Abstract
Transplant renal artery stenosis (TRAS) is a well-recognized vascular complication after kidney transplant. It occurs most frequently in the first 6 months after kidney transplant, and is one of the major causes of graft loss and premature death in transplant recipients. Renal hypoperfusion occurring in TRAS results in activation of the renin–angiotensin–aldosterone system; patients usually present with worsening or refractory hypertension, fluid retention and often allograft dysfunction. Flash pulmonary edema can develop in patients with critical bilateral renal artery stenosis or renal artery stenosis in a solitary kidney, and this unique clinical entity has been named Pickering Syndrome. Prompt diagnosis and treatment of TRAS can prevent allograft damage and systemic sequelae. Duplex sonography is the most commonly used screening tool, whereas angiography provides the definitive diagnosis. Percutaneous transluminal angioplasty with stent placement can be performed during angiography if a lesion is identified, and it is generally the first-line therapy for TRAS. However, there is no randomized controlled trial examining the efficacy and safety of percutaneous transluminal angioplasty compared with medical therapy alone or surgical intervention.
Keywords: flash pulmonary edema, hypertensive crisis, Pickering syndrome, renal artery pseudoaneurysm, transplant renal artery stenosis
Introduction
Poorly controlled hypertension is common among renal transplant recipients and associated with graft failure and high mortality [1]. Transplant renal artery stenosis (TRAS) is the narrowing of the transplant renal artery, impeding blood flow to the allograft. It accounts for 1–5% cases of post-transplant hypertension [2–4]. Especially, since the introduction of calcineurin inhibitors and other immunosuppressive agents, the incidence of allograft rejection has substantially decreased [5], making TRAS one of the important causes of graft loss and premature death in transplant recipients. In this study, we describe a case of transplant renal artery narrowing caused by compression of a pseudoaneurysm with clinical features of TRAS, followed by a thorough review on TRAS. The key teaching points are listed in Table 1.
Table 1.
Key teaching points
|
Case presentation
A 42-year-old African-American man, who underwent kidney transplantation for end-stage renal disease due to hypertension, presented to the emergency department with lightheadedness, palpitations and a reported home blood pressure of >220/110 mmHg. Six weeks prior, he had received a kidney from a 30-year-old deceased donor who died from a gunshot wound. The donor renal anatomy was notable for three renal arteries: two main renal arteries on a common aortic patch and a third superior pole renal artery on a separate aortic patch. The superior pole artery supplied ∼20% of the graft. It had been transected during procurement, but was repaired in an end-to-end fashion with running continuous sutures. During transplantation, two separate aortic cuff anastomoses were made to the external iliac artery. Reperfusion of the kidney was normal and the kidney functioned immediately. The immunosuppression regimen included basiliximab induction and maintenance tacrolimus, mycophenolate mofetil and corticosteroids. The postoperative course was uncomplicated except for new onset of atrial fibrillation, which was resolved with cardioversion. The blood pressure after kidney transplantation ranged between 125/70 and 175/105 mmHg on metoprolol tartrate 100 mg and nifedipine 90 mg twice daily. After addressing medication compliance, antihypertensive medications were adjusted. On the day of the presentation to the emergency department, the patient's antihypertensive medications included clonidine 0.2 mg three times a day, labetalol 200 mg and nifedipine 90 mg twice daily. He appeared diaphoretic, with a blood pressure of 235/122 mmHg and a heart rate of 87 bpm. Physical examination revealed bilateral lung rales without peripheral edema or abdominal pain.
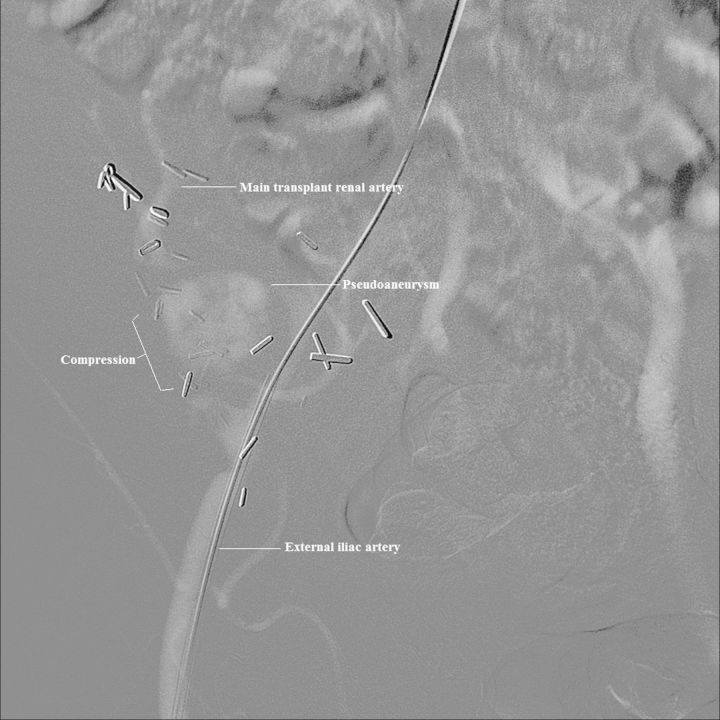
Laboratory tests were significant for a serum creatinine, which had increased from a post-transplant baseline of 1.4 to 2.2 mg/dL over 4 days. The tacrolimus trough level was slightly elevated at 18.3 ng/mL (target 8–15 ng/mL), which suggested against allograft rejection. Urine and blood cultures were negative. Chest X-ray showed evidence of pulmonary edema. Electrocardiogram revealed no evidence of cardiac ischemia or arrhythmia. The patient was admitted to the intensive care unit and administered a nicardipine drip, but his blood pressure remained elevated. Given the presence of refractory hypertension and flash pulmonary edema, TRAS was suspected. Duplex sonography was performed and revealed a hilar pseudoaneurysm adjacent to one of the main donor renal arteries measuring 3.1 × 3.2 × 3.1 cm, with a peak systolic velocity of 457 cm/s in the transplant renal artery. Angiography demonstrated a bi-lobed pseudoaneurysm arising from the distal anastomosis and extrinsically compressing the main transplant artery and limiting flow (Figure 1).
