Abstract
Low-protein diets (LPDs) have encountered various fortunes, and several questions remain open. No single study, including the famous Modification of Diet in Renal Disease, was conclusive and even if systematic reviews are in favour of protein restriction, at least in non-diabetic adults, implementation is lagging. LPDs are considered difficult, malnutrition is a threat and compliance is poor. LPDs have been reappraised in this era of reconsideration of dialysis indications and timing. The definition of a normal-adequate protein diet has shifted in the overall population from 1 to 1.2 to 0.8 g/kg/day. Vegan–vegetarian diets are increasingly widespread, thus setting the groundwork for easier integration of moderate protein restriction in Chronic Kidney Disease. There are four main moderately restricted LPDs (0.6 g/kg/day). Two of them require careful planning of quantity and quality of food: a ‘traditional’ one, with mixed proteins that works on the quantity and quality of food and a vegan one, which integrates grains and legumes. Two further options may be seen as a way to simplify LPDs while being on the safe side for malnutrition: adding supplements of essential amino and keto acids (various doses) allows an easier shift from omnivorous to vegan diets, while protein-free food intake allows for an increase in calories. Very-low-protein diets (vLPDs: 0.3 g/kg/day) combine both approaches and usually require higher doses of supplements. Moderately restricted LPDs may be adapted to virtually any cuisine and should be tailored to the patients' preferences, while vLPDs usually require trained, compliant patients; a broader offer of diet options may lead to more widespread use of LPDs, without competition among the various schemas.
Keywords: CKD, compliance, keto acids and amino acids, low-protein diets, very-low-protein diets
Low protein diets: a new garden?
In a wonderful book, ‘Cradle to Cradle, Remaking the Way We Make Things’, a manifesto of the ‘new industrial revolution’, a chemist and an architect search for a new production strategy combining abundance, safety and efficacy. One of the many anecdotes concerns the mother of one of the authors, who used to cultivate a wild garden full of diverse flowers and unusual trees. In 1982, the woman was fined by the local administration because her garden was ‘too messy’. The lady continued paying a yearly fine for about a decade, until she won an award for creating a habitat for songbirds. The authors concluded that it was not a change in the garden; it was a change in the prevailing aesthetic. It was a change in the society [1].
A similar story may be told for low-protein diets (LPDs). Since the pioneering studies by Thomas Addis, who foresaw Brenner's theory of ‘workload’ on the remnant nephrons and systematically used diets in clinical practice, LPDs have encountered alternate fortunes [2–9]. Strong supporters and determined adversaries contributed to the controversy, reflecting changes in the near-world of renal replacement therapies, and the wider-world of nutrition [10–12].
The conflicting interpretation of the largest randomized controlled trial on diet in kidney disease, the Modification of Diet in Renal Disease (MDRD) study, which failed to demonstrate an advantage in the primary intention-to-treat analysis, but supported a positive effect in the secondary per-protocol analyses, further radicalized the positions in favour of or against LPDs [9, 13–17].
The MDRD study highlighted the crucial role of compliance, not yet fully appreciated in chronic diseases, and added two important tools: the MDRD equation for the assessment of GFR and the only dietary satisfaction questionnaire validated in kidney patients [18, 19].
The issue of the diet was not solved by a Cochrane review in favour of protein restriction in non-diabetic adults, leaving the definition of the optimal protein intake in LPDs up for debate [20, 21].
The present cultural drive favours a lower protein intake in the overall population: several studies have tried to define a healthy diet, i.e. one that is rich in ‘healthy foods’, such as vegetables, fruits, nuts, olive oil and grains, and poor in red or processed meat [22–24]. Globalization confronts the current European and North American diets with different ones, which are often lower in animal proteins, and this has also led to an in-depth review of recommended daily allowances [25–27]. Rediscovery of the Mediterranean diets, rich in vegetables, legumes and cereals, and demonstration of their association with longer survival, call for attention to traditional diets which are more easily ‘transformed’ into LPDs [28–30]. Meanwhile, evidence of the increase in all-cause mortality, cardiovascular diseases and cancer related to diets that are rich in red and processed meat calls for attention to the ‘too much’ [31, 32].
In counter-tendency to the classical food marketing strategy that highlighted the presence of ‘valuable’ ingredients, a world-wide campaign is being carried out to regulate additives in food [33–35]. Growing attention to the quality rather than the quantity of what we eat activates virtuous cycles of ‘zero km’ production, with renewed interest in non-preserved, additive-free food.
In this context, vegetarian and vegan diets are becoming increasingly popular, setting the groundwork for easier integration of vegetarian protein-restricted diets in our patients who are no longer seen as ‘outliers’ in a carnivorous world [36–38].
Vegan–vegetarian diets usually contain between 0.6 and 0.8 g/kg/day of vegetable proteins and may be less effective in enhancing renal hyper-filtration, also since vegetable proteins are less bioavailable than animal proteins [39–42]. Provided that deficits are controlled and corrected (in particular, iron, vitamin B12 and vitamin D), these diets are considered safe by the most eminent associations, including the American Dietary Association, in all phases of life, including growth, pregnancy and lactation [36–38].
This paradigm shift allows ‘our’ LPDs to be more easily integrated in a society in which ‘eating together’ is a pivotal moment, with easier access to vegan menus, available on intercontinental flights as well as in most international restaurants.
No alibi: it is possible to reduce protein content and to follow at least a moderately protein-restricted diet almost everywhere. As for the wild garden, our society is moving towards a reappraisal of traditional diets, characterized by lower animal protein content, better quality of food and fewer additives [29–31, 43–47].
Are we answering the unanswered questions?
Almost 30 years after the provocative paper ‘Dietary treatment for chronic renal failure, ten unanswered questions’ and its tentative answers, many questions are still unanswered (Does chronic renal failure always progress? How should we assess progression, nutritional status or compliance? Is there a placebo effect in diet trials? When should a low-protein diet start? What are the risks of a low-protein diet? What is the cost of a low-protein diet?) [10, 11]. In this narrative review, we will focus on the most practical one: ‘Which low-protein diet?’ as a key for implementing the ‘right diet’ with the ‘right patient’.
What is a diet?
On Wikipedia, ‘diet is the sum of food consumed by a person or other organism’. We will employ the word ‘diet’ in this broad meaning, preferring it for the sake of simplicity to the more elegant, but less practical ‘nutritional approach’ ‘nutritional therapy’, however without the restrictive meaning ‘diet as reduction of … ’.
Diet is all that we eat. Implicit in this is the opinion that for Chronic Kidney Disease (CKD) patients, being on a diet should consist of changing their habits and not (simply) in restricting the use of some (many-most) foods.
What is a normal protein diet and what is an LPD?
