Abstract
Background
Patients with acute decompensated heart failure (ADHF) and cardiorenal syndrome (CRS) 1 have poor outcomes. Ultrafiltration (UF) is used to mechanically remove salt and water in ADHF patients with diuretic resistance. However, little is known about the outcomes of ADHF patients on inotropes and/or vasopressors who require continuous renal replacement therapy (CRRT) for both UF and solute clearance in severe acute kidney injury.
Methods
We retrospectively analyzed 37 consecutive critically ill patients who were admitted for ADHF from 2005–13 and were on inotropes and/or vasopressors at the time of CRRT initiation. The primary outcome was in-hospital mortality.
Results
In-hospital mortality rate was 62%. Median survival was 15.5 days after CRRT initiation, and 10 months following hospital discharge. When comparing renal and cardiovascular variables for survivors and non-survivors at baseline, admission and CRRT initiation, survivors were less likely to need vasopressors. After controlling for multiple predictors, vasopressor use remained associated with time to death (HR 9.9; 95% CI 2.3–43.3; P = 0.002). Patients with isolated right ventricular dysfunction had an in-hospital mortality of 45% compared with 69% in those with left ventricular dysfunction (P = 0.27). Age of >70 years was associated with 100% in-hospital mortality.
Conclusions
Rescue therapy using CRRT in refractory CRS1 was associated with high in-hospital mortality, especially when vasopressors were used and when patient age exceeded 70 years. Additionally, survivors had a poor long-term prognosis.
Keywords: AKI, cardiorenal syndrome, CRRT
Introduction
Acute decompensation of chronic heart failure (HF) leads to over 1 million hospitalizations annually [1] and places a significant burden on the health system with an estimated in-hospital mortality of 3–5% [2–5]. Patients with advanced heart failure (New York Heart Association classes III and IV) admitted with acute decompensated heart failure (ADHF) often do not tolerate angiotensin-converting enzyme (ACE) inhibitors, beta-blockers or vasodilators due to hypotension and may require inotropic agents and/or vasopressors. In addition, they develop diuretic resistance and require higher diuretic doses, leading to electrolyte imbalances, further hypotension and decreased effective intravascular volume with neurohumoral activation, reduced glomerular flow and progressive acute kidney injury (AKI). This worsening of renal function in the context of worsening HF has been labeled as ‘cardiorenal syndrome’, and five subtypes have been defined [6]. Cardiorenal syndrome 1 (CRS1) is defined by acute worsening of renal function in the setting of HF exacerbation [6].
Up to 40% of patients admitted with ADHF will have AKI at admission or during hospitalization which is associated with poor short- and long-term outcomes [7]. The Acute Decompensated Heart Failure National Registry assessed the impact of renal dysfunction and hypotension and found patients with a systolic blood pressure (SBP) of <115 mmHg, creatinine of >2.75 mg/dL and blood urinary nitrogen (BUN) of >43 mg/dL at admission had an in-hospital mortality of >20% [4]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry showed a 16.3% in-hospital mortality when patients had an SBP of <100 mmHg and creatinine of >2.0 mg/dL upon admission [8]. These findings demonstrate that both renal dysfunction at admission and worsening AKI during hospitalization are independent predictors of increased length of hospitalization and increased mortality in patients with ADHF, especially in the presence of hypotension.
Since diuretics are often ineffective in reversing volume overload in cardiorenal syndrome 1, ultrafiltration (UF) with or without renal replacement therapy (RRT), depending on the severity of the AKI, has been used in ADHF to try to relieve congestion and improve patient outcomes. Two randomized clinical trials have compared the use of UF with diuretics. The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure trial (UNLOAD) showed greater weight loss and decreased readmission rates in the UF group [9], whereas the Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial (CARESS-HF) showed increased risk of AKI and no differences in weight loss or readmission rates in the UF group [10]. Results of these trials highlight the uncertainty of the use of UF in clinical practice for ADHF patients. Importantly, both trials excluded patients with severe renal dysfunction (serum creatinine of >3.0 mg/dL for UNLOAD and >3.5 mg/dL for CARESS-HF), hypotension and ongoing use of intravenous vasodilators or inotropic agents. Data on the use of UF (with or without the need for RRT) for relieving congestion in the setting of severe AKI from cardiorenal syndrome 1 in ADHF patients receiving inotropes or vasopressors are lacking.