Fig. 1.
Angiography of the transplant renal artery with digital subtraction angiography. A large pseudoaneurysm measuring 3.1 × 3.2 × 3.1 cm causing extrinsic compression on the main transplant renal artery limiting flow.
The following day, the patient was taken to the operating room for pseudoaneurysm repair. Due to extensive abdominal adhesions from prior surgeries, repair of the pseudoaneurysm was aborted, and the pseudoaneurysm was controlled by placement of a covered endoluminal stent to the external iliac artery. This led to surgical embolization of the allograft. The blood pressure improved significantly postoperatively. Finally, the allograft was removed. On the pathologic examination of the explanted kidney, there was extensive coagulative necrosis, reactive acute inflammation and vascular thrombi, which was compatible with infarction. In the residual viable kidney parenchyma, there were vascular changes suggestive of malignant hypertension. There was no evidence of rejection. In summary, this is a case of hypertensive crisis and flash pulmonary edema in a kidney transplant recipient, as a result of transplant renal artery narrowing due to compression of a pseudoaneurysm.
Epidemiology
Owing to the difference in diagnostic modalities, the reported incidence of TRAS varies widely, ranging from 1 to 23% [6, 7]. Most of these studies were performed retrospectively in a single center. For example, Rengel et al. [8] estimated the incidence of TRAS to be 4.5% among 286 kidney transplant recipients from 1990 to 1997 in their institution. The study published by Hurst et al. [9] in 2009 was the only study performed using a national sample of renal transplant recipients. Using the United States Renal Data System (USRDS) registry, they identified 42 403 Medicare primary renal transplant recipients from 2000 to 2005, and found a cumulative incidence of TRAS to be 2% at 3 years and an overall incidence rate to be 8.3 cases per 1000 patient-years [95% confidence interval (CI) 7.8–8.9]. In children, the prevalence of TRAS seems to be lower compared with adults. In a single center, a retrospective study with 216 pediatric patients, the prevalence of TRAS was 4.6% among patients transplanted between 2001 and 2011 [10]. The lower prevalence of TRAS in the pediatric population is likely due to a minor extent of vascular changes in younger donors. TRAS accounts for 1–5% cases of post-transplant hypertension and ∼75% of post-transplant vascular complications [2–4]. It is a major cause of graft loss and premature death in transplant recipients. According to the USRDS registry, the adjusted hazard ratio for death and graft loss was 2.84 (95% CI 1.70–4.72) in transplant recipients with TRAS compared with those without TRAS [9].
Clinical manifestation
TRAS usually occurs between 3 months and 2 years after renal transplantation, with the highest frequency in the first 6-month post-transplant but it may present at any time [9, 11, 12]. Patients with TRAS usually present with worsening or refractory hypertension, fluid retention and/or graft dysfunction without evidence of rejection [7]. Similar to bilateral renal artery stenosis or unilateral stenosis in a solitary kidney, the renin–angiotensin–aldosterone system (RAAS) is activated in TRAS. This leads to sodium and fluid retention, and patients may develop edema, congestive heart failure or recurrent bouts of pulmonary edema. In the present case, the patient presented with hypertensive crisis and flash pulmonary edema. Patients with flash pulmonary edema classically present with sudden onset of severe, unprovoked dyspnea with normal left ventricular systolic function [13]. Renal artery stenosis and flash pulmonary edema is a unique entity with distinct pathophysiological, clinical and therapeutic features. It has been named Pickering Syndrome to honor Thomas G. Pickering, who first described it [13]. In 1988, Pickering et al. [14] reported a series of 11 patients with atheromatous renovascular hypertension and history of multiple episodes of pulmonary edema. Since then, there have been a number of case reports confirming this entity. In the present case, the presence of flash pulmonary edema in the setting of hypertension crisis led to prompt diagnosis of TRAS followed by therapeutic interventions, specifically surgical embolization of the allograft and then transplant nephrectomy, thus preventing potential complications from the hypertensive crisis.