From attention to under-nutrition to attention to over-nutrition
If the definition of diet is simple, the definition of normal protein intake is not. Following the increased availability of food, in the Western world, a ‘healthy’ diet progressively switched from a diet containing a ‘minimum requirement to avoid deficits’ to one not surpassing an ‘ideal intake’ to avoid over-nutrition [48].
The FDA Reference Daily Intake or Recommended Daily Intake (that substitutes the older ‘Recommended Daily Allowance’) is the daily intake that suffices to meet the requirements of 97–98% of healthy individuals in every demographic in the USA: this is presently identified as 0.8 g/kg/day of proteins, lower than the previous 1.0 g/kg/day, considered the hallmark of ‘normal’ protein intake [25–27].
As a consequence, what we used to call moderately restricted ‘LPDs’ (0.8 g/kg/day) now have ‘normal protein’ content, while diets at 1.0–1.2 g/kg/day should be considered ‘high protein’. This is not only semantic: it casts a different light on the classical 0.6 g/kg/day diets, no longer radically different from ‘normal’ diets (Table 1).
Table 1.
Characteristics of the main LPDs, defined according to protein content
| Diet definition | Type of proteins | Scheme | Advantages—problems | Supplements protein-free food |
|---|---|---|---|---|
| Moderate protein restriction: 0.6 g/kg/day | ||||
| ‘Traditional’ LPD | 0.6 g/kg/day, mixed, with at least 50% of animal origin (or ‘high biological value’) | The diet requires adaptation to the local habits; 30–35 kcal/kg are reached by implementing protein-poor calorie-rich natural food (for example, rice, potatoes, tapioca, fruit, oil and butter) | Advantage: no supplement or special food needed; limits: skilled dietician is needed. The caloric intake may be difficult to reach in particular in ‘small’ patients | None |
| Vegan LPD | 0.6 g/kg/day, vegetable origin | This diet is strictly vegan; it is based upon a combination of different proteins of vegetable origin, mainly in grains, legumes and soy protein | Advantage: no supplement or special food are needed; limits: the combination of legumes and cereals at each meal is demanding. May not be suited to patients with diverticulosis or intestinal problems | None |
| Vegan supplemented with keto and amino acids | 0.6 g/kg/day, vegetable origin | This diet is strictly vegan; it may be based upon forbidden (all food of animal origin) and allowed food (all food of vegetable origin). Supplements allow integration without need for combining grains and legumes at each meal | Advantage: simplified scheme based upon allowed and forbidden food; may be used in patients who do not like or tolerate legumes. Limits: need for supplementation of many pills (free of charge only in a few countries). | Alpha-keto acids and amino acids, min: 1:10, max 1:5 kg BW |
| LPD with protein-free food | 0.6 g/kg/day, mixed origin | Cereals are all or in part replaced by protein-free food (mainly pasta and bread). This allows moderate quantities of proteins of animal origin | Advantage: no need to drastically change food habits. Problems: easily integrated in Mediterranean diets, less in Northern diets. Limited availability (high cost) of protein-free food in several countries. | Protein-free food |
| vLPD: 0.3 g/kg/day | ||||
| vLPD-vegan supplemented | 0.3 g/kg/day, vegetable origin | This diet is strictly vegan; it may be based upon forbidden (all food of animal origin) and allowed food (all food of vegetable origin). More easily carried out if bread and pasta are substituted with protein-free food. | Advantage: probably more effective in postponing dialysis. Problems: requires separate cooking, is monotonous and requires a high number of pills. Limited availability (high cost) of protein-free food in several countries. | Alpha-keto acids and amino acids, 1:5 kg BW; optional: protein-free food. |
In pregnancy: 0.6–0.8 LPD–vegan vegetarian—supplemented: mainly vegetable proteins; small doses of milk and yogurt allowed. Alpha-keto acids and amino acids 1:8–10 kg BW in the first trimester, increased to 1:5–8 kg BW in the last.
A practical nomenclature: vegan–vegetarian diets
The systematic distinction between vegan and vegetarian diets is relatively new: the term vegan was coined by Donald Watson in 1944: ‘vegan’ from the beginning and end of vegetarian ‘because veganism starts with vegetarianism and carries it through to its logical conclusion’ [49]. The two terms were often used interchangeably in the past, or, most often, vegetarian diets were subdivided into ‘strict’, corresponding to the current vegan diets, and ‘ovo-lacto’ vegetarian diets [36–38, 49].
Vegetarian diets are free from fish, meat and poultry, i.e. from living sources of food; there is however, a long list of variants, including ovo-vegetarian (eggs only), lacto-vegetarian (milk and dairy products) and pescetarian (fish is allowed). Honey is excluded by some diets, while others take care in avoiding all animal-derived components which are common in food processing, for example, sugars whitened with bone char, cheeses that use animal rennet or gelatin from animal collagen. Watson, who was registered as ‘a conscientious objector’ in World War II, expanded his philosophy to object to any harm to living creatures. This latter attitude is extended in the Fruitarianism, based upon fruit, nuts, seeds and food gathered without ‘harming the plant’ [50].
The cultural roots of vegetarianism are deep: the list of eminent vegetarians includes Buddha, Leonardo Da Vinci, Gandhi, Tolstoy, George Bernard Shaw (who foresaw the participation at his funeral of all the animals he had not devoured), Isaac Bashevic Singer (I did not become a vegetarian for my health, I did it for the health of the chickens), but also music stars, such as Paul McCartney (If slaughterhouses had glass walls, everyone would be a vegetarian).
Even if the demonstration of the possibility to combine public success and vegetarian diet is reassuring, the motivations of a ‘vegetarian by choice’, be it for the sake of the chickens or for the sake of the soul, may be different from those of a ‘vegetarian for health’, in whom the motivation is sustaining health, or avoiding dialysis. We need different strategies, while learning from the ‘vegans–vegetarians’ by choice.
LPDs in CKD: learning from the beginning
Tracking the development of LPDs is fascinating: the first attempts to counterbalance the hyper-azotaemia of advanced kidney disease date to the first half of the Twentieth Century [3, 51–53]. However, most of the approaches that proved to be effective in clinical practice were developed in the 50–60s; our current diets are the result of the strategies to overcome the difficulties identified in these early studies.
The first problem was combining LPDs with a sufficient caloric intake, because a hyper-catabolic state leads to an increase in blood urea [54–57]. Different sources of calories were tested, and the cultural differences were highlighted: surrogates of pasta and bread were compatible only with Italian diets, while in Anglo-Saxon countries mixtures of lipids and carbohydrates were tested, overall with poor success [54–58].
These first diets were very hard: the effort of having to keep all foods under control made them exceedingly complex: this is, for example, a typical dinner published in the late ‘60s with a low-protein mixed diet: 120 g carrots (cooked weight); 100 g runner beans; 100 g potatoes (raw weight); 15 g salt-free butter; 135 g tinned peaches (100 g peach, 35 g juice); 15 g single cream and 10 g white bread. The modified Giovannetti diet was no easier: this is a ‘supper’ from a 1970 paper: ‘50 g egg; 15 g lettuce; 55 g tomato; 30 g beetroot (cooked weight); 50 g low-protein bread; 15 g unsalted butter; 100 g mandarins (tinned) (80 g fruit + 20 g juice); 15 g single cream’ [57].