In this study, we present the characteristics and outcomes of 37 consecutive advanced HF patients requiring inotropes and/or vasopressors for acute decompensation of chronic HF who were treated with continuous renal replacement therapy (CRRT) for diuretic resistance and progressive AKI. We hypothesized that CRRT has limited clinical utility in hemodynamically unstable ADHF patients and would not lead to improved survival or AKI resolution.
Methods
Study population
We analyzed 37 consecutive ADHF patients who were admitted to the cardiac intensive care unit from 2005 to 2013 on inotropes and/or vasopressors and required CRRT for worsening renal failure. Only patients with acute decompensation of chronic HF were included. We excluded patients with dialysis-dependent end-stage kidney disease, chronic kidney disease (CKD) stage 5, AKI from sepsis or AKI from intravenous contrasted studies. Patients who had cardiogenic shock from acute myocardial infarction were also excluded, as well as patients with ventricular assist devices. The University of Alabama-Birmingham Institutional Review Board approved the study.
CRRT protocol
Continuous venovenous hemodiafiltration (CVVHDF) was performed using the Prismaflex device with an HF1000 hemofilter through a double-lumen 12-French catheter inserted into the internal jugular or femoral vein. The blood flow rate was initiated at 200 mL/min with an effluent rate of 25–30 mL/kg/h. Regional citrate anticoagulation was used to maintain circuit patency unless contraindicated.
Data collection
Information regarding demographic and clinical characteristics, medical history, management strategies, therapeutic effects and clinical outcomes was collected from medical records. Data collection included medications, CRRT parameters, laboratory studies, vitals, hemodynamic data and echocardiographic studies when available. AKI was defined as an increase in serum creatinine by 0.3 mg/dL or ≥1.5 times baseline within 48 [11]. Baseline creatinine was determined as the lowest documented creatinine within the 3 months prior to admission. The Modification of Diet in Renal Disease (MDRD) equation was used for estimating glomerular filtration rate (GFR). CKD staging was based on the MDRD equation or documentation of CKD staging by medical records. Hemodynamic measurements were collected from right heart catheterization or from pulmonary artery catheter measurements as the day closest to CRRT initiation.
Study outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included 12-month mortality and dialysis dependency at hospital discharge.
Statistical analysis
Differences in continuous variables were tested using two-group t-tests or Wilcoxon rank-sum tests, depending on their distribution. Pearson chi-square tests or Fisher exact tests were used to test for associations between categorical variables. Multiple-variable logistic regression was performed to determine which predictor variables may be associated with the likelihood of death during hospitalization. To determine which variables to include in the multivariable models, univariate comparisons by the outcome of interest (death during hospitalization) were performed for each potential covariate. Variables with a P-value of <0.05 were included in the multivariable model. Race, gender and age were retained in all final models. Survival was assessed using the Kaplan–Meier method. Patients who did not die were censored. Cox proportional hazards regression modeling was used to study the association between potential predictor variables and time to death during hospitalization, while controlling for confounders. The proportional hazards assumption was assessed using the log–log survival function.
Statistical tests were two-sided. Results with P ≤ 0.05 were considered significant. Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC, USA) and JMP, version 10.
Results
Cohort description
A total of 37 patients were included in the study. Table 1 summarizes the outpatient baseline characteristics of the patient cohort prior to admission. All patients had been admitted at least twice for ADHF in the 12 months prior to the observed admission. However, none of them previously required RRT. Twenty-six patients (70%) had left ventricular systolic dysfunction whereas 11 (30%) had isolated right ventricular systolic dysfunction. Of the 37 patients, 4 (11%) had CKD stage 4, 27 (73%) had CKD stage 3 and 6 (16%) had CKD stage 2. Mean baseline estimated GFR was 42.8 ± 14.7 mL/min/1.73 m2 and mean baseline creatinine was 1.8 ± 0.4 mg/dL.