Pathophysiology
TRAS has multiple causes, and usually occurs close to the sites of surgical anastomoses. In a case series of 38 patients, stenosis at different sites was recognized: stenosis of the recipient artery (pre-anastomotic, in the iliac artery), stenosis of the suture line and stenosis of the donor renal artery [12]. The causes that have been identified include atheroma in the donor artery, suture techniques, trauma to the donor or recipient artery during procurement or transplantation as well as immune-mediated vascular damage [7, 12]. Suture errors were thought more likely to occur in end-to-end anastomoses because of the difference in texture and caliber between the donor and recipient vessels [12]. In addition, end-to-end anastomoses may cause turbulent flow or disturbed hemodynamics, which could be responsible for the development of stenosis. However, the evidence is contradictory [15, 16]. Stenosis that occurs years after transplantation usually reflects atherosclerotic disease either of the transplant renal artery or of the adjacent proximal iliac artery [17]. Diffuse stenosis may reflect immune-mediated endothelial damage. This was evidenced by the similar histologic changes seen between the stenosed arteries and the vessels of renal allograft rejection [12, 18]. Furthermore, post-anastomotic TRAS has been shown to be associated with de novo Class II donor-specific antibodies [19]. In rare cases, TRAS or narrowing can occur due to extrinsic mechanical compression. For example, there has been a case report of transplant renal artery compression by enlarged native polycystic kidneys causing clinical features [20]. Similarly, in the present case, mechanical compression of a pseudoaneurysm caused transplant renal artery narrowing and the clinical features of TRAS.
Renovascular hypertension that occurs in the setting of TRAS is similar to Goldblatt's ‘one-kidney, one-clip’ experimental model on hypertension [21]. Pathologist Harry Goldblatt established the first animal model of hypertension in 1934, by testing the effect of experimentally induced renal ischemia on blood pressure in dogs [22]. In his ‘two-kidney, one-clip’ model, a clip is applied to the renal artery of one kidney to induce ipsilateral renal hypoperfusion. This activates the RAAS, causing hypertension with sodium and fluid retention. The elevated blood pressure leads to pressure natriuresis in the contralateral normal kidney. Therefore, the overall volume status is normal or decreased, and the renin level is high in the ‘two-kidney, one-clip’ model. In the ‘one-kidney, one-clip’ model, a clip is applied to one kidney's artery while the contralateral kidney is removed. Renal hypoperfusion again results in activation of the RAAS leading to sodium retention and volume expansion, but there is no compensatory mechanism. Thus, in a steady state, there is sustained hypertension and volume expansion, and plasma renin activity is normal or low. One difference between TRAS and the ‘one-kidney, one-clip’ model is that the transplant kidney is denervated, thus kidney hypoperfusion does not directly elicit the sympathetic response that is normally triggered by renal ischemia [23]. In dogs, the critical degree of renal artery stenosis to cause a decrease in renal perfusion pressure was determined to be >50% of the diameter, and the minimum degree of stenosis needed to cause hypertension was 70% of the diameters, with the kidney being either innervated or denervated [24].
In Pickering Syndrome, flash pulmonary edema develops in patients with critical bilateral renal artery stenosis or renal artery stenosis in a solitary kidney. It occurs when there is an abrupt imbalance of pulmonary fluid homeostasis and damage to the pulmonary capillary endothelium, leading to accumulation of fluid within the pulmonary interstitium and alveoli [25]. It is a dramatic form of acute decompensated heart failure. Flooding of the alveolar space can occur within minutes resulting in a life-threatening condition. Messerli et al. [13] proposed three pathophysiological mechanisms that may predispose patients with bilateral renal artery stenosis to develop flash pulmonary edema. The three mechanisms were defective natriuresis leading to sodium and fluid retention, exacerbation of diastolic dysfunction from increased blood pressure and damage of pulmonary capillary blood-gas barrier from increased intracapillary pressure and release of neurohumoral mediators, such as angiotensin II, catecholamines and endothelin-1. In other words, RAAS activation and sympathetic nervous system overactivity in TRAS could lead to damage to pulmonary capillary and fluid accumulation in pulmonary interstitium and alveoli, thus the development of flash pulmonary edema.
Differential diagnosis
There are several causes of hypertension after kidney transplantation. The risk factors for post-transplant hypertension include native kidney disease in the recipients, donor age, cold ischemia time, delayed graft function, allograft rejection and use of immunotherapy such as corticosteroid and calcineurin inhibitors [26]. Calcineurin inhibitors, cyclosporine more than tacrolimus, play a dominant role in raising blood pressure after kidney transplantation [27, 28]. This is also true in other solid organ transplantation. Among heart transplant recipients, Shiba et al. [29] found a 10-year incidence of 83.8% in developing hypertension in the cyclosporine era. One of the mechanisms that calcineurin inhibitors raise blood pressure by is increasing the release of vasoconstrictors, such as endothelin [30, 31]. In this patient, worsening hypertension was initially attributed to the use of immunotherapy, specifically tacrolimus and corticosteroids. However, despite escalating the dosage of antihypertensive medications, he still developed hypertensive crisis.
