited to 12 months, and monitoring for recurrent AF varied considerably.25–26,75
Given the limitation of these trials, registry data offer important insights into the application, success, and limitations of ablation therapy (Table 3). The AF ablation pilot study of the European Heart Rhythm Association, the first systematic, prospective international study specifically aimed at collecting information on AF ablation practices in Europe, suggests that patients selected for ablation are younger and more symptomatic of the arrhythmia, with up to one third seeking to reduce or eliminate the need for medications.31 This preliminary study showed that the most commonly employed ablation strategy is electrical isolation of the pulmonary veins and that, at discharge, 91.4% of patients were in sinus rhythm and 88.3% of patients were receiving vitamin K antagonists and 67% AADs. After 1 year, of 944 patients, 73.7% were free of recurrent AF based on periodic ECG monitoring, 56.6% without AADs, and conduction block was present in approximately 60%.31 The overall complication rate was 7.7%, in the range of other multicenter surveys.76
Other insights come from a world‐wide survey of catheter ablations that focused on methodology, rather than patient characteristics and outcomes. In this survey, success rates were higher in patients with paroxysmal than persistent and long‐standing AF, and 70% of patients did not require further AAD treatment during follow‐up.77 Reports from Canada of the costs, compared to medical therapy, for paroxysmal AF found catheter ablation a fiscally viable alternative, with cost equivalence after 4 years.78 LAAC devices, such as the ACP and the Watchman device, have been proposed as alternatives to anticoagulation in patients at high risk of stroke (Table 4).
The Future of AF Registries
Currently available registries are providing a wealth of data that will help guide management of AF patients. Trial data have confirmed the effectiveness of novel anticoagulants, including dabigatran, rivaroxaban, apixaban, NOACs and edoxaban,65,79–81 and other agents may be introduced in the future. Efforts are underway to address the comparative effectiveness of these agents,11,82–83 but ongoing registry data will establish evolving clinical practices regarding their use relative to warfarin and one another.4,15 Another important function of longitudinal registries is to identify safety concerns surrounding these agents.
Registry data can have important limitations that should not be overlooked. Among these are data from single centers involving short follow‐up in which patient demographics and patterns of AF are not well characterized. Unlike RCTs, which include highly selected patients that limit generalizability, registries are population based and therefore subject to bias. Registries lack controls from which to draw comparative assessments. Furthermore, data are lacking for some regions, and the majority have focused on Europe and the United States, although studies such as RECORD‐AF Asia Pacific seek to address this. It has been also suggested, by the 2014 AHA/ACC/Heart Rhythm Society guidelines for management of patients with AF, that comparisons between registries from different sociocultural backgrounds may be obscured by the difficulty to quantify and report individualized antithrombotic therapy based on a shared physician‐patient decision.84 An important factor to consider when studying registry data is whether the registry is sponsored through industry, given that this may introduce the possibility of bias into the outcomes.
Some challenges face those attempting to initiate registries, such as specifying the purpose of the registry early on, how the data will be used, who will analyze it, who will have access to it, and, most important, who will fund it.36,85 This information can, for example, determine whether patients in the registry are similar to those enrolled in RCTs, generate hypotheses for future clinical trials, and determine the effectiveness of therapy in subpopulations. Obtaining sufficient funding for a registry is also a critical challenge. Previous registries have been either voluntary or linked to reimbursement.36 Determining the best funding mechanism for a registry early on is one of the most critical first steps to the success of such a registry. Investment in a national registry certainly requires substantial resources. Such investments must be balanced with other important safety and clinical effectiveness studies, such as CABANA and EAST.36,86
AF typically coexists with other conditions as both risk factors and complications. Registry data can inform our knowledge of the relationship between AF and other diseases to facilitate tailored, patient‐specific approaches.
Conclusions
As highlighted in this review, registries extend knowledge of the natural history, risk factors, treatment practices, and long‐term outcomes and risks of management strategies within a truly representative population of AF patients managed in clinical practice. Together with patient database evaluations, these resources can greatly improve understanding of the needs and gaps in care for diverse patient subgroups. Though a comprehensive review of administrative databases was not within the scope of this review, increasingly relevant literature derived from mining of patient data can inform the management of patients with AF in an array of care settings.