The definition of the protein target was a crucial issue. The underlying idea was simple: protein intake should be ‘the lowest the best’, provided that hyper-catabolism was avoided [3, 52–62].
Different levels of protein restriction were tested, initially defined according to total protein content (the 40 g versus the 20–19–18 g diets [54–57]) and later according to body weight, finally leading to a pragmatic subdivision into LPDs at 0.8–0.6–0.3 g of protein per kg/body weight (Tables 1 and 2, Figure 1).
Table 2.
Energy, minerals and vitamins in the different LPDs
| ‘Traditional’ LPD | LPD with protein-free food | Vegan LPD |
Vegan supplemented with keto and amino acids | VLPD-vegan supplemented | ||
|---|---|---|---|---|---|---|
| Calories | 30–35 kg body weight: this is the most crucial point to avoid protein malnutrition, in particular in elderly patients with cardiovascular disease (MIA syndrome); this is a basic prescription for all LPD; the risk of hyper-catabolism may increase in ‘very low’ protein diets | |||||
| Calcium | Calcium is usually added | Calcium is contained in the supplements; further addition may not be routinely needed | ||||
| Phosphate | Phosphate content strictly depends upon the quota of animal proteins. It may be increased if canned, preserved or frozen foods are employed | Phosphate content is usually low in vegan diets, in particular if only fresh food is used. Bioavailability of vegetable phosphate may be different. Readily bioavailable phosphate may be remarkably increased if canned or frozen food is routinely consumed (usually frozen vegetables and canned legumes) | ||||
| Vit D | Supplementation is commonly needed; however the quota of animal proteins may protect from severe deficits | Vitamin D is commonly needed; bioavailable vit D is less represented in vegetable-based products | ||||
| Folate | Supplementation may be needed if the patient eats low quantities of fresh fruits and vegetables | Supplementation usually not needed if fresh food is used | ||||
| B12 | Supplementation may be needed; however, the quota of animal proteins usually protects from severe deficits | Supplementation is commonly needed; B12 deficit is particularly prevalent and may lead to severe problems in vegan–vegetarian pregnancy | ||||
| Iron | Supplementation is commonly needed; however, the quota of animal proteins may protect from severe deficits | Supplementation is usually needed; the bioavailability of vegetable iron is usually lower, even if well-designed vegan diets are not necessarily associated with a deficit in iron | ||||
We did not consider in this review the intake of two main nutrients: sodium and potassium, on the account of their dependence from several other elements, including, for the former the type of disease (interstitial versus vascular or glomerular nephropathy), the blood pressure level and the anti-hypertensive therapy, and for the latter the acidosis status, the use of diuretics and the eventual potassium-containing food additives.
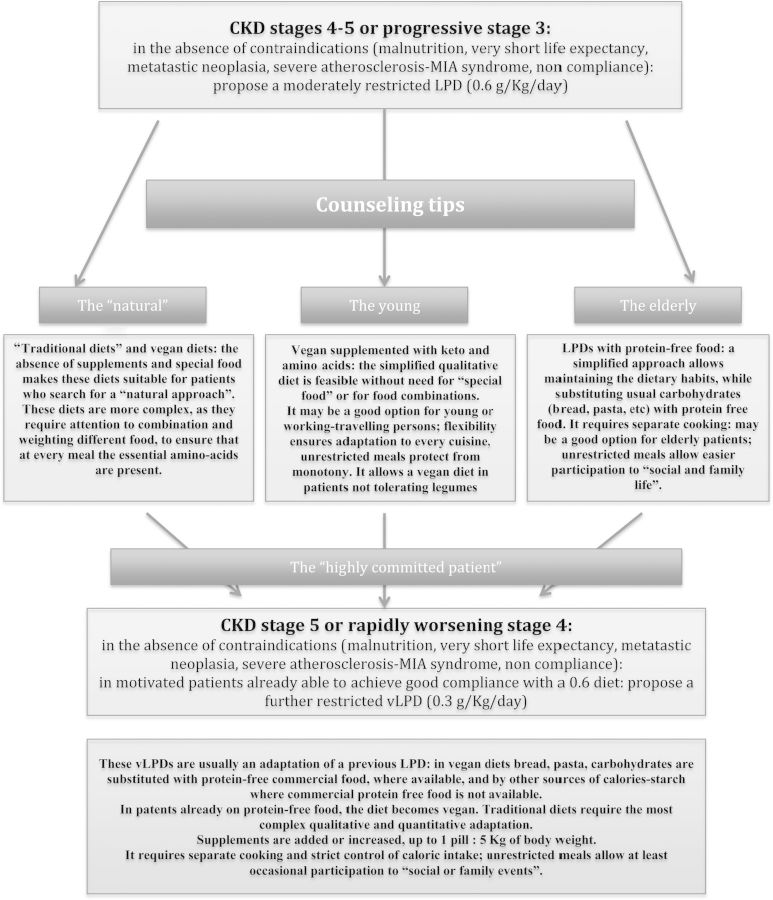
Fig. 1.
A diet flowchart and counseling tips.
Early studies attempted to assess the minimum protein intake requirement. ‘Very-low-protein diets’ (vLPDs) derive from data suggesting that 0.3 g/kg/day is the lowest, safely achievable protein intake, provided supplementation with essential amino and/or keto acids is included.
Conversely, the other ‘magic number’ of 0.6 g/kg/day was believed to identify the upper level of protein restriction exerting a measurable effect on CKD progression [3, 52–62].
The concept of malnutrition came later, when the availability of dialysis allowed to carry out a long-term analysis of the diet and of its carry-over effect after the start of renal replacement therapy [61–64].
Interestingly, these early studies calculated that LPDs allowed life span to be prolonged by about 1 year (in the absence of dialysis) and slowed progression when started earlier [55, 61]. These results are remarkably similar to more recently reported ones, with different, more palatable, LPDs, in a context of widespread dialysis availability [8, 16, 20, 21, 62–71].
Why is the evidence on LPDs in CKD inconclusive: personalized medicine, or RCTs?
The controversy over LPDs in CKD is indirect proof of the lack of conclusive data [7–11, 14–16, 63, 64]. After the MDRD study, several well-conducted Randomized Controlled Trials (RCTs) and one Cochrane review on non-diabetic adults were in favour of protein restriction [14, 65–67, 71–73]. However, no study is devoid of biases and none represented a paradigm shift towards a systematic acceptance of LPDs in CKD.