In the present case, Page kidney was also a differential diagnosis after renal ultrasound revealed a large mass located adjacent to the allograft. Page kidney occurs when a large subcapsular hematoma causes pressure-induced ischemia, leading to RAAS activation and systemic hypertension [32, 33]. Page kidney was first described by Irvine H. Page in 1939 when he wrapped kidneys in cellophane to cause renal parenchymal compression and noted the induction of hypertension [34]. Page kidney is often associated with blunt trauma [35] and iatrogenic intervention, such as allograft or native kidney biopsy [36, 37]. Although spontaneous Page kidney has been described in the kidney transplant literature [38], it usually occurs as a complication of allograft biopsy [39, 40]. Page kidney in an allograft is generally associated with acute hypertension and renal insufficiency [35, 37]. Surgical evacuation of the subcapsular hematoma is sometimes warranted to preserve renal function and normalize renin-mediated hypertension. In this patient, Page kidney became a differential diagnosis for hypertensive crisis and acute kidney injury after a large mass was discovered adjacent to the allograft on ultrasound. However, subsequent computed tomographic (CT) scan revealed that hematoma was not subcapsular and therefore did not cause renal parenchymal compression or Page kidney.
Diagnosis
The definitive diagnosis of TRAS requires the use of invasive angiography: conventional or digital subtraction angiography [3, 41]. Conventional angiography utilizes a relatively large amount of iodinated contrast, and thus poses a risk of developing contrast-induced acute kidney injury [42, 43]. Digital subtraction angiography has been shown to correlate highly with conventional angiography and uses a smaller amount of contrast material. This makes digital subtraction angiography a more suitable choice in the evaluation of TRAS [44, 45]. Although angiography procedures are diagnostic, they are invasive and may cause various complications, such as thromboembolism, pseudoaneurysms, traumatic arteriovenous fistulas and hematomas [3]. Therefore, angiographic techniques are not used as a screening tool, but are reserved for either patients with inconclusive results on the non-invasive screening tests or patients with TRAS requiring treatment [3].
The following non-invasive tests are reasonable alternatives for initial testing of TRAS: duplex sonography, isotope renography, CT angiography and contrast-enhanced magnetic resonance angiography (MRA). Duplex sonography is commonly utilized as an initial tool to investigate allograft dysfunction since it can be performed safely regardless of renal function [46]. Acceleration time in the transplant renal and intrarenal arteries ≥0.1 s, peak systolic velocity in the transplant renal artery >200 cm/s and a ratio of peak systolic velocity in the transplant renal-to-external iliac arteries >1.8 are used to diagnose TRAS [47]. Elevated peak systolic velocity in the transplant renal artery is the most sensitive Doppler criterion for the detection of high-grade TRAS [48]. In the present case, peak systolic velocity in the transplant renal artery was 457 cm/s. This led to the diagnosis of TRAS and the subsequent digital subtraction angiography. However, Duplex sonography is highly operator-dependent, and there may be technical difficulties in assessing transplant vessels [3, 46]. Contrast-enhanced ultrasound can complement standard sonographic examination in the evaluation of TRAS by providing a quick and non-invasive assessment of graft perfusion [49–51]. Longer time of contrast agent inflow indicates the presence of stenosis, and the rate of contrast agent inflow has been shown to be positively correlated with severity of arterial stenosis on cross-sectional imaging [49]. Isotope renography (basal or after renin-angiotensin system stimulation) can also be used as a non-invasive screening procedure for TRAS, but it is limited by its relatively low specificity of 67% [52]. CT angiography is a widely available and utilized tool for accurate and non-invasive diagnosis of TRAS [53, 54]. This technique provides three-dimensional images of the vascular anatomy and depicts stenotic areas that are highly correlated with the findings on selective angiography [3, 55]. In addition to being non-invasive, CT angiography requires lesser volume of iodinated contrast than angiography [3, 55]. Similar to CT angiography, contrast-enhanced MRA can also accurately depict arterial anatomy, detect and grade transplant artery stenosis with the advantage of avoiding radiation exposure and uses relatively non-nephrotoxic gadolinium-based contrast agents [46, 56–58]. In a small study (n = 27), no significant difference was observed in diagnostic accuracy between contrast-enhanced CT angiography and gadolinium-enhanced MRA in the assessment of hemodynamically significant TRAS [59]. However, impaired renal function may prevent administration of gadolinium-based agents due to the risk of nephrogenic systemic sclerosis [58, 60]. Advanced non-contrast MRA techniques also allow imaging of post-transplant vascular anatomy with a high degree of accuracy [61–63]. For instance, an MRA technique using spatial labeling and multiple inversion pulses was reported to have a positive predictive value of 91% for diagnosing high-grade stenosis in the transplant artery, while electrocardiogram-gated non-enhanced three-dimensional steady-state free precession MRA also had 91% in diagnostic accuracy [61, 62]. The pros and cons of the non-invasive tests are listed in Table 2. Duplex sonography is typically the initial test of choice to diagnose TRAS.
Table 2.