Registry data are the means by which to determine whether patients are managed in line with evidence‐based guidelines—but they offer more than just a reflection of whether everyday practice adheres to accepted norms or ideals. Follow‐up of large numbers of patients allows assessment of long‐term outcomes and infrequent complications. There is scope to gather more information on the impact of AF and its management on quality of life as well as to provide insights into the overall health economic burden of AF. These registries have already helped shape and improve the way we manage patients, and well‐designed registries have the potential to yield hypotheses to guide future clinical and basic research.
Sources of Funding
Lip, Al Khatib, Cosio, Ruskin, Nattel, Halperin, Kirchhof, and Camm attended a meeting in London to discuss the content for this article, for which they received financial compensation for their time (apart from Al Khatib) and expenses, from an unrestricted educational grant by Sanofi. No reimbursement was received for drafting or reviewing the article. Sanofi had no input into the meeting agenda or discussions nor into the contents of the manuscript. Logistical and editorial support was provided by HealthCare21 Communications Ltd. (Macclesfield, UK) and was supported by Sanofi.
Disclosures
Lip: consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Daiichi‐Sankyo, Biotronik, Portola, and Boehringer Ingelheim; speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, and Sanofi. Ruskin: consultant: Atricure Inc, Arrhythmia Education Inc, Astellas/Cardiome, Biosense Webster, Inc, Bristol Myers Squibb, CardioInsight, InfoBionic (equity), Medtronic, Inc, Pfizer, Portola (equity), Sanofi Aventis, and Third Rock Ventures; fellowship support: Biosense Webster, Inc, Boston Scientific Corp, Medtronic, Inc, and St. Jude Medical. Al Khatib: has no conflicts of interest to declare and did not receive any honoraria for her participation in the meetings that led to development of this manuscript. Cosio: fellowship program support by Medtronic and Sorin; speaker's honoraria from Sanofi, St. Jude. Banerjee, Blendea, Conroy, Hess, Guasch, and Cosio have no conflict of interest to declare. Savelieva: advisor/speaker/investigator for: Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Cardiome, Daiichi, Pfizer, and Sanofi. Nattel: consultant/advisor to Xention; listed as inventor on the following patents awarded or pending belonging to the Montreal Heart Institute: Preventing atrial fibrillation with the use of statin drugs; TRPC3 channels are critical for regulating fibroblast proliferation in the heart; and MiR21 as a target in prevention of atrial fibrillation. De Bono: research funding from BMS and travel funding from St. Jude Medical, Boston Scientific, and Medtronic. Halperin: consulting fees from Astellas Pharma US, Inc, Atricure/Boston Biomedical Associates, AstraZeneca, Bayer AG HealthCare, Boehringer Ingelheim, Pharmaceuticals, Inc, Bristol‐Meyers Squibb, Daiichi Sankyo, Ortho‐McNeil‐Janssen Pharmaceuticals, Inc, Johnson & Johnson, Pfizer, Inc, Sanofi and Biotronik, Inc. Kirchhof: consulting fees and honoraria from 3M Medica, MEDA Pharma, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Daiichi‐Sankyo, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer/BMS, Sanofi, Servier, Siemens, and TAKEDA; research grants from 3M Medica/MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, Sanofi, St. Jude Medical, German Federal Ministry for Education and Research, Fondation Leducq, German Research Foundation, and the European Union; travel support received from the European Society of Cardiology, the European Heart Rhythm Association, and from the German Atrial Fibrillation Competence NETwork. Camm: consultant/advisor to St. Jude, Medtronic, Boston Scientific, Sanofi, Cardiome, Pfizer, BMS, Bayer, and Boehringer Ingelheim.