There are many reasons why studies may be non-conclusive or biased. Diet, for which compliance is key to success, is highly intrusive in a patient's life, as demonstrated by the attrition bias according to which patients tend to switch to their preferred diets despite the original prescriptions [14, 74–78].
Personalized, patient-centred medicine is an emerging clinical model, that is hardly compatible with the rigidity of RCTs [79–82]. Following the lesson of the complementary medicines, the focus may shift from the demonstration of the superiority of a treatment to an observational multidimensional ‘whole system’ approach [83].
In fact, to cite a BMJ educational report, ‘when the effectiveness of the intervention depends on the subject's active participation’, a reflection of the patient's preferences, ‘a randomized trial may be inappropriate because the very act of random allocation may reduce the effectiveness of the intervention’ [84]. Thus, while RCTs are best for studying short-term efficacy, observational studies may be more appropriate for analysing their implementation and highlighting the interactions with patients' preferences and compliance [76, 84–86]. RCTs and observational studies have complementary roles: ‘high quality observational studies may extend evidence over a wider population and are likely to be dominant in the identification of harms and when RCTs would be unethical or impractical’ [87].
The methodological drawbacks of studies on diets in CKD are similar to those of dialysis: randomization of peritoneal dialysis versus haemodialysis is considered ‘unethical and unpractical’. The endless search for the best dialysis treatment shows that the main reason for supporting treatment choice is the demonstration of its equivalence in terms of hard end points [88]. The diet is probably as intrusive as dialysis is in daily life. The price of an appealing randomized study design not involving ‘indifferent’ therapies is a low enrolment rate or an important attrition bias: indeed how many of us would accept being randomized to a restricted versus an unrestricted diet? [14, 15, 65, 66, 71].
A practical nomenclature: moderate protein restriction: low-protein ‘0.6-diets’: ‘traditional’, vegan, vegan-supplemented and with protein-free food
The approaches to ‘moderate protein restriction’ which is synonymous with a diet having a protein intake of 0.6 g/kg/day encompass various combinations of protein content, prevalence of vegetable proteins, use of dietary supplements and protein-free food (Tables 1 and 2, and Figure 1).
There are four main policies for achieving a balanced ‘0.6 diet’: we will call them ‘traditional’, vegan, vegan-supplemented and with protein-free food.
Although all protein-restricted diets share the risk for malnutrition, which is obviously enhanced by baseline malnutrition, severe atherosclerotic disease and inflammation (the Malnutrition-Inflammation-Atherosclerosis (MIA) syndrome), the risks are low in moderately restricted diets and may be more frequent in the case of stricter protein restriction. A ‘hidden’ but crucial point in the management of all types of LPDs is indeed the exclusion of the cases with baseline malnutrition, long-term steroid therapy, very advanced age, with or without MIA [89, 90]. Differences in exclusion policy may account for the different results obtained in various studies, in particular as for the safety issues.
‘Traditional’ 0.6-diets
There is substantial agreement that moderate protein restriction is feasible without the need for supplements or protein-free food [12, 72, 73, 75, 91].
Achieving this goal may be easier where the traditional cuisine is mainly based upon vegetable proteins, such as in India or in Mediterranean countries, and is integrated with small quantities of animal proteins, but may be difficult in Anglo-Saxon or Northern European countries [28–32, 43–46].
Designing LPDs with mixed protein content may start from an adaptation of local diets: for example, Mediterranean diets integrate legumes and cereals, and the addition of a very small amount of dairy products or meat-fish supplies the essential amino acids that are needed; on the other side of the world, in the ‘Okinawa diet’, very small amounts of animal protein were added to a diet that is rich in fruit and vegetables [23, 29, 30, 92]. Adaptation may be more difficult in other contexts where the baseline diet is less rich in vegetables and cereals, however protein content has only recently risen in the Western world, and most traditional cuisines have a lower protein intake in ‘everyday food’ [28–31].
Vegan 0.6–0.8 diets
As already mentioned, non-restricted vegan diets usually provide a protein intake of 0.6–0.8 g/kg/day and are nutritionally adequate if carried out with attention to the integration of essential amino acids [36–38]. As a rule, legumes and cereals and/or soy should be combined at every meal, and a high variety of legumes and cereals ensures integration of essential amino acids. Some interesting schemas are available from different parts of the world, such as the Italian Barsotti scheme, or the Israel approach by Soroka. Both Barsotti and Sokora employed dietary regimens at 0.7–0.8 g/kg/day on the account of the lower bioavailability of vegetable proteins [40, 41, 93].
Supplemented, vegan 0.6-diets
Supplementation with amino and keto acids and substitution with protein-free commercial food represent two strategies to ‘be on the safe side’ as for malnutrition. The first strategy provides supplementation of essential amino acids (in the form of amino and keto acids, metabolized in vitro with an additional urea-sparing effect), the second one adds calories in the form of protein-free food [89, 94].
In the past, various mixtures/combinations of amino acids and keto acids were used; presently only two of them, both of which are marketed by the same company, are available: Alfa Kappa (in Italy) or Ketosteril (all over the world) [94, 95]. Their composition is identical but for the presence of 23 mg per pill of l-tryptophan in Ketosteril. The Italian formula is tryptophan free due to its possible role as a precursor of pro-fibrotic cytokines and indoles [96, 97]. In the absence of a clear demonstration of any clinical difference, they are usually considered equivalent and have been used interchangeably in some studies, also on account of manufacturing changes that have taken place over time [69, 70].
According to a consensus statement, supplements are not strictly needed in vegan diets at 0.6 g/kg/day of proteins [89]. However, they may be useful when omnivorous patients are ‘forced’ into a vegan regimen, or when a patient does not like or cannot tolerate legumes. Supplementation allows a simplified quantitative approach that excludes animal-derived food, but leaves a free choice of vegetables, fruits, legumes and cereals [69, 70]. On the basis of our group's experience, this is also a good strategy in fragile patients, including diabetics, pregnant women or patients recovering renal function [69, 70, 89, 98–101].
The dose of supplements with the 0.6 diets is not standardized; our group indicates 1 pill every 10 kg of body weight, as a compromise between ‘too many pills’ and risk of malnutrition [69, 70, 98–101].
0.6-Diets with protein-free food
The issue of protein-free foods is complex [102]. The idea was born in the era of limited dialysis availability: natural protein-free foods (tapioca, butter, sugar, fruits and vegetables) resulted in very monotonous diets. Following the English example of starch-containing food for coeliac patients, the first protein-free pasta was produced in 1966, followed in the late ‘70s by an industrial production of pasta, flour, biscuits, bread and crispbread. More recently, protein-free drinks, snacks, and partially cooked foods have become commercially available [102]. However, to the best of our knowledge, protein-free food is available free of charge to CKD patients only in Italy, thus limiting the systematic diffusion of this approach [70].