Table 1.
Baseline outpatient characteristics
| Age in years | Mean: 58.8 (range 33–87) |
| Gender | M = 24, F = 13 |
| Race | White 18, African American 19 |
| DM | 51% (n = 19) |
| HTN | 54% (n = 20) |
| Cause of cardiac dysfunction | |
| Ischemic | 24% (n = 9) |
| Non-ischemic | 41% (n = 15) |
| Valvular | 5% (n = 2) |
| Right-sided | 30% (n = 11) |
| LVEF | Mean: 30% SD 16.5 |
| RVEF | Mean: 32% SD 12.2 |
| Medication prior to admission | |
| ASA | 59% (n = 22) |
| Beta-blocker | 70% (n = 26) |
| ACE/ARB | 43% (n = 16) |
| Aldosterone antagonist | 32% (n = 12) |
| Digoxin | 32% (n = 12) |
| Hydralazine/ISDN | 32% (n = 12) |
| Loop diuretic | 95% (n = 35) |
| Loop diuretic dose (mg of furosemide equivalent) | Mean: 174 (range 0–400) |
| Second-site diuretic | 24% (n = 9) |
| Home inotropes | 24% (n = 9) |
| Baseline creatinine in mg/dL | 1.75 ± 0.43 |
DM, diabetes mellitus; HTN, hypertension; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction; ASA, aspirin; ACE/ARB, angiotensin-converting enzyme/ angiotensin receptor blocker; ISDN, isosorbide dinitrate; SD, standard deviation.
CRRT was initiated on average 6.1 ± 5.7 days (median 4.0 days) after patient admission. At CRRT initiation, no patients were on ACE inhibitors, beta-blockers or vasodilators due to hypotension; 23 patients (65%) were on inotropes, 2 (5%) on vasopressors and 12 (32%) on both inotropes and vasopressors. The main indication for CRRT was refractory volume overload with worsening azotemia and symptomatic uremia. Mean patient weight at CRRT initiation was 106 ± 42 kg. Mean BUN and creatinine at CRRT initiation were 90 ± 41 and 4.0 ± 1.5 mg/dL, respectively. Mean 24-h urine output prior to CRRT was 760 ± 696 mL (median 588 mL).
Clinical outcomes
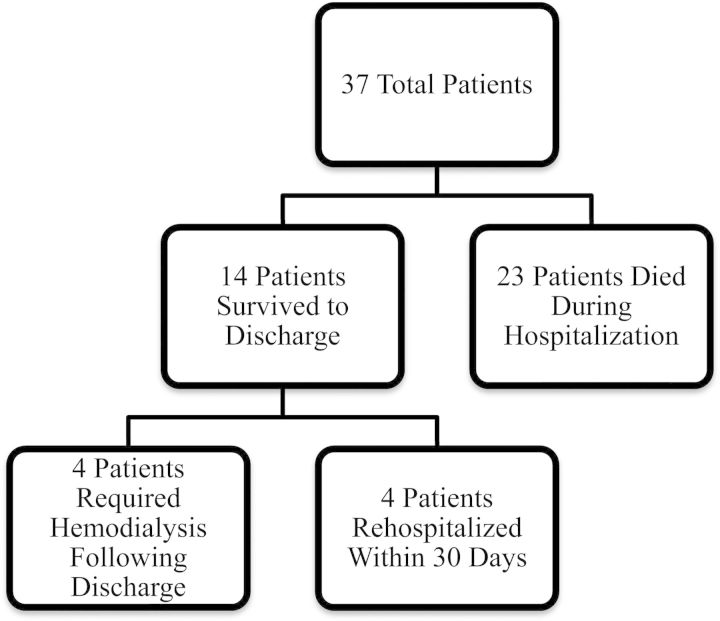
Although all patients achieved negative volume status with CRRT, 25 of the 37 patients (68%) required increasing vasopressor support after CRRT initiation. Patients were on CRRT for a median of 6 days (range 1–24 days). None of the patients experienced complications from CRRT or citrate anticoagulation. Twenty-three patients died in the hospital with an in-hospital mortality of 62%. Of the 14 patients who survived hospitalization, 2 (14%) were discharged to home hospice and 4 (29%) were re-hospitalized within 30 days (Figure 1). Two of the four patients re-hospitalized were receiving outpatient intermittent hemodialysis. Median survival was 15.5 days from CRRT initiation; for those who survived to hospital discharge, median survival was 10 months (Figure 2). Five patients (14%) were alive at 1 year after discharge; of those, one died within 2 weeks after reaching the 1-year survival date.