References
- 1.Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, Schotten U, Wegscheider K, Boriani G, Brandes A, Ezekowitz M, Diener H, Haegeli L, Heidbuchel H, Lane D, Mont L, Willems S, Dorian P, Aunes‐Jansson M, Blomstrom‐Lundqvist C, Borentain M, Breitenstein S, Brueckmann M, Cater N, Clemens A, Dobrev D, Dubner S, Edvardsson NG, Friberg L, Goette A, Gulizia M, Hatala R, Horwood J, Szumowski L, Kappenberger L, Kautzner J, Leute A, Lobban T, Meyer R, Millerhagen J, Morgan J, Muenzel F, Nabauer M, Baertels C, Oeff M, Paar D, Polifka J, Ravens U, Rosin L, Stegink W, Steinbeck G, Vardas P, Vincent A, Walter M, Breithardt G, Camm AJ. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options—a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2011; 14:8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavazzi L. Do we need clinical registries? Eur Heart J. 2014; 35:7-9. [DOI] [PubMed] [Google Scholar]
- 3.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008; 61:344-349. [DOI] [PubMed] [Google Scholar]
- 4.Lopes RD, Shah BR, Olson DM, Zhao X, Pan W, Bushnell CD, Peterson ED. Antithrombotic therapy use at discharge and 1 year in patients with atrial fibrillation and acute stroke: results from the AVAIL Registry. Stroke. 2011; 42:3477-3483. [DOI] [PubMed] [Google Scholar]
- 5.Pandey DK, Cursio JFInvestigators CS. Data feedback for quality improvement of stroke care: CAPTURE Stroke experience. Am J Prev Med. 2006; 31:S224-S229. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, Lopez‐Sendon J, Vardas PE, Aliot E, Santini M, Crijns HJ. Antithrombotic treatment in real‐life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006; 27:3018-3026. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY, Mantovani LG, Verheugt FW, Jamal W, Misselwitz F, Rushton‐Smith S, Turpie AG. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J. 2012; 163:e11. [DOI] [PubMed] [Google Scholar]
- 8.Huisman MV, Lip GYH, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Teutsch C, Zint K, Ackermann D, Clemens A, Bartels DB. Design and rationale of GLORIA‐AF: a global registry program on long‐term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J. 2014; 167:329-334. [DOI] [PubMed] [Google Scholar]
- 9.Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa HInvestigators JRR. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J‐RHYTHM Registry. Circ J. 2011; 75:1328-1333. [DOI] [PubMed] [Google Scholar]
- 10.Lopes RD, Li L, Granger CB, Wang TY, Foody JM, Funk M, Peterson ED, Alexander KP. Atrial fibrillation and acute myocardial infarction: antithrombotic therapy and outcomes. Am J Med. 2012; 125:897-905. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee A, Lane DA, Torp‐Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012; 107:584-589. [DOI] [PubMed] [Google Scholar]
- 12.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011; 162:e601. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboembolic events–European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014; 16:6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkel TA, Hoeks SE, Schouten O, Zeymer U, Limbourg T, Baumgartner I, Bhatt DL, Steg PG, Goto S, Rother J, Cacoub PP, Verhagen HJ, Bax JJ, Poldermans D. Prognosis of atrial fibrillation in patients with symptomatic peripheral arterial disease: data from the REduction of Atherothrombosis for Continued Health (REACH) Registry. Eur J Vasc Endovasc Surg. 2010; 40:9-16. [DOI] [PubMed] [Google Scholar]
- 15.Healey J, Oldgren J, Parekh A, Zhu J, Pais P, Commerford P, Ezekowitz M, Wallentin L, Connolly SJ, Yusuf S. Global variations in the 1‐year rates of death and stroke in 15,432 patients presenting to the emergency department with atrial fibrillation in 47 countries: The RE‐LY AF Registry. Escardio. 2012:711005-711006. [Google Scholar]