Replacing the usual cereals with protein-free food allows the patient to achieve a high caloric intake, thus giving him/her more freedom to plan the rest of the diet. This is especially favourable in Mediterranean countries where up to half of the proteins derive from bread and pasta (12 g of proteins per 100 g), and replacing them allows the patients to reach the target protein intake without substantially changing their dietary habits. Hence, in our experience, these diets are preferred by elderly patients, while younger ones tend to prefer vegan supplemented diets [70, 71].
Adding calorie-rich food to avoid malnutrition was also attempted by other supplements (for example, maltodextrin and oil creamer), once more in keeping with the local dietary habits [103, 104].
vLPDs: a combination of vegan, supplemented diets and protein-free food
A supplemented LPD is not synonymous with a vLPD [89]. However, vLPDs (usually at 0.3 g/kg/day) require integration to avoid malnutrition [14–16, 66–68, 71, 72, 90, 104]. The amount of amino and keto acid supplements, conventionally measured in pills per kg/day, is 1 pill/5 kg of body weight [89].
The combination of a vegan diet, requiring many pills, possibly together with commercial protein-free food, makes these ‘artificial diets’ extremely complex and an option for only a small number of patients. The Bucharest group, which to date has probably achieved the best compliance with vLPDs, enrolled about 10% of the initially screened patients into their trial [71, 105]. Similar difficulties were encountered in the Brunori trial [65–67]. Interestingly, in our observational study, which allowed patients to freely choose their own diet, the prevalence vLPDs was also about 10% with respect to all LPDs [70]. Despite the difficulties, vLPDs are usually considered more effective in postponing dialysis in compliant patients [71–75]. A ‘missing point’ in the literature is the actual menu of these diets.
In Italy, the availability of protein-free food makes it easier to reach the ideal caloric intake, however, when protein-free food is not available, the diet may be even more demanding [16, 66–68, 71, 72, 105].
While this review was focussed on the ‘classical’ indications for CKD; however, it may be worth mentioning that vLPDs may have a specific indication in the ‘rescue treatment’ of nephrotic syndrome resistant to the therapies specifically addressed at the pathophysiologic mechanisms [106, 107].
Quality matters, but compliance matters more; different proteins may have different effects, and what about ‘unrestricted meals’?
The quality of the protein matters: some studies suggest there is a favourable effect of soy proteins in kidney transplant patients, diabetic patients or experimental animals [108–111]. However, the evidence is limited and shares the same methodological drawbacks as trials on LPDs: compliance is crucial in the long term and it is difficult to achieve if the diet is too rigid.
Various policies have been employed to improve compliance: one option is to alternate different LPDs in order to increase the variety of food [112].
The approach followed by our group is a qualitative simplification of the diet, based upon forbidden and allowed food for the vegan diets, or upon substitution of carbohydrates with protein-free food, without otherwise changing the diet [69, 70, 98–101]. An occasional unrestricted meal (one to three times per week) or one day a week off-diet is another strategy for ensuring compliance. At least two groups have systematically reported inclusion of free meals, thus leading to good compliance [69, 70, 75, 98–101, 113].
A warning: an LPD is not synonymous with a healthy diet
A decrease in kidney function is associated with an increased risk in adverse drug reactions. Less is known about the toxic effect of food additives, preserving agents or taste enhancers. Recent studies highlighted the importance of added polyphosphates and warned about sodium and potassium content [114–117]. The list of potentially toxic additives is long and the effect in the context of reduced kidney function has not yet been investigated. While waiting for further studies to be carried out, attention should be paid to all food components and not only to the quantity of proteins, and should be a warning to patients and manufacturers alike.
This review did not consider other important elements, including sodium and potassium, on account of their highly complex inter-dependence with other issues, including type of disease, blood pressure level and anti-hypertensive therapy, acidosis balance and food additives. While each of these issues may be worth a specific review, reminding them may underline the complexity of a comprehensive dietary approach to CKD patients [118, 119].
Working conclusions: which diet for which patient?
If we extend the approach that has been developed for dialysis, i.e. allowing each patient to choose the most acceptable treatment and encouraging empowerment and self-care, we may design a multiple choice diet system with easy access to different options so that each patient may find the best diet (or at least the least intrusive one).
A stepwise option, starting from moderate protein restriction, could be proposed to all patients with progressive or advanced CKD in an attempt to allocate each patient to the simplest and most feasible diet option (such as vegan simplified diets for younger patients, traditional and with protein-free food for elderly patients), while strict 0.3 diets will probably play a role in highly motivated, well-trained patients, or in selected patients for whom postponing dialysis may be of particular relevance (Figure 1).
In such a setting, there may be no competition among different diets, and the availability of more schemas may allow a greater number of patients to follow an LPD for a longer period, with potential benefits for the overall CKD population.
Conflict of interest statement
G.B.P. belongs to the Ketosteril advisory board (Fresenus Kabi); F.N.V. received a Fresenus Kabi research fellowship through a University grant to G.B.P. (University of Torino). The other authors have no potential conflict of interest. The results presented in this paper have not been published previously in whole or part.