Comparison of non-invasive tests for transplant renal artery stenosis
| Non-invasive tests | Advantage | Disadvantage |
|---|---|---|
| Duplex sonography [3, 46] | No use of contrast agents, no radiation, inexpensive, high sensitivity (87–94%), high specificity (86–100%) | Operator-dependent, time-consuming, can be technically difficult especially in patients with complex anatomy of vessels |
| Isotope renography [52] | Good sensitivity (75%) may be predictive of physiologically meaningful renal artery stenosis | Low specificity (67%) |
| Computed tomography angiography [53–55] | Three-dimensional images allow direct visualization of vessels in optimal projection, shorter examination, not operator- dependent | Radiation, use of iodinated contrast |
| Magnetic resonance angiography [3, 46, 53, 54, 56–58] | Three-dimensional images, high sensitivity (67–100%), high specificity (75–100%), no radiation, no iodinated contrast | Artifacts from adjacent surgical clips, claustrophobia, high cost, patient hardware compatibility, use of gadolinium, limited availability |
Therapy
If a patient presents with Pickering Syndrome, hemodynamic unloading using antihypertensive drugs usually results in the prompt resolution of flash pulmonary edema. A loop diuretic may be used to initiate natriuresis to overcome sodium retention [13]. Although inhibitors of the RAAS could improve flash pulmonary edema empirically, they may further compromise renal perfusion. Therefore, these should not be used unless serum creatinine and potassium levels are in the normal range. In this patient, he developed oliguric acute kidney injury and ended up needing emergent renal replacement therapy to avoid acute respiratory decompensation.
Recognition of TRAS is important because it is potentially treatable. There are three treatment modalities: medical therapy alone, percutaneous transluminal angioplasty and surgical revascularization each with medical therapy. If renal function is stable and there is no hemodynamically significant stenosis on imaging, conservative treatment with antihypertensive medications can be used to control blood pressure [3]. If there is uncontrolled hypertension, worsening renal function or progression of stenosis, revascularization is warranted. In dogs with hypertension induced by constriction of the renal artery to a sole remaining kidney, release of renal artery constriction decreased the mean arterial blood pressure over a 3-day period and resulted in a significant negative sodium and fluid balance [64]. Renal revascularization improves renal perfusion, and decreases circulating angiotensin and aldosterone levels. This results in improvement in kidney function, natriuresis and a decrease in blood pressure.
Percutaneous transluminal angioplasty with stent placement is thought to be the first-line therapy to correct the stenosis. In general, it works well for lesions that are short, linear and distal from the anastomosis [65]. In a retrospective review of 547 renal transplants performed over a 6-year period, Greenstein et al. [16] reported that percutaneous transluminal angioplasty resulted in immediate cure or improvement in 76% of patients at a mean follow-up period of 30 months. Similarly, Chew et al. [66] reported an overall clinical success rate of 76.9% in a 10-year retrospective study. However, there are also other studies reporting a lower success rate. For example, Merkus et al. [67] reported only a third of percutaneous transluminal angioplasty procedures resulted in a definitive correction of the stenosis in their case series. Furthermore, in the USRDS registry, 145 of the 823 patients with TRAS underwent an angioplasty procedure, and no significant improvement in overall allograft survival was observed with angioplasty compared with without angioplasty (P = 0.4) [9]. There are also several complications associated with percutaneous intervention. They include renal artery dissection, stent restenosis, thromboembolism, hematoma and pseudoaneurysms at the puncture site [68]. There is currently no randomized controlled trial examining the efficacy of percutaneous transluminal angioplasty over medical therapy alone.
Open revision surgery is considered as a rescue therapy and reserved for cases of unsuccessful angioplasty. Surgical techniques include resection and revision of the anastomosis, saphenous vein bypass graft of the stenotic segment, localized endarterectomy and excision/reimplantation of the renal artery [69]. Success rate seems to be comparable with percutaneous transluminal angioplasty [3]. However, there is a high risk of surgical complications that can result in graft loss and high mortality.
In the present case, transplant renal artery narrowing was caused by compression of a pseudoaneurysm. There were three donor renal arteries. Two of them were on a common aortic patch, while the third one was on a separate aortic patch. The third renal artery was transected during procurement and repaired. Two separate aortic cuff anastomoses were made to the external iliac artery. These multiple anastomoses could have contributed to the development of the pseudoaneurysm. Because transplant renal artery narrowing in this case was caused by the extrinsic compression of the pseudoaneurysm, the therapeutic strategy was different from a typical case of TRAS. Therefore, we focused the treatment on the control of the renal artery pseudoaneurysm, which can be repaired through either open or endovascular techniques. Historically, open repair was the standard of the care, but endovascular techniques are becoming more popular nowadays [70]. In this case, renal ultrasound with duplex showed the radiographic features of TRAS and the presence of a pseudoaneurysm. This was confirmed and further characterized by angiography. During the angiogram, endovascular repair such as coil embolization or balloon-assisted coiling [71] was not attempted, because patient continued to have hypertensive crisis and developed respiratory distress. The following day, he went to the operating room after he received renal replacement therapy with ultrafiltration. In the operation room, we attempted endovascular repair of the pseudoaneurysm, but were unsuccessful due to the complexity of the transplant renal vessel anatomy. In addition, due to extensive abdominal adhesion from prior surgeries, open surgical repair such as aneurysmectomy or arteriorrhaphy could not be performed either. Finally, a covered endoluminal stent was placed to the external iliac artery, and this led to surgical embolization of the allograft and subsequent allograft nephrectomy.