- 16.Alegret JM, Vinolas X, Sagrista J, Hernandez‐Madrid A, Berruezo A, Moya A, Martinez Sande JL, Pastor A. [Clinical characteristics of patients with persistent atrial fibrillation referred for cardioversion: Spanish Cardioversion Registry (REVERSE)]. Rev Esp Cardiol. 2008; 61:630-634. [PubMed] [Google Scholar]
- 17.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012; 33:1500-1510. [DOI] [PubMed] [Google Scholar]
- 18.Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo ALInvestigators. ASACa. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry). Am J Cardiol. 2010; 105:1122-1129. [DOI] [PubMed] [Google Scholar]
- 19.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Europace. 2006; 8:651-745. [DOI] [PubMed] [Google Scholar]
- 20.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011; 100:897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade JG, Connolly SJ, Dorian P, Green M, Humphries KH, Klein GJ, Sheldon R, Talajic M, Kerr CR. Antiarrhythmic use from 1991 to 2007: insights from the Canadian Registry of Atrial Fibrillation (CARAF I and II). Heart Rhythm. 2010; 7:1171-1177. [DOI] [PubMed] [Google Scholar]
- 22.Zubaid M, Rashed WA, Alsheikh‐Ali AA, Almahmeed W, Shehab A, Sulaiman K, Al‐Zakwani I, Alqudaimi A, Asaad N, Amin HGulf Survey of Atrial Fibrillation Events I. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circ Cardiovasc Qual Outcomes. 2011; 4:477-482. [DOI] [PubMed] [Google Scholar]
- 23.Said SM, Esperer HD, Kluba K, Genz C, Wiedemann AK, Boenigk H, Herold J, Schmeisser A, Braun‐Dullaeus RC. Efficacy and safety profile of dronedarone in clinical practice. Results of the Magdeburg Dronedarone Registry (MADRE study). Int J Cardiol. 2013; 167:2600-2604. [DOI] [PubMed] [Google Scholar]
- 24.Alam M, Bandeali SJ, Shahzad SA, Lakkis N. Real‐life global survey evaluating patients with atrial fibrillation (REALISE‐AF): results of an international observational registry. Expert Rev Cardiovasc Ther. 2012; 10:283-291. [DOI] [PubMed] [Google Scholar]
- 25.Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey PR, Le Heuzey JY, Merioua I, Pedrazzini L, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub W. Real‐life observations of clinical outcomes with rhythm‐ and rate‐control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation). J Am Coll Cardiol. 2011; 58:493-501. [DOI] [PubMed] [Google Scholar]
- 26.Kowey PR, Briethardt G, Camm J, Crijns H, Dorian P, Le Heuzey JY, Pedrazzini L, Prystowsky EN, Salette G, Schwartz PJ, Torp‐Pedersen C, Weintraub W. Physician stated atrial fibrillation management in light of treatment guidelines: data from an international, observational prospective survey. Clin Cardiol. 2010; 33:172-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Heuzey JY, Breithardt G, Camm J, Crijns H, Dorian P, Kowey PR, Merioua I, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub W. The RecordAF study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010; 105:687-693. [DOI] [PubMed] [Google Scholar]
- 28.Amerena J, Chen SA, Sriratanasathavorn C, Cho JG, Huang D, Omar R, Tse HF, King A. Insights into management of atrial fibrillation in Asia Pacific gained from baseline data from REgistry on cardiac rhythm disORDers (RecordAF‐Asia Pacific [AP]) registry. Am J Cardiol. 2012; 109:378-382. [DOI] [PubMed] [Google Scholar]
- 29.Lip GY, Gitt AK, Le Heuzey JY, Bash LD, Morabito CJ, Bernhardt AA, Sisk CM, Chazelle F, Crijns HJ. Overtreatment and undertreatment with anticoagulation in relation to cardioversion of atrial fibrillation (the RHYTHM‐AF study). Am J Cardiol. 2014; 113:480-484. [DOI] [PubMed] [Google Scholar]
- 30.Gitt AK, Smolka W, Michailov G, Bernhardt A, Pittrow D, Lewalter T. Types and outcomes of cardioversion in patients admitted to hospital for atrial fibrillation: results of the German RHYTHM‐AF Study. Clin Res Cardiol. 2013; 102:713-723. [DOI] [PubMed] [Google Scholar]
- 31.Arbelo E, Brugada J, Hindricks G, Maggioni A, Tavazzi L, Vardas P, Anselme F, Inama G, Jais P, Kalarus Z, Kautzner J, Lewalter T, Mairesse G, Perez‐Villacastin J, Riahi S, Taborsky M, Theodorakis G, Trines S. ESC‐EURObservational Research Programme: the Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace. 2012; 14:1094-1103. [DOI] [PubMed] [Google Scholar]