Fig. 1.
Breakdown of clinical outcomes.
Fig. 2.
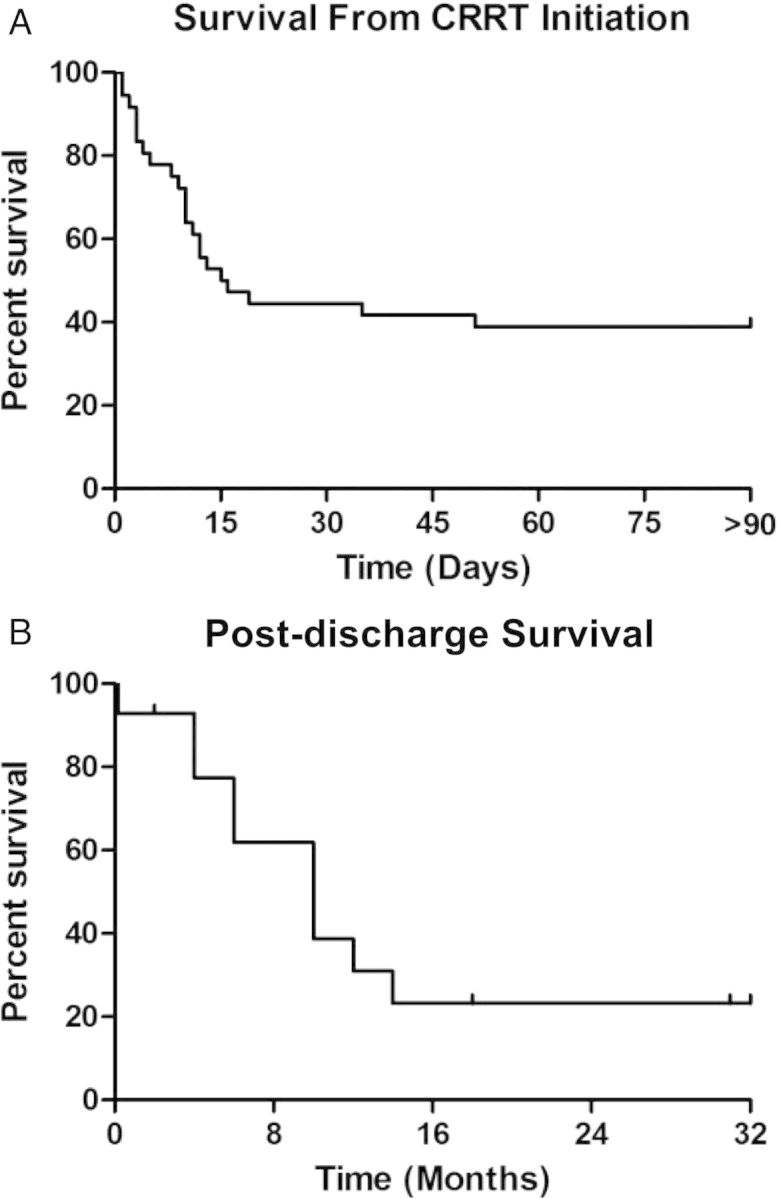
Kaplan–Meier survival curves from date of CRRT initiation (n = 37) (A) and hospital discharge (n = 14) (B). Median survival was 15.5 days from CRRT initiation and 10 months from hospital discharge.