Conclusion
It is important to recognize TRAS in patients with post-transplant hypertension because it is associated with allograft loss and high mortality rate, and more importantly because it is potentially treatable. TRAS should be high on the differential diagnosis list especially when a kidney transplant recipient presents with hypertensive crisis and flash pulmonary edema, or Pickering Syndrome. Duplex sonography is commonly used as a screening tool, but a more definitive diagnosis requires invasive angiography. Percutaneous transluminal angioplasty with stent placement is generally thought to be the first-line therapy for TRAS. However, due to the nature of the disease, most of the studies were retrospective and the conclusions were drawn based on single-center experience. There is no randomized controlled trial to examine the efficacy and safety of percutaneous transluminal angioplasty compared with medical therapy alone or surgical intervention.
Funding
This publication was supported by National Institutes of Health (NIH) grant T32 DK007110 (WC) and by Clinical and Translational Science Award (CTSA) grants 1 UL1 TR001073 (WC) from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of interest statement
None declared.
References
- 1.Kasiske BL, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–1081. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Aguera Fernandez LG, Zudaire JJ, Isa WA, et al. [Vascular complications in 237 recipients of renal transplant from cadaver] Actas Urol Esp. 1992;16:292–295. [PubMed] [Google Scholar]
- 3.Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15:134–141. doi: 10.1097/01.asn.0000099379.61001.f8. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava A, Kumar J, Sharma S, et al. Vascular complication in live related renal transplant: an experience of 1945 cases. Indian J Urol. 2013;29:42–47. doi: 10.4103/0970-1591.109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 6.Patel NH, Jindal RM, Wilkin T, et al. Renal arterial stenosis in renal allografts: retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219:663–667. doi: 10.1148/radiology.219.3.r01jn30663. [DOI] [PubMed] [Google Scholar]
- 7.Fervenza FC, Lafayette RA, Alfrey EJ, et al. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31:142–148. doi: 10.1053/ajkd.1998.v31.pm9428466. [DOI] [PubMed] [Google Scholar]
- 8.Rengel M, Gomes-Da-Silva G, Inchaustegui L, et al. Renal artery stenosis after kidney transplantation: diagnostic and therapeutic approach. Kidney Int Suppl. 1998;68:S99–S106. doi: 10.1038/sj.ki.4490573. [DOI] [PubMed] [Google Scholar]
- 9.Hurst FP, Abbott KC, Neff RT, et al. Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am J Nephrol. 2009;30:459–467. doi: 10.1159/000242431. [DOI] [PubMed] [Google Scholar]
- 10.Ghirardo G, De Franceschi M, Vidal E, et al. Transplant renal artery stenosis in children: risk factors and outcome after endovascular treatment. Pediatr Nephrol. 2014;29:461–467. doi: 10.1007/s00467-013-2681-7. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JP, Ascher NL, Fryd DS, et al. Transplant renal artery stenosis. Transplantation. 1989;48:580–583. [PubMed] [Google Scholar]
- 12.Lacombe M. Arterial stenosis complicating renal allotransplantation in man: a study of 38 cases. Ann Surg. 1975;181:283–288. doi: 10.1097/00000658-197503000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messerli FH, Bangalore S, Makani H, et al. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. Eur Heart J. 2011;32:2231–2235. doi: 10.1093/eurheartj/ehr056. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Herman L, Devereux RB, et al. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet. 1988;2:551–552. doi: 10.1016/s0140-6736(88)92668-2. [DOI] [PubMed] [Google Scholar]
- 15.Morris PJ, Yadav RV, Kincaid-Smith P, et al. Renal artery stenosis in renal transplantation. Med J Aust. 1971;1:1255–1257. [PubMed] [Google Scholar]
- 16.Greenstein SM, Verstandig A, McLean GK, et al. Percutaneous transluminal angioplasty. The procedure of choice in the hypertensive renal allograft recipient with renal artery stenosis. Transplantation. 1987;43:29–32. [PubMed] [Google Scholar]
- 17.Becker BN, Odorico JS, Becker YT, et al. Peripheral vascular disease and renal transplant artery stenosis: a reappraisal of transplant renovascular disease. Clin Transplant. 1999;13:349–355. doi: 10.1034/j.1399-0012.1999.130412.x. [DOI] [PubMed] [Google Scholar]
- 18.Porter KA, Thomson WB, Owen K, et al. Obliterative vascular changes in four human kidney homotransplants. Br Med J. 1963;2:639–645. doi: 10.1136/bmj.2.5358.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willicombe M, Sandhu B, Brookes P, et al. Postanastomotic transplant renal artery stenosis: association with de novo class II donor-specific antibodies. Am J Transplant. 2014;14:133–143. doi: 10.1111/ajt.12531. [DOI] [PubMed] [Google Scholar]