- 32.Lip GYH, Laroche C, Dan G‐A, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. ‘Real‐world’ antithrombotic treatment in atrial fibrillation: the EURObservational Research Programme Atrial Fibrillation General Pilot survey. Am J Med. 2014; 127:e1. [DOI] [PubMed] [Google Scholar]
- 33.Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD. Patients treated with catheter ablation for atrial fibrillation have long‐term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011; 22:839-845. [DOI] [PubMed] [Google Scholar]
- 34.Bertaglia E, Stabile G, Pappone A, Themistoclakis S, Tondo C, Sanctis VD, Soldati E, Tritto M, Solimene F, Grimaldi M, Zoppo F, Pandozi C, Augello G, Calò L, Pappone C. Updated National Multicenter Registry on Procedural Safety of Catheter Ablation for Atrial Fibrillation. J Cardiovasc Electrophysiol. 2013; 24:1069-1074. [DOI] [PubMed] [Google Scholar]
- 35.Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, Pimentel R, Mansour MC, D'Avila A, Sanchez JE, Burkhardt JD, Chalhoub F, Mohanty P, Coffey J, Shaik N, Monir G, Reddy VY, Ruskin J, Natale A. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012; 59:1168-1174. [DOI] [PubMed] [Google Scholar]
- 36.Al‐Khatib SM, Calkins H, Eloff BC, Packer DL, Ellenbogen KA, Hammill SC, Natale A, Page RL, Prystowsky E, Jackman WM, Stevenson WG, Waldo AL, Wilber D, Kowey P, Yaross MS, Mark DB, Reiffel J, Finkle JK, Marinac‐Dabic D, Pinnow E, Sager P, Sedrakyan A, Canos D, Gross T, Berliner E, Krucoff MW. Planning the Safety of Atrial Fibrillation Ablation Registry Initiative (SAFARI) as a Collaborative Pan‐Stakeholder Critical Path Registry Model: a Cardiac Safety Research Consortium “Incubator” Think Tank. Am Heart J. 2010; 159:17-24. [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, Lopez‐Minquez JR, Meerkin D, Valdes M, Ormerod O, Leithauser B. Left atrial appendage closure with amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011; 77:700-706. [DOI] [PubMed] [Google Scholar]
- 38.Reddy VY, Mobius‐Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: The ASAP study (ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013; 61:2551-2556. [DOI] [PubMed] [Google Scholar]
- 39.Al‐Khatib SM, Calkins H, Eloff BC, Kowey P, Hammill SC, Ellenbogen KA, Marinac‐Dabic D, Waldo AL, Brindis RG, Wilbur DJ, Jackman WM, Yaross MS, Russo AM, Prystowsky E, Varosy PD, Gross T, Pinnow E, Turakhia MP, Krucoff MW. Developing the Safety of Atrial Fibrillation Ablation Registry Initiative (SAFARI) as a collaborative pan‐stakeholder critical path registry model: a Cardiac Safety Research Consortium “Incubator” Think Tank. Am Heart J. 2010; 160:619-626. [DOI] [PubMed] [Google Scholar]
- 40.Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GY, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007; 335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2‐VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0‐1: a nationwide cohort study. Thromb Haemost. 2012; 107:1172-1179. [DOI] [PubMed] [Google Scholar]
- 42.Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp‐Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012; 367:625-635. [DOI] [PubMed] [Google Scholar]
- 43.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009; 151:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012; 125:2298-2307. [DOI] [PubMed] [Google Scholar]
- 45.Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J. 2014 [DOI] [PubMed] [Google Scholar]
- 46.Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, Lip GY. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 2014; 145:1370-1382. [DOI] [PubMed] [Google Scholar]
- 47.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870. [DOI] [PubMed] [Google Scholar]
- 48.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart survey on atrial fibrillation. Chest. 2010; 137:263-272. [DOI] [PubMed] [Google Scholar]
