Abstract
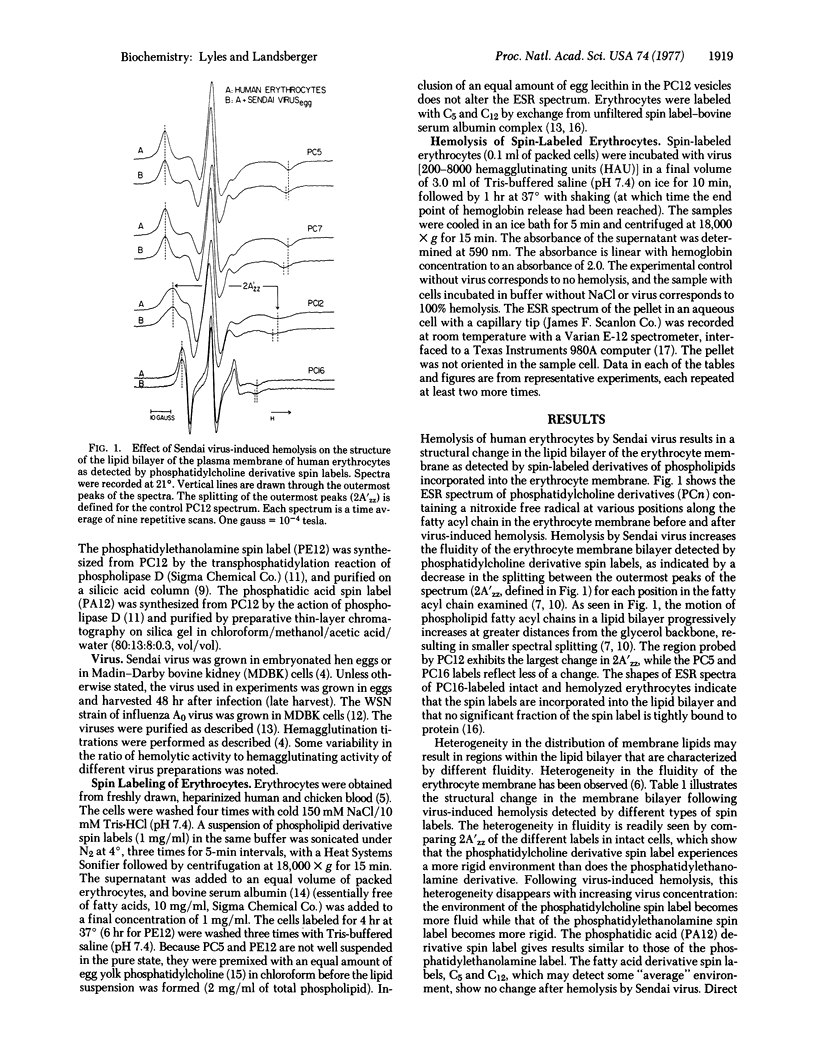
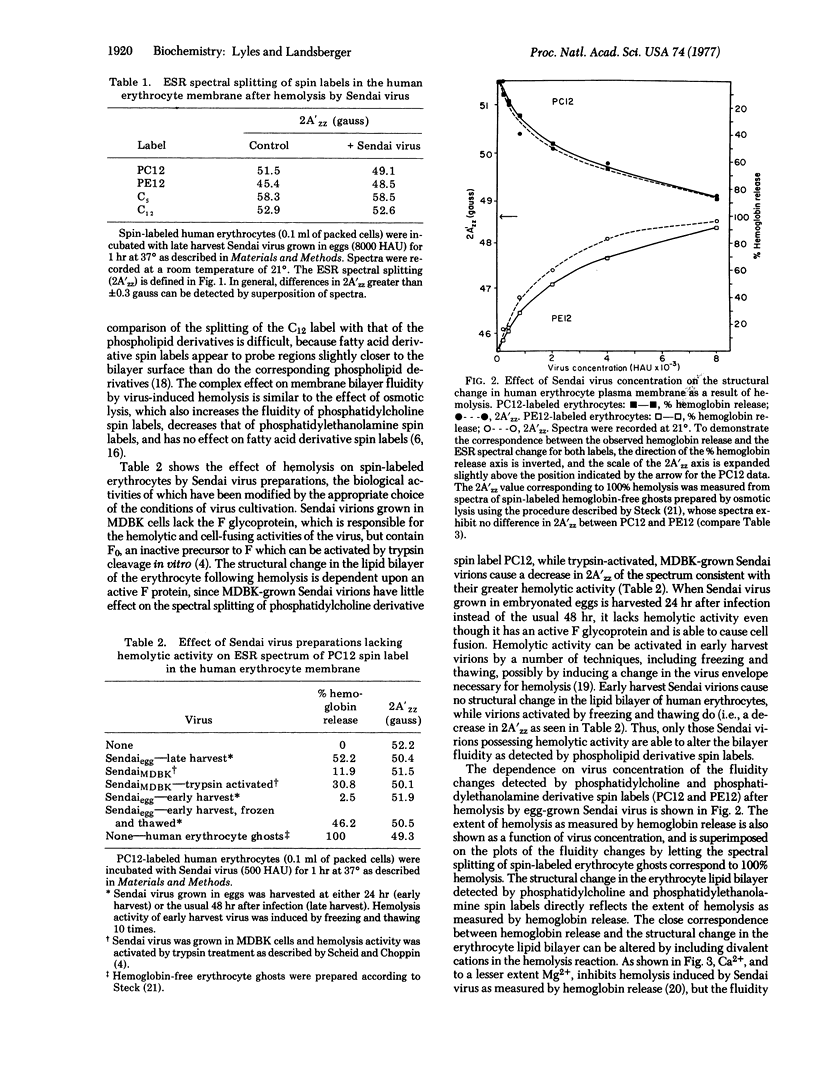
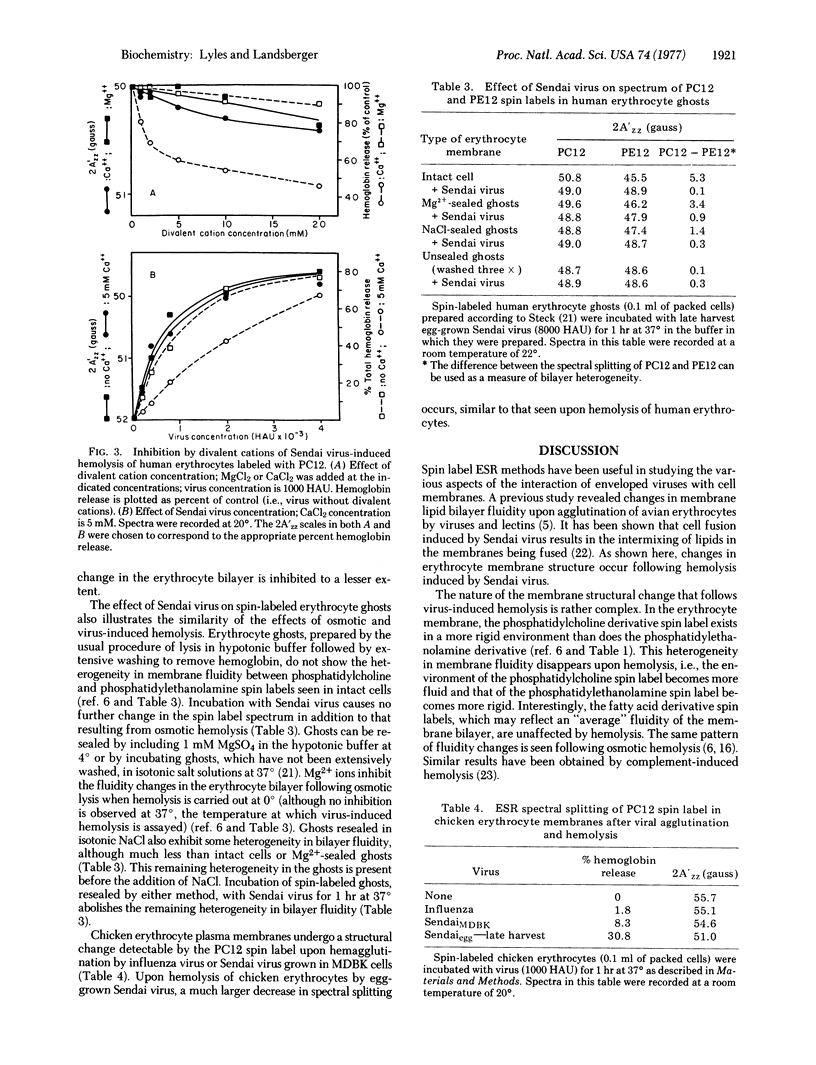
Hemolysis of human or chicken erythrocytes by Sendai virus causes a change in the structure of the erythrocyte membrane lipid bilayer that can be detected by spin label electron spin resonance. In the intact erythrocyte, the phosphatidylcholine derivative spin label exists in a more rigid environment than the corresponding phosphatidylethanolamine label. Virus-induced hemolysis tends to abolish this difference in fluidity, i.e., the region of the phosphatidylcholine spin label becomes more fluid and that of the phosphatidylethanolamine spin label becomes more rigid. Fatty acid derivative spin labels, which may detect some "average" environment, show no change in fluidity. The fluidity change is detected at several different positions in the fatty acyl chain of the phosphatidylcholine spin label. Sendai virions grown in Madin-Darby bovine kidney (MDBK) cells or grown in eggs and harvested early, which lack hemolytic activity, cause no significant change in bilayer structure. Hemolytic activity and the ability to alter erythrocyte bilayer fluidity can be activated in MDBK-grown Sendai virions by trypsin treatment in vitro and in early-harvest egg-grown Sendai virions by freezing and thawing. Erythrocyte ghosts prepared by osmotic hemolysis and resealed by treatment with Mg2+ or elevated ionic strength exhibit a difference in fluidity between phosphatidylcholine and phosphatidylethanolamine spin labels, although less than that observed in whole cells. Incubation of resealed ghosts with Sendai