- 20.Lee L, Gunaratnam L, Sener A. Transplant renal artery stenosis secondary to mechanical compression from polycystic kidney disease: a case report. Can Urol Assoc J. 2013;7:E251–E253. doi: 10.5489/cuaj.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldblatt H, Lynch J, Hanzal RF, et al. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Epps HL. Harry Goldblatt and the discovery of renin. J Exp Med. 2005;201:1351. doi: 10.1084/jem.2019fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz E, Amann K, Fliser D. The sympathetic nervous system and the kidney: its importance in renal diseases. Blood Press Suppl. 1998;3:14–19. doi: 10.1080/080370598438429. [DOI] [PubMed] [Google Scholar]
- 24.Imanishi M, Akabane S, Takamiya M, et al. Critical degree of renal arterial stenosis that causes hypertension in dogs. Angiology. 1992;43:833–842. doi: 10.1177/000331979204301006. [DOI] [PubMed] [Google Scholar]
- 25.Rimoldi SF, Yuzefpolskaya M, Allemann Y, et al. Flash pulmonary edema. Prog Cardiovasc Dis. 2009;52:249–259. doi: 10.1016/j.pcad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Mangray M, Vella JP. Hypertension after kidney transplant. Am J Kidney Dis. 2011;57:331–341. doi: 10.1053/j.ajkd.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Textor SC, Canzanello VJ, Taler SJ, et al. Cyclosporine-induced hypertension after transplantation. Mayo Clin Proc. 1994;69:1182–1193. doi: 10.1016/s0025-6196(12)65772-3. [DOI] [PubMed] [Google Scholar]
- 28.First MR, Neylan JF, Rocher LL, et al. Hypertension after renal transplantation. J Am Soc Nephrol. 1994;4(Suppl 8):S30–S36. doi: 10.1681/ASN.V48s30. [DOI] [PubMed] [Google Scholar]
- 29.Shiba N, Chan MC, Kwok BW, et al. Analysis of survivors more than 10 years after heart transplantation in the cyclosporine era: Stanford experience. J Heart Lung Transplant. 2004;23:155–164. doi: 10.1016/S1053-2498(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y, Miyamori I, Wu P, et al. Effects of an endothelin receptor antagonist in rats with cyclosporine-induced hypertension. Hypertension. 1995;26(6 Pt 1):932–936. doi: 10.1161/01.hyp.26.6.932. [DOI] [PubMed] [Google Scholar]
- 31.Perico N, Ruggenenti P, Gaspari F, et al. Daily renal hypoperfusion induced by cyclosporine in patients with renal transplantation. Transplantation. 1992;54:56–60. doi: 10.1097/00007890-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Massumi RA, Andrade A, Kramer N. Arterial hypertension in traumatic subcapsular perirenal hematoma (Page kidney). Evidence for renal ischemia. Am J Med. 1969;46:635–639. doi: 10.1016/0002-9343(69)90082-5. [DOI] [PubMed] [Google Scholar]
- 33.Spark RF, Berg S. Renal trauma and hypertension: the role of renin. Arch Intern Med. 1976;136:1097–1100. [PubMed] [Google Scholar]
- 34.Page IH. A method for producing persistent hypertension by cellophane. Science. 1939;89:273–274. doi: 10.1126/science.89.2308.273. [DOI] [PubMed] [Google Scholar]
- 35.Smyth A, Collins CS, Thorsteinsdottir B, et al. Page kidney: etiology, renal function outcomes and risk for future hypertension. J Clin Hypertens (Greenwich) 2012;14:216–221. doi: 10.1111/j.1751-7176.2012.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung J, Caumartin Y, Warren J, et al. Acute Page kidney following renal allograft biopsy: a complication requiring early recognition and treatment. Am J Transplant. 2008;8:1323–1328. doi: 10.1111/j.1600-6143.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- 37.McCune TR, Stone WJ, Breyer JA. Page kidney: case report and review of the literature. Am J Kidney Dis. 1991;18:593–599. doi: 10.1016/s0272-6386(12)80656-1. [DOI] [PubMed] [Google Scholar]
- 38.Butt FK, Seawright AH, Kokko KE, et al. An unusual presentation of a Page kidney 24 days after transplantation: case report. Transplant Proc. 2010;42:4291–4294. doi: 10.1016/j.transproceed.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 39.Cromie WJ, Jordan MH, Leapman SB. Pseudorejection: the Page kidney phenomenon in renal allografts. J Urol. 1976;116:658–659. doi: 10.1016/s0022-5347(17)58953-3. [DOI] [PubMed] [Google Scholar]
- 40.Patel TV, Goes N. Page kidney. Kidney Int. 2007;72:1562. doi: 10.1038/sj.ki.5002580. [DOI] [PubMed] [Google Scholar]
- 41.Gedroyc WM, Reidy JF, Saxton HM. Arteriography of renal transplantation. Clin Radiol. 1987;38:239–243. doi: 10.1016/s0009-9260(87)80055-7. [DOI] [PubMed] [Google Scholar]
- 42.Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 43.Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268:719–728. doi: 10.1148/radiol.13122276. [DOI] [PubMed] [Google Scholar]
- 44.Picus D, Neeley JP, McClennan BL, et al. Intraarterial digital subtraction angiography of renal transplants. AJR Am J Roentgenol. 1985;145:93–96. doi: 10.2214/ajr.145.1.93. [DOI] [PubMed] [Google Scholar]
- 45.Rath M, Castro L, Schuler M, et al. Digital subtraction angiography in the diagnosis of arterial complications after renal transplantation. Eur J Radiol. 1984;4:34–37. [PubMed] [Google Scholar]