Comparing survivors with non-survivors
We compared renal and cardiovascular clinical variables at baseline, admission and CRRT initiation and found no significant differences between in-hospitalization survivors and non-survivors (Tables 2 and 3). There was no difference in mortality based on anticoagulation for CRRT; all five patients (100%) on CRRT without anticoagulation died compared with 18 patients (56%) on citrate anticoagulation (P = 0.13). Survivors tended to have better left ventricular cardiac function as compared with non-survivors (LVEF based on echocardiograph 36.6 ± 15.8% versus 25.9 ± 15.9%, P = 0.06). Survivors required significantly less vasopressors after CRRT initiation as compared with those who died: 29% (4/14) versus 91% (21/23), respectively (P < 0.0001) (Table 4). There was no difference between groups in terms of inotrope use. All seven patients over 70 years of age died during hospitalization; this was significantly different (P = 0.03) when compared with patients of ≤70 years of age.
Table 2.
Comparison of renal variables between survivors and non-survivors
| Variable | Survivors (mean ± SD) | Non-survivors (mean ± SD) | P-value |
|---|---|---|---|
| Baseline creatinine (mg/dL) | 1.8 ± 0.5 | 1.7 ± 0.4 | 0.85 |
| Baseline estimated GFR (mL/min/1.73 m2) | 44.2 ± 14.6 | 41.7 ± 15.0 | 0.64 |
| CKD stage | 2.9 ± 0.6 | 3.0 ± 0.5 | 0.88 |
| Home loop dose (mg of oral furosemide equivalent) | 221 ± 138 | 146 ± 122 | 0.09 |
| Second-site diuretic | 21%± (n = 3) | 13%± (n = 3) | 0.13 |
| Admission BUN (mg/dL) | 81.8 ± 51.3 | 73.3 ± 30.9 | 0.59 |
| Admission creatinine (mg/dL) | 3.1 ± 0.8 | 3.1 ± 1.3 | 0.92 |
| Urine output prior CRRT (mL/kg/24 h) | 10.8 ± 10.2 | 7.3 ± 6.5 | 0.26 |
| Loop diuretic dose prior to CRRT (mg IV furosemide equivalent) | 141.7 ± 156.2 | 195.3 ± 165.7 | 0.15 |
| Urine/loop diuretic dose prior to CRRT (mL/kg/24 h/mg furosemide equivalent) | 0.06 ± 0.06 | 0.03 ± 0.02 | 0.15 |
| BUN prior to CRRT (mg/dL) | 98.7 ± 55.5 | 83.8 ± 26.9 | 0.36 |
| Creatinine prior to CRRT (mg/dL) | 3.8 ± 1.7 | 4.2 ± 1.3 | 0.42 |
| CRRT dose (mL/kg/h) | 33.3 ± 7.4 | 35.1 ± 10.6 | 0.65 |
CKD, chronic kidney disease; BUN, blood urea nitrogen; CRRT, continuous renal replacement therapy; IV, intravenous.
Table 3.
Comparison of cardiovascular variables between survivors and non-survivors
| Variable | Survivors (mean ± SD) | Non-survivors (mean ± SD) | P-value |
|---|---|---|---|
| SBP admission (mmHg) | 104.7 ± 20.4 | 110.4 ± 19.8 | 0.49 |
| MAP admission (mmHg) | 74.6 ± 13.3 | 78.2 ± 12.9 | 0.50 |
| LVEF (%) | 36.6 ± 15.8 | 25.9 ± 15.9 | 0.06 |
| Right ventricular ejection fraction (%) | 35.4 ± 9.4 | 28.7 ± 13.8 | 0.14 |
| Left ventricular in diastole diameter (cm) | 6.1 ± 1.4 | 6.2 ± 1.5 | 0.82 |
| Right atrial pressure (mmHg) | 19.8 ± 12.4 (n = 4) | 24.1 ± 6.4 (n = 10) | 0.40 |
| Mean pulmonary arterial pressure (mmHg) | 41.5 ± 15.7 (n = 4) | 49.4 ± 11.9 (n = 10) | 0.33 |
| Pulmonary capillary wedge pressure (mmHg) | 25.3 ± 13.4 (n = 4) | 29.8 ± 7.4 (n = 10) | 0.42 |
| Cardiac Index (L/min/m2) | 2.7 ± 0.8 (n = 4) | 2.5 ± 0.9 (n = 10) | 0.73 |
| SBP at CRRT Initiation (mmHg) | 93.7 ± 18.2 | 88.4 ± 12.9 | 0.32 |
| MAP at CRRT Initiation (mmHg) | 59.9 ± 11.4 | 57.3 ± 7.9 | 0.44 |
CRRT, continuous renal replacement therapy.