References
- 1.McDonough W, Braungart M. Cradle to Cradle, Remaking the Way We Make Things. New York: North Point Press; 2002. [Google Scholar]
- 2.Piccoli GB. Patient-based continuum of care in nephrology: why read Thomas Addis’ ‘Glomerular Nephritis’ in 2010? J Nephrol. 2010;23:164–167. [PubMed] [Google Scholar]
- 3.Addis T, Lew W. Diet and death in acute uremia. J Clin Invest. 1939;18:773–775. doi: 10.1172/JCI101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addis T. Glomerular Nephritis: Diagnosis and Treatment. New York: Macmillan; 1949. [Google Scholar]
- 5.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249(3 Pt 2):F324–F337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom J. Discovery and rediscovery of low protein diet. Clin Nephrol. 1984;21:29–35. [PubMed] [Google Scholar]
- 8.Mitch WE, Remuzzi G. Diets for patients with chronic kidney disease, still worth prescribing. J Am Soc Nephrol. 2004;15:234–237. doi: 10.1097/01.asn.0000106014.20274.c7. [DOI] [PubMed] [Google Scholar]
- 9.Walser M, Mitch WE, Maroni BJ, et al. Should protein intake be restricted in predialysis patients? Kidney Int. 1999;55:771–777. doi: 10.1046/j.1523-1755.1999.055003771.x. [DOI] [PubMed] [Google Scholar]
- 10.el Nahas AM, Coles GA. Dietary treatment of chronic renal failure: ten unanswered questions. Lancet. 1986;1:597–600. doi: 10.1016/s0140-6736(86)92819-9. [DOI] [PubMed] [Google Scholar]
- 11.Giovannetti S on behalf of the Steering Committee of the European Study Groups for the conservative treatment of chronic renal failure. Answers to ten questions on the dietary treatment of chronic renal failure. Lancet. 1986;328:1140–1142. doi: 10.1016/s0140-6736(86)90542-8. [DOI] [PubMed] [Google Scholar]
- 12.Fouque D, Aparicio M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:383–392. doi: 10.1038/ncpneph0524. [DOI] [PubMed] [Google Scholar]
- 13.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Greene T, Beck GJ, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? J Am Soc Nephrol. 1999;10:2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 15.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 16.Walser M, Hill S. Can renal replacement be deferred by a supplemented very low protein diet? J Am Soc Nephrol. 1999;10:110–116. doi: 10.1681/ASN.V101110. [DOI] [PubMed] [Google Scholar]
- 17.Milas NC, Nowalk MP, Akpele L, et al. Factors associated with adherence to the dietary protein intervention in the modification of diet in renal disease study. J Am Diet Assoc. 1995;95:1295–1300. doi: 10.1016/s0002-8223(95)00340-1. [DOI] [PubMed] [Google Scholar]
- 18.Coyne T, Olson M, Bradham K, et al. Dietary satisfaction correlated with adherence in the modification of diet in renal disease study. J Am Diet Assoc. 1995;95:1301–1306. doi: 10.1016/s0002-8223(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen LI, Guh JY, Wu KD, et al. Modification of diet in renal disease (MDRD) study and CKD epidemiology collaboration (CKD-EPI) equations for Taiwanese adults. PLoS One. 2014;9:e99645. doi: 10.1371/journal.pone.0099645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD001892.pub3. CD001892. [DOI] [PubMed] [Google Scholar]
- 21.Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD002181.pub2. CD002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 23.Sotos-Prieto M, Moreno-Franco B, Ordovás JM, et al. Design and development of an instrument to measure overall lifestyle habits for epidemiological research: the Mediterranean Lifestyle (MEDLIFE) index. Public Health Nutr. 2014;15:1–9. doi: 10.1017/S1368980014001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkler D, Dehghan M, Teo KK, et al. Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern Med. 2013;173:1682–1692. doi: 10.1001/jamainternmed.2013.9051. [DOI] [PubMed] [Google Scholar]
- 25.Millward DJ, Jackson AA. Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: implications for recommended protein and amino acid intakes. Public Health Nutr. 2004;7:387–405. doi: 10.1079/PHN2003545. [DOI] [PubMed] [Google Scholar]
- 26.Volpi E, Campbell WW, Dwyer JT, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afolabi PR, Jahoor F, Gibson NR, et al. Response of hepatic proteins to the lowering of habitual dietary protein to the recommended safe level of intake. Am J Physiol Endocrinol Metab. 2004;287:E327–E330. doi: 10.1152/ajpendo.00036.2004. [DOI] [PubMed] [Google Scholar]
- 28.Keys A, Aravanis C, Buchem FSP, et al. The diet and all-causes death rate in the Seven Countries Study. Lancet. 1981;2:58–61. [PubMed] [Google Scholar]
- 29.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55(11 Pt 1):383–389. doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 30.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 31.Trichopoulou A, Martínez-González MA, Tong TY, et al. Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med. 2014;12:112. doi: 10.1186/1741-7015-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179:282–289. doi: 10.1093/aje/kwt261. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson MF, Havas S, McCarter R. Changes in sodium levels in processed and restaurant foods, 2005 to 2011. JAMA Intern Med. 2013;173:1285–1291. doi: 10.1001/jamainternmed.2013.6154. [DOI] [PubMed] [Google Scholar]
- 34.Sylvetsky AC, Dietz WH. Nutrient-content claims—guidance or cause for confusion? N Engl J Med. 2014;371:195–198. doi: 10.1056/NEJMp1404899. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Evaluation of certain food additives. World Health Organ Tech Rep Ser. 2012:1–183. back cover. [PubMed] [Google Scholar]
- 36.Craig WJ, Mangels AR American Dietetic Association. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 37.American Dietetic Association; Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: vegetarian diets. Can J Diet Pract Res. 2003;64:62–81. doi: 10.3148/64.2.2003.62. [DOI] [PubMed] [Google Scholar]
- 38.Craig WJ. Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract. 2010;25:613–620. doi: 10.1177/0884533610385707. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Liu J, Su J, et al. The effects of soy protein on chronic kidney disease: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2014;68:987–993. doi: 10.1038/ejcn.2014.112. [DOI] [PubMed] [Google Scholar]
- 40.Barsotti G, Morelli E, Cupisti A, et al. A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron. 1996;74:390–394. doi: 10.1159/000189341. [DOI] [PubMed] [Google Scholar]
- 41.Soroka N, Silverberg DS, Greemland M, et al. Comparison of a vegetable-based (soya) and an animal-based low-protein diet in predialysis chronic renal failure patients. Nephron. 1998;79:173–180. doi: 10.1159/000045021. [DOI] [PubMed] [Google Scholar]
- 42.Buzio C, Mutti A, Perazzoli F, et al. Protein-induced changes in kidney function depend on the time of administration but not on the dietary source. Nephron. 1990;56:234–240. doi: 10.1159/000186146. [DOI] [PubMed] [Google Scholar]
- 43.Fujita R, Braun KL, Hughes CK. The traditional Hawaiian diet: a review of the literature. Pac Health Dialog. 2004;11:250–259. [PubMed] [Google Scholar]