- 49.Mason PK, Lake DE, DiMarco JP, Ferguson JD, Mangrum JM, Bilchick K, Moorman LP, Moorman JR. Impact of the CHA2DS2‐VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:e601-e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jover E, Roldan V, Gallego P, Hernandez‐Romero D, Valdes M, Vicente V, Lip GY, Marin F. Predictive value of the CHA2DS2‐VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl). 2012; 65:627-633. [DOI] [PubMed] [Google Scholar]
- 51.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011; 342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin‐associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011; 58:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RMInvestigators. RASCa. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once‐daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013; 127:224-232. [DOI] [PubMed] [Google Scholar]
- 54.Roldan V, Marin F, Fernandez H, Manzano‐Fernandez S, Gallego P, Valdes M, Vicente V, Lip GY. Predictive value of the HAS‐BLED and ATRIA bleeding scores for the risk of serious bleeding in a ‘real world’ anticoagulated atrial fibrillation population. Chest. 2013; 143:179-184. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee A, Fauchier L, Vourc'h P, Andres CR, Taillandier S, Halimi JM, Lip GY. Renal impairment and ischaemic stroke risk assessment in patients with atrial fibrillation: The Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol. 2013; 61:2079-2087. [DOI] [PubMed] [Google Scholar]
- 56.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE‐LY (Randomized Evaluation of Long‐term Anticoagulation Therapy) trial analysis. Circulation. 2014; 129:961-970. [DOI] [PubMed] [Google Scholar]
- 57.Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KH, Tu JV, Behlouli H, Guo H, Pilote L. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014; 129:1196-1203. [DOI] [PubMed] [Google Scholar]
- 58.Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, Krause‐Steinrauf H, Kurtzke JF, Nazarian SM, Radford MJ. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992; 327:1406-1412. [DOI] [PubMed] [Google Scholar]
- 59.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo‐controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989; 1:175-179. [DOI] [PubMed] [Google Scholar]
- 60.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999; 131:492-501. [DOI] [PubMed] [Google Scholar]
- 61.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006; 367:1903-1912. [DOI] [PubMed] [Google Scholar]
- 62.Saposnik G, Black SE, Hakim A, Fang J, Tu JV, Kapral MKInvestigators of the Registry of the Canadian Stroke N, Stroke Outcomes Research Canada Working G. Age disparities in stroke quality of care and delivery of health services. Stroke. 2009; 40:3328-3335. [DOI] [PubMed] [Google Scholar]
- 63.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006; 151:713-719. [DOI] [PubMed] [Google Scholar]
- 64.Lin LY, Lee CH, Yu CC, Tsai CT, Lai LP, Hwang JJ, Chen PC, Lin JL. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation—a nationwide database analysis. Atherosclerosis. 2011; 217:292-295. [DOI] [PubMed] [Google Scholar]
- 65.Mahmud A, Bennett K, Okechukwu I, Feely J. National underuse of anti‐thrombotic therapy in chronic atrial fibrillation identified from digoxin prescribing. Br J Clin Pharmacol. 2007; 64:706-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin LCommittee R‐LS Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-1151. [DOI] [PubMed] [Google Scholar]
- 67.Hohnloser SH, Camm AJ. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta‐analysis of the literature. Europace. 2013; 15:1407-1411. [DOI] [PubMed] [Google Scholar]
- 68.Haines DE, Mead‐Salley M, Salazar M, Marchlinski FE, Zado E, Calkins H, Yarmohammadi H, Nademanee K, Amnueypol M, Skanes AC, Saklani P. Dabigatran versus warfarin anticoagulation before and after catheter ablation for the treatment of atrial fibrillation. J Interv Card Electrophysiol. 2013; 37:233-239. [DOI] [PubMed] [Google Scholar]