Table 4.
Use of vasoactive medications
| Medication | Survivors, n = 14 | Non-survivors, n = 23 | P-value |
|---|---|---|---|
| Dobutamine | 64% (9) | 57% (13) | 0.96 |
| Milrinone | 50% (7) | 70% (16) | 0.20 |
| Vasopressors | 29% (4) | 91% (21) | <0.0001 |
Univariate logistic analyses demonstrated that vasopressor use was associated with higher odds of death during hospitalization. Age, race, gender, weight, diabetes, MAP, LVEF, RVEF, inotrope use, CKD stage and CRRT dose were not significantly associated with death during hospitalization. In a multivariable analysis that controlled for age, race and gender, vasopressor use remained significantly associated with death during hospitalization (OR 22.7; 95% CI 3.5–142.9; P < 0.001). Time to death was assessed by proportional hazards modeling, with adjustment for other variables. After controlling for age, race and gender, vasopressor use remained associated with time to death (HR 9.9; 95% CI 2.3–43.3; P = 0.002). Proportional hazards assumptions were satisfied for time to death models.
RV dysfunction versus LV dysfunction
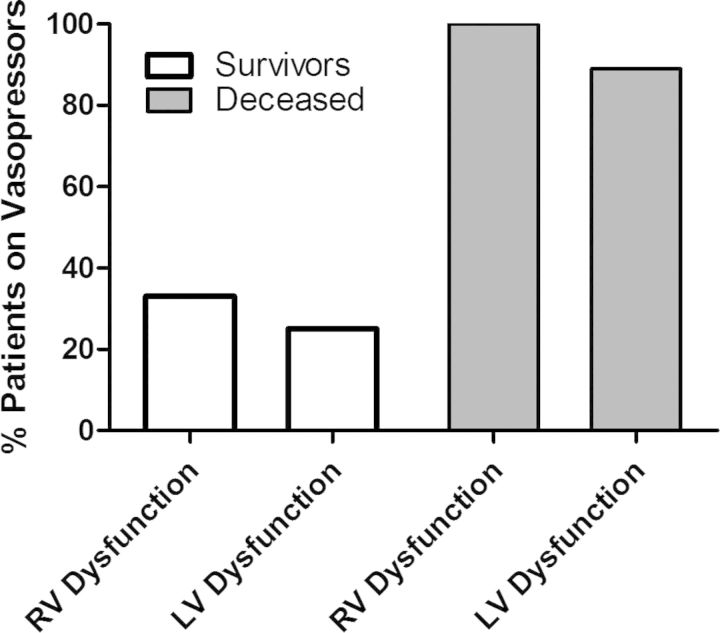
We further analyzed patients by comparing those with RV dysfunction with those with LV dysfunction. Overall hospital mortality was 45% (5/11) in the RV dysfunction group and 69% (18/26) in the LV dysfunction group (P = 0.27). Patients with RV dysfunction who survived hospitalization tended to receive less vasopressors than those who died (33% versus 100%, P = 0.06) (Figure 3). Two of the eight patients (25%) with LV dysfunction who survived required vasopressors, as compared with 16 of the 18 (89%) who died (P = 0.003).
Fig. 3.
Association of vasopressors with in-hospital mortality based on ventricular dysfunction.