virus abolishes the difference in fluidity. Unsealed ghosts that have been extensively washed show no heterogeneity in membrane bilayer fluidity, and incubation with Sendai virus causes no further fluidity change. Virus-induced hemolysis as measured by hemoglobin release is more sensitive to inhibition by Ca2+ than is the associated fluidity change in the bilayer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss W. F., Kelley C. J., Landsberger F. R. A novel synthesis of spin label derivatives of phosphatidylcholine. Anal Biochem. 1975 Mar;64(1):289–292. doi: 10.1016/0003-2697(75)90432-7. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. I. The effects of protein removal. J Cell Biol. 1974 Dec;63(3):1018–1036. doi: 10.1083/jcb.63.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Jost P., Libertini L. J., Hebert V. C., Griffith O. H. Lipid spin labels in lecithin multilayers. A study of motion along fatty acid chains. J Mol Biol. 1971 Jul 14;59(1):77–98. doi: 10.1016/0022-2836(71)90414-1. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger F. R., Paxton J., Lenard J. A study of intact human erythrocytes and their ghosts using stearic acid spin labels. Biochim Biophys Acta. 1972 Apr 14;266(1):1–6. doi: 10.1016/0005-2736(72)90113-7. [DOI] [PubMed] [Google Scholar]
- Lenard J., Tsai D. K., Compans R. W., Landsberger F. R. Observations on the membrane organization of standard and incomplete influenza grown in MDBK cells. Virology. 1976 Jun;71(2):389–394. doi: 10.1016/0042-6822(76)90366-4. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. V irus and lectin agglutination of erythrocytes: spin label study of membrane lipid-protein interactions. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3497–3501. doi: 10.1073/pnas.73.10.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Asano A., Oki K., Okada Y., Onishi S. A spin-label study on fusion of red blood cells induced by hemagglutinating virus of Japan. Biochemistry. 1975 Aug 26;14(17):3736–3741. doi: 10.1021/bi00688a003. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ohnishi S., Kitamura H., Inai S. Membrane fluidity change in erythrocytes induced by complement system. Biochemistry. 1976 Nov 2;15(22):4838–4843. doi: 10.1021/bi00667a013. [DOI] [PubMed] [Google Scholar]
- Peretz H., Toister Z., Laster Y., Loyter A. Fusion of intact human erythrocytes and erythrocyte ghosts. J Cell Biol. 1974 Oct;63(1):1–11. doi: 10.1083/jcb.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet A., Guthmann C., Matricon J., Bienvenue A., Devaux P. F. Study of the transverse diffusion of spin labeled phospholipids in biological membranes. I. Human red bloods cells. Biochim Biophys Acta. 1976 Mar 19;426(3):357–371. doi: 10.1016/0005-2736(76)90382-5. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K. I., Ohnishi S. Heterogeneity in the fluidity of intact erythrocyte membrane and its homogenization upon hemolysis. Biochim Biophys Acta. 1976 Mar 5;426(2):218–231. doi: 10.1016/0005-2736(76)90333-3. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Kingzett T. J., Rottschaefer S., Griffith O. H., Keith A. D. A spin-labeled lipid for probing biological membranes. Chem Phys Lipids. 1969 Sep;3(3):245–253. doi: 10.1016/0009-3084(69)90016-4. [DOI] [PubMed] [Google Scholar]