- 44.McKerchar C, Bowers S, Heta C, et al. Enhancing Māori food security using traditional kai. Glob Health Promot. 2014 doi: 10.1177/1757975914543573. pii: 1757975914543573 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 45.Willcox BJ, Willcox DC, Todoriki H, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 46.Laird BD, Goncharov AB, Egeland GM, et al. Dietary advice on Inuit traditional food use needs to balance benefits and risks of mercury, selenium, and n3 fatty acids. J Nutr. 2013;143:923–930. doi: 10.3945/jn.112.173351. [DOI] [PubMed] [Google Scholar]
- 47.Li JR, Hsieh YH. Traditional Chinese food technology and cuisine. Asia Pac J Clin Nutr. 2004;13:147–155. [PubMed] [Google Scholar]
- 48.Webber L, Divajeva D, Marsh T, et al. The future burden of obesity-related diseases in the 53 WHO European-Region countries and the impact of effective interventions: a modelling study. BMJ Open. 2014;4:e004787. doi: 10.1136/bmjopen-2014-004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson D. 1944. Vegan News, No. 1. November,
- 50.Gilsing AM, Weijenberg MP, Goldbohm RA, et al. The Netherlands Cohort Study−Meat Investigation Cohort; a population-based cohort over-represented with vegetarians, pescetarians and low meat consumers. Nutr J. 2013;12:156. doi: 10.1186/1475-2891-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis DS. On the influence of a diet with high protein content on the kidney. Can Med Assoc J. 1921;11:682–683. [PMC free article] [PubMed] [Google Scholar]
- 52.Mackay LL, Addis T, Mackay EM. The degree of compensatory renal hypertrophy following unilateral nephrectomy: II. The influence of the protein intake. J Exp Med. 1938;67:515–519. doi: 10.1084/jem.67.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borst JG. Protein katabolism in uraemia; effects of protein-free diet, infections, and blood-transfusions. Lancet. 1948;1:824–829. [PubMed] [Google Scholar]
- 54.Giovannetti S, Maggiore Q. A low-nitrogen diet with proteins of high biological value for severe chronic uraemia. Lancet. 1964;1:1000–1003. doi: 10.1016/s0140-6736(64)91919-1. [DOI] [PubMed] [Google Scholar]
- 55.Kerr ND, Robson A, Ashcroft R. Diet in chronic renal failure. Proc R Soc Med. 1967;60:115–116. [PMC free article] [PubMed] [Google Scholar]
- 56.Berlyne GM, Janabi KM, Shaw AB. Dietary treatment of chronic renal failure. Proc R Soc Med. 1966;59:665–667. [PMC free article] [PubMed] [Google Scholar]
- 57.Wright PL, Brereton PJ, Snell DEM. Effectiveness of modified Giovannetti diet compared with mixed low-protein diet. Metabolism. 1970;19:201–213. doi: 10.1016/0026-0495(70)90053-3. [DOI] [PubMed] [Google Scholar]
- 58.Hood CEA, Beale DJ, Housley J, et al. Dialysed egg as nitrogen source in dietary control of chronic renal failure. Lancet. 1969;1:479–482. doi: 10.1016/s0140-6736(69)91586-4. [DOI] [PubMed] [Google Scholar]
- 59.Franklin SS, Gordon A, Kleeman CR, et al. Use of a balanced low-protein diet in chronic renal failure. JAMA. 1967;202:477–484. [PubMed] [Google Scholar]
- 60.Berlyne GM, Gaan D, Ginks WR. Dietary treatment of chronic renal failure. Am J Clin Nutr. 1968;21:547–552. doi: 10.1093/ajcn/21.6.547. [DOI] [PubMed] [Google Scholar]
- 61.Giordano C, Pluvio M, Di Guida G, et al. Modulated nitrogen intake for patients on low-protein diets. Am J Clin Nutr. 1980;33:1638–1641. doi: 10.1093/ajcn/33.7.1638. [DOI] [PubMed] [Google Scholar]
- 62.Guarnieri G, Faccini L, Lipartiti T, et al. Simple methods for nutritional assessment in hemodialyzed patients. Am J Clin Nutr. 1980;33:1598–1607. doi: 10.1093/ajcn/33.7.1598. [DOI] [PubMed] [Google Scholar]
- 63.Johnson DW. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: the case against. Nephrology (Carlton) 2006;11:58–62. doi: 10.1111/j.1440-1797.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- 64.Maroni BJ. Protein restriction and malnutrition in renal disease: fact or fiction? Miner Electrolyte Metab. 1997;23:225–228. [PubMed] [Google Scholar]
- 65.Maiorca R, Brunori G, Viola BF, et al. Diet or dialysis in the elderly? The DODE study: a prospective randomized multicenter trial. J Nephrol. 2000;13:267–270. [PubMed] [Google Scholar]
- 66.Brunori G, Viola BF, Parrinello G, et al. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis. 2007;49:569–580. doi: 10.1053/j.ajkd.2007.02.278. [DOI] [PubMed] [Google Scholar]
- 67.Scalone L, Borghetti F, Brunori G, et al. Cost–benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol Dial Transplant. 2010;25:907–913. doi: 10.1093/ndt/gfp572. [DOI] [PubMed] [Google Scholar]
- 68.Friedman AN. New evidence for an old strategy to help delay the need for dialysis. Am J Kidney Dis. 2007;49:563–565. doi: 10.1053/j.ajkd.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Piccoli GB, Ferraresi M, Deagostini MC, et al. Vegetarian low-protein diets supplemented with keto analogues: a niche for the few or an option for many? Nephrol Dial Transplant. 2013;28:2295–2305. doi: 10.1093/ndt/gft092. [DOI] [PubMed] [Google Scholar]
- 70.Piccoli GB, Deagostini MC, Vigotti FN, et al. Which low-protein diet for which CKD patient? An observational, personalized approach. Nutrition. 2014;30:992–999. doi: 10.1016/j.nut.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Mircescu G, Gârneaţă L, Stancu SH, et al. Effects of a supplemented hypoproteic diet in chronic kidney disease. J Ren Nutr. 2007;17:179–188. doi: 10.1053/j.jrn.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 72.Cianciaruso B, Pota A, Pisani A, et al. Metabolic effects of two low protein diets in chronic kidney disease stage 4–5—a randomized controlled trial. Nephrol Dial Transplant. 2008;23:636–644. doi: 10.1093/ndt/gfm576. [DOI] [PubMed] [Google Scholar]
- 73.Zeller K, Whittaker E, Sullivan L, et al. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324:78–84. doi: 10.1056/NEJM199101103240202. [DOI] [PubMed] [Google Scholar]
- 74.Combe C, Deforges-Lasseur C, Caix J, et al. Compliance and effects of nutritional treatment on progression and metabolic disorders of chronic renal failure. Nephrol Dial Transplant. 1993;8:412–418. [PubMed] [Google Scholar]
- 75.Giordano M, Ciarambino T, Castellino P, et al. Light and shadows of dietary protein restriction in elderly with chronic kidney disease. Nutrition. 2013;29:1090–1093. doi: 10.1016/j.nut.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 76.Thilly N. Low-protein diet in chronic kidney disease: from questions of effectiveness to those of feasibility. Nephrol Dial Transplant. 2013;28:2203–2205. doi: 10.1093/ndt/gft235. [DOI] [PubMed] [Google Scholar]
- 77.Paes-Barreto JG, Silva MI, Qureshi AR, et al. Can renal nutrition education improve adherence to a low-protein diet in patients with stages 3 to 5 chronic kidney disease? J Ren Nutr. 2013;23:164–171. doi: 10.1053/j.jrn.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Cianciaruso B, Capuano A, D'Amaro E, et al. Dietary compliance to a low protein and phosphate diet in patients with chronic renal failure. Kidney Int Suppl. 1989;27:S173–S176. [PubMed] [Google Scholar]
- 79.Gray JA. The shift to personalised and population medicine. Lancet. 2013;382:200–201. doi: 10.1016/s0140-6736(13)61590-1. [DOI] [PubMed] [Google Scholar]