Renal outcomes
None of the 23 patients who died during hospitalization recovered renal function; they remained on CRRT until death or until CRRT was withdrawn for palliative care. Six of the 14 survivors (43%) did not recover renal function; 4 (29%) were discharged on chronic intermittent hemodialysis and 2 were discharged to home hospice without renal recovery. Of the eight survivors (57%) who recovered renal function, one was readmitted 5 weeks after discharge and required re-initiation of CRRT.
Discussion
Our study demonstrated that patients with refractory ADHF on inotropes and/or vasopressors who require CRRT for severe AKI from cardiorenal syndrome 1 have an overall poor prognosis and do not recover renal function despite volume removal. A single center study by the Cleveland Clinic evaluated ADHF patients who required nephrology consultation for slow continuous ultrafiltration (SCUF) due to worsening renal function despite standard medical therapy [12]. Of the 63 patients analyzed in the study, 38% were on inotropes at initiation of SCUF and none were reported to be on vasopressors. In the Cleveland study, 59% required transition from SCUF to CRRT for solute clearance due to progressive azotemia; 14% were dialysis dependent at hospital discharge and 32% of all patients either died prior to discharge or were discharged to hospice care. In contrast to the Cleveland Clinic study and other studies that used UF and/or CRRT for treatment of ADHF, our patient cohort was more critically ill, with 95% of patients on inotropes and 38% on vasopressors at initiation of CRRT. In addition, the average creatinine at initiation of CRRT was 4.0 mg/dL. This creatinine level was nearly twice the creatinine of patients initiated on SCUF in the Cleveland Clinic study (mean creatinine 2.2 mg/dL) and would have been an exclusion criteria for both the UNLOAD and CARESS-HF trials [9, 10].
Although we did not see significant differences, there was a trend toward better cardiac function in the surviving group with left ventricular ejection fraction (LVEF) being closest to statistical significance. It is important to consider that our patient population was a mix of patients with left ventricular dysfunction and right ventricular dysfunction, and the patients with right ventricular dysfunction had lower in-hospital death rates (45% versus 69%). Thus, the increase in LVEF in the surviving group may have been skewed because of the surviving patients with RV dysfunction. However, we cannot rule out slightly improved cardiac function played a role in survival to hospital discharge.
Not surprisingly, our results and those from the Cleveland Clinic study demonstrate that more severe renal dysfunction is associated with higher mortality in cardiorenal syndrome 1. When UF was used for volume control in the Cleveland Clinic study, 12% of patients died in the hospital, but when renal dysfunction worsened with UF, and CRRT was implemented for volume and solute control, in-hospital mortality rose to 43%. Furthermore, as seen with other studies, SBP at admission was found to be an important predictor of long-term survival. In our study, in-hospital mortality was 62% for all patients requiring CRRT and 88% for patients requiring both inotropes and vasopressors on CRRT. These results raise the question of whether inotropic and vasopressor-dependent ADHF patients with severe AKI should be offered CRRT when there is no plan for destination therapy (i.e. ventricular assist devices or listing for cardiac transplant) in the setting of progressive pump failure, especially in patients of >70 years of age. If such salvage therapy is offered, it seems imperative to address goals of care and realistic expectations with the patient and family members, and to consider early involvement of palliative medicine, given the likely poor outcome. Additionally, CRRT adds substantial cost to the care of these patients without a proven benefit.
Even though CRRT was able to remove fluid and maintain negative volume status, the patients who died became progressively hypotensive with fluid removal and required increasing amount of vasopressors. The high rate of non-renal recovery in our cohort is likely related to continuous hypotension and hypoperfusion, propagating continued acute tubular necrosis or worsening intrinsic renal disease. It is unclear whether earlier implementation of CRRT in the course of ADHF would be beneficial. Only one randomized trial has studied the efficacy of CRRT in patients with ADHF and less severe AKI [13]. In the trial by Badawy et al., 40 ADHF patients were randomized to treatment for 72 h with CVVHDF or intravenous furosemide. Mean serum creatinine at enrollment for both groups was 1.4 mg/dL. Although weight loss and total fluid removal were significantly greater in the CRRT group, there was no difference in 30-day mortality (25% in the furosemide group versus 15% in the CRRT group, P = NS) or dialysis dependency (6.7% in the furosemide group versus 5.9% in the CRRT group, P = NS). Furthermore, in this small study, patients were excluded with SBP of <85 mmHg, and no patients were on vasopressors.