- 69.De Vos CB, Breithardt G, Camm AJ, Dorian P, Kowey PR, Le Heuzey JY, Naditch‐Brûlé L, Prystowsky EN, Schwartz PJ, Torp‐Pedersen C, Weintraub WS, Crijns HJ. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm‐control therapy. Am Heart J. 2012; 163:887-893. [DOI] [PubMed] [Google Scholar]
- 70.Poulin F, Khairy P, Roy D, Levesque S, Talajic M, Guertin JR, Lelorier J. Atrial fibrillation and congestive heart failure: a cost analysis of rhythm‐control vs. rate‐control strategies. Can J Cardiol. 2013; 29:1256-1262. [DOI] [PubMed] [Google Scholar]
- 71.Andersen SS, Hansen ML, Gislason GH, Schramm TK, Folke F, Fosbol E, Abildstrom SZ, Madsen M, Kober L, Torp‐Pedersen C. Antiarrhythmic therapy and risk of death in patients with atrial fibrillation: a nationwide study. Europace. 2009; 11:886-891. [DOI] [PubMed] [Google Scholar]
- 72.Goehring E, Bohn R, Pezzullo J, Jones J. Real‐world outcomes for dronedarone initiators compared to other antiarrhythmic agents in the treatment of atrial fibrillation in a large United States population. J Am Coll Cardiol. 2013; 61:E269 [Google Scholar]
- 73.Jensen PN, Dublin S, Johnson K, Floyd J, Heckbert SR. Mini‐sentinel systematic evaluation of health outcome of interest definitions for studies using administrative data. Cerebrovascular accident/transient ischemic attack report. HOI evidence reviews. 2011. Available at: http://www.mini-sentinel.org/work_products/HealthOutcomes/Mini-Sentinel-HOI-Evidence-Review-CVA-TIA-Report.pdf. Accessed July 16, 2014.
- 74.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012; 21suppl 1:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiang CE, Naditch‐Brûlé L, Murin J, Goethals M, Inoue H, O'Neill J, Silva‐Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real‐life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012; 5:632-639. [DOI] [PubMed] [Google Scholar]
- 76.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005; 111:1100-1105. [DOI] [PubMed] [Google Scholar]
- 77.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010; 3:32-38. [DOI] [PubMed] [Google Scholar]
- 78.Khaykin Y, Morillo CA, Skanes AC, McCracken A, Humphries K, Kerr CR. Cost comparison of catheter ablation and medical therapy in atrial fibrillation. J Cardiovasc Electrophysiol. 2007; 18:907-913. [DOI] [PubMed] [Google Scholar]
- 79.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RMInvestigators RA. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883-891. [DOI] [PubMed] [Google Scholar]
- 80.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin LCommittees A Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992. [DOI] [PubMed] [Google Scholar]
- 81.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EMInvestigators EA‐T. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369:2093-2104. [DOI] [PubMed] [Google Scholar]
- 82.Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta‐analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012; 5:711-719. [DOI] [PubMed] [Google Scholar]
- 83.Mantha S, Ansell J. An indirect comparison of dabigatran, rivaroxaban and apixaban for atrial fibrillation. Thromb Haemost. 2012; 108:476-484. [DOI] [PubMed] [Google Scholar]
- 84.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014 [DOI] [PubMed] [Google Scholar]
- 85.Hammill SC, Kremers MS, Stevenson LW, Kadish AH, Heidenreich PA, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Curtis JP, Lang CM, Harder JC, Brindis RG. Review of the registry's second year, data collected, and plans to add lead and pediatric ICD procedures. Heart Rhythm. 2008; 5:1359-1363. [DOI] [PubMed] [Google Scholar]
- 86.Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas P, Wegscheider K. Improving outcomes in patients with atrial fibrillation: rationale and design of the early treatment of atrial fibrillation for stroke prevention trial. Am Heart J. 2013; 166:442-448. [DOI] [PubMed] [Google Scholar]