- 80.Kitsios GD, Kent DM. Personalised medicine: not just in our genes. BMJ. 2012;344:e2161. doi: 10.1136/bmj.e2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basch E. Toward patient-centered drug development in oncology. N Engl J Med. 2013;369:397–400. doi: 10.1056/NEJMp1114649. [DOI] [PubMed] [Google Scholar]
- 82.Lin GA. Patient education: one size does not fit all. JAMA Intern Med. 2013;173:1376. doi: 10.1001/jamainternmed.2013.7402. [DOI] [PubMed] [Google Scholar]
- 83.Verhoef MJ, Lewith G, Ritenbaugh C, et al. Complementary and alternative medicine whole systems research: beyond identification of inadequacies of the RCT. Complement Ther Med. 2005;13:206–212. doi: 10.1016/j.ctim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 86.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barton S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ. 2000;321:255–256. doi: 10.1136/bmj.321.7256.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piccoli GB. The never-ending search for the perfect dialysis. Should we move from the best treatment to the best system? Nephrol Dial Transplant. 2011;26:1128–1131. doi: 10.1093/ndt/gfr123. [DOI] [PubMed] [Google Scholar]
- 89.Stenvinkel P, Heimbürger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–960. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 90.Abraham G, Varsha P, Mathew M, et al. Malnutrition and nutritional therapy of chronic kidney disease in developing countries: the Asian perspective. Adv Ren Replace Ther. 2003;10:213–221. doi: 10.1053/j.arrt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Aparicio M, Bellizzi V, Chauveau P, et al. Keto acid therapy in predialysis chronic kidney disease patients: final consensus. J Ren Nutr. 2012;22(2 Suppl):S22–S24. doi: 10.1053/j.jrn.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Oba S, Nagata C, Nakamura K, et al. Diet based on the Japanese Food Guide Spinning Top and subsequent mortality among men and women in a general Japanese population. J Am Diet Assoc. 2009;109:1540–1547. doi: 10.1016/j.jada.2009.06.367. [DOI] [PubMed] [Google Scholar]
- 93.Chauveau P, Combe C, Fouque D, et al. Vegetarianism: advantages and drawbacks in patients with chronic kidney diseases. J Ren Nutr. 2013;23:399–405. doi: 10.1053/j.jrn.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Liou HH. What can a keto acid/amino acid-supplemented protein-restricted diet do for the ‘butterfly effect’ in chronic kidney disease patients? J Ren Nutr. 2009;19(5 Suppl):S15–S18. doi: 10.1053/j.jrn.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Walser M, Mitch WE, Abras E. Supplements containing amino acids and keto acids in the treatment of chronic uremia. Kidney Int Suppl. 1983;16:S285–S289. [PubMed] [Google Scholar]
- 96.Schefold JC, Zeden JP, Fotopoulou C, et al. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant. 2009;24:1901–1908. doi: 10.1093/ndt/gfn739. [DOI] [PubMed] [Google Scholar]
- 97.Sallée M, Dou L, Cerini C, et al. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piccoli GB, Attini R, Vasario E, et al. Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: report of 12 pregnancies in 11 patients. Nephrol Dial Transplant. 2011;26:196–205. doi: 10.1093/ndt/gfq333. [DOI] [PubMed] [Google Scholar]
- 99.Piccoli GB, Leone F, Attini R, et al. Association of low-protein supplemented diets with fetal growth in pregnant women with CKD. Clin J Am Soc Nephrol. 2014;9:864–873. doi: 10.2215/CJN.06690613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piccoli GB, Motta D, Martina G, et al. Low-protein vegetarian diet with alpha-chetoanalogues prior to pre-emptive pancreas-kidney transplantation. Rev Diabet Stud. 2004;1:95–102. doi: 10.1900/RDS.2004.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piccoli GB, Guzzo G, Vigotti FN, et al. Tailoring dialysis and resuming low-protein diets may favor chronic dialysis discontinuation: report on three cases. Hemodial Int. 2014;18:590–595. doi: 10.1111/hdi.12168. [DOI] [PubMed] [Google Scholar]
- 102.D'Alessandro C, Rossi A, Innocenti M, et al. Dietary protein restriction for renal patients: don't forget protein-free foods. J Ren Nutr. 2013;23:367–371. doi: 10.1053/j.jrn.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Wu HL, Sung JM, Kao MD, et al. Nonprotein calorie supplement improves adherence to low-protein diet and exerts beneficial responses on renal function in chronic kidney disease. J Ren Nutr. 2013;23:271–276. doi: 10.1053/j.jrn.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–1177. doi: 10.3945/ajcn.112.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garneata L, Mircescu G. Effect of low-protein diet supplemented with keto acids on progression of chronic kidney disease. J Ren Nutr. 2013;23:210–213. doi: 10.1053/j.jrn.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 106.Walser M, Hill S, Tomalis EA. Treatment of nephrotic adults with a supplemented, very low-protein diet. Am J Kidney Dis. 1996;28:354–364. doi: 10.1016/s0272-6386(96)90492-8. [DOI] [PubMed] [Google Scholar]
- 107.D'Amico G, Remuzzi G, Maschio G, et al. Effect of dietary proteins and lipids in patients with membranous nephropathy and nephrotic syndrome. Clin Nephrol. 1991;35:237–242. [PubMed] [Google Scholar]
- 108.Anderson JW, Blake JE, Turner J, et al. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr. 1998;68(6 Suppl):1347S–1353S. doi: 10.1093/ajcn/68.6.1347S. [DOI] [PubMed] [Google Scholar]
- 109.Azadbakht L, Esmaillzadeh A. Soy–protein consumption and kidney-related biomarkers among type 2 diabetics: a crossover, randomized clinical trial. J Ren Nutr. 2009;19:479–486. doi: 10.1053/j.jrn.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Cupisti A, Ghiadoni L, D'Alessandro C, et al. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229–234. doi: 10.1093/ndt/gfl553. [DOI] [PubMed] [Google Scholar]
- 111.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cupisti A, Morelli E, Meola M, et al. Vegetarian diet alternated with conventional low-protein diet for patients with chronic renal failure. J Ren Nutr. 2002;12:32–37. doi: 10.1053/jren.2002.29595. [DOI] [PubMed] [Google Scholar]
- 113.Giordano M, Ciarambino T, Castellino P, et al. Long-term effects of moderate protein diet on renal function and low-grade inflammation in older adults with type 2 diabetes and chronic kidney disease. Nutrition. 2014;30:1045–1049. doi: 10.1016/j.nut.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Cupisti A, Kalantar-Zadeh K. Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol. 2013;33:180–190. doi: 10.1016/j.semnephrol.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 116.Sarathy S, Sullivan C, Leon JB, et al. Fast food, phosphorus-containing additives, and the renal diet. J Ren Nutr. 2008;18:466–470. doi: 10.1053/j.jrn.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin J Am Soc Nephrol. 2009;4:1370–1373. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Middleton JP, Lehrich RW. Prescriptions for dietary sodium in patients with chronic kidney disease: how will this shake out? Kidney Int. 2014;86:457–459. doi: 10.1038/ki.2014.124. [DOI] [PubMed] [Google Scholar]
- 119.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–995. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]