Finally, our data add to the growing body of literature that show progression of cardiorenal syndrome 1 is not entirely explained by hemodynamics and suggests other yet-undefined variables play a role. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial [14], there was no correlation between central hemodynamics and worsening renal function. In the Cleveland Clinic study, patients developed worsening renal function despite improvement in hemodynamic parameters such as pulmonary artery pressure, central venous pressure and pulmonary wedge pressure [12]. This strongly argues that other variables play a role in cardiorenal syndrome 1. One such possibility is that inflammation from heart failure exacerbation subsequently worsens renal dysfunction. Studies have shown that TNF-α and IL-6 are elevated in ADHF [15–18] and may play a role in AKI [19, 20]. Perhaps, differences in generation rates of inflammatory molecules may explain why some patients develop worsening AKI despite similar hemodynamics. Further investigation into the role of inflammation in cardiorenal syndrome 1 may help answer this question.
Our study is limited by the single center, retrospective design, small sample and observational nature. However, little data exist on outcomes in critically ill ADHF patients who require CRRT for AKI from cardiorenal syndrome 1, and our study is the largest series to date utilizing CVVHDF in the setting of vasopressors and inotropes. In summary, we found that rescue therapy using CRRT for refractory cardiorenal syndrome 1 was associated with a high in-hospital mortality rate, especially when vasopressors were added and when patient age exceeded 70 years. Moreover, the majority of patients did not recover renal function, and survivors had a poor long-term prognosis. Additional studies are needed to uncover the mechanisms leading to irreversible AKI in ADHF and better determine whether UF and CRRT have any beneficial effect on the long-term outcomes of patients with advanced ADHF. The sobering results of this study along with others, such as CARRESS-HF and the Cleveland Clinic study, suggest that UF with or without RRT is not an effective form of salvage therapy in advanced ADHF patients with diuretic resistance and does not lead to renal recovery or improved survival, despite volume removal. Early implementation of palliative care interventions may be warranted in this patient population.
Funding
None
Conflict of interest statement
None declared.
Acknowledgements
The authors thank Dr Amit Gaggar for helpful discussions.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 3.Cuffe MS, Califf RM, Adams KF, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Adams KF, Abraham WT, et al. ADHERE Scientific Advisory Committee SuG, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Heywood JT, Heidenreich PA, et al. Investigators ASACa. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 2013;3:26–37. doi: 10.1159/000347037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 10.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol. 2012;60:1906–1912. doi: 10.1016/j.jacc.2012.08.957. [DOI] [PubMed] [Google Scholar]
- 13.Badawy SS, Fahmy A. Efficacy and cardiovascular tolerability of continuous veno-venous hemodiafiltration in acute decompensated heart failure: a randomized comparative study. J Crit Care. 2012;27:106. doi: 10.1016/j.jcrc.2011.05.013. e7–13. [DOI] [PubMed] [Google Scholar]
- 14.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen KH, Lassus J, Harjola VP, et al. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396–403. doi: 10.1016/j.ejheart.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Milani RV, Mehra MR, Endres S, et al. The clinical relevance of circulating tumor necrosis factor-alpha in acute decompensated chronic heart failure without cachexia. Chest. 1996;110:992–995. doi: 10.1378/chest.110.4.992. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Sato R, Sato T, et al. Time-course of changes in the levels of interleukin 6 in acutely decompensated heart failure. Int J Cardiol. 2005;100:415–420. doi: 10.1016/j.ijcard.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Tziakas D, Chalikias G, Parissis JT, et al. Prolonged activation of tumor necrosis factor (TNF)-alpha and its soluble receptors in chronic heart failure patients both in the compensated and decompensated state. Interplay between their levels and metalloproteinase-3. Eur Cytokine Netw. 2004;15:231–239. [PubMed] [Google Scholar]
- 19.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 20.Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–1115. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]