- 46.Browne RF, Tuite DJ. Imaging of the renal transplant: comparison of MRI with duplex sonography. Abdom Imaging. 2006;31:461–482. doi: 10.1007/s00261-005-0394-5. [DOI] [PubMed] [Google Scholar]
- 47.de Morais RH, Muglia VF, Mamere AE, et al. Duplex Doppler sonography of transplant renal artery stenosis. J Clin Ultrasound. 2003;31:135–141. doi: 10.1002/jcu.10147. [DOI] [PubMed] [Google Scholar]
- 48.Baxter GM, Ireland H, Moss JG, et al. Colour Doppler ultrasound in renal transplant artery stenosis: which Doppler index? Clin Radiol. 1995;50:618–622. doi: 10.1016/s0009-9260(05)83291-x. [DOI] [PubMed] [Google Scholar]
- 49.Grzelak P, Kurnatowska I, Nowicki M, et al. Detection of transplant renal artery stenosis in the early postoperative period with analysis of parenchymal perfusion with ultrasound contrast agent. Ann Transplant. 2013;18:187–194. doi: 10.12659/AOT.883896. [DOI] [PubMed] [Google Scholar]
- 50.Rennert J, Farkas S, Georgieva M, et al. Identification of early complications following pancreas and renal transplantation using contrast enhanced ultrasound (CEUS)—first results. Clin Hemorheol Microcirc. 2013 doi: 10.3233/CH-131675. Feb 4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Ciccone MM, Cortese F, Fiorella A, et al. The clinical role of contrast-enhanced ultrasound in the evaluation of renal artery stenosis and diagnostic superiority as compared to traditional echo-color-Doppler flow imaging. Int Angiol. 2011;30:135–139. [PubMed] [Google Scholar]
- 52.Erley CM, Duda SH, Wakat JP, et al. Noninvasive procedures for diagnosis of renovascular hypertension in renal transplant recipients—a prospective analysis. Transplantation. 1992;54:863–867. doi: 10.1097/00007890-199211000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann LV, Smith PA, Kuszyk BS, et al. Three-dimensional helical CT angiography in renal transplant recipients: a new problem-solving tool. AJR Am J Roentgenol. 1999;173:1085–1089. doi: 10.2214/ajr.173.4.10511184. [DOI] [PubMed] [Google Scholar]
- 54.Sebastia C, Quiroga S, Boye R, et al. Helical CT in renal transplantation: normal findings and early and late complications. Radiographics. 2001;21:1103–1117. doi: 10.1148/radiographics.21.5.g01se131103. [DOI] [PubMed] [Google Scholar]
- 55.Sun IO, Hong YA, Kim HG, et al. Clinical usefulness of 3-dimensional computerized tomographic renal angiography to detect transplant renal artery stenosis. Transplant Proc. 2012;44:691–693. doi: 10.1016/j.transproceed.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Hwang JK, Chun HJ, Kim JM, et al. Contrast-enhanced magnetic resonance angiography in the early period after kidney transplantation. Transplant Proc. 2013;45:2925–2930. doi: 10.1016/j.transproceed.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 57.Gufler H, Weimer W, Neu K, et al. Contrast enhanced MR angiography with parallel imaging in the early period after renal transplantation. J Magn Reson Imaging. 2009;29:909–916. doi: 10.1002/jmri.21726. [DOI] [PubMed] [Google Scholar]
- 58.Jain R, Sawhney S. Contrast-enhanced MR angiography (CE-MRA) in the evaluation of vascular complications of renal transplantation. Clin Radiol. 2005;60:1171–1181. doi: 10.1016/j.crad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Gaddikeri S, Mitsumori L, Vaidya S, et al. Comparing the diagnostic accuracy of contrast-enhanced computed tomographic angiography and gadolinium-enhanced magnetic resonance angiography for the assessment of hemodynamically significant transplant renal artery stenosis. Curr Probl Diagn Radiol. 2014;43:162–168. doi: 10.1067/j.cpradiol.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Bellin MF, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008;66:160–167. doi: 10.1016/j.ejrad.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 61.Tang H, Wang Z, Wang L, et al. Depiction of transplant renal vascular anatomy and complications: unenhanced MR angiography by using spatial labeling with multiple inversion pulses. Radiology. 2014;271:879–887. doi: 10.1148/radiol.14131800. [DOI] [PubMed] [Google Scholar]
- 62.Lanzman RS, Voiculescu A, Walther C, et al. ECG-gated nonenhanced 3D steady-state free precession MR angiography in assessment of transplant renal arteries: comparison with DSA. Radiology. 2009;252:914–921. doi: 10.1148/radiol.2531082260. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Berg N, Sheehan J, et al. Renal transplant: nonenhanced renal MR angiography with magnetization-prepared steady-state free precession. Radiology. 2009;251:535–542. doi: 10.1148/radiol.2512081094. [DOI] [PubMed] [Google Scholar]
- 64.Liard JF, Cowley AW, Jr, McCaa RE, et al. Renin, aldosterone, body fluid volumes, and the baroreceptor reflex in the development and reversal of Goldblatt hypertension in conscious dogs. Circ Res. 1974;34:549–560. doi: 10.1161/01.res.34.4.549. [DOI] [PubMed] [Google Scholar]
- 65.Benoit G, Moukarzel M, Hiesse C, et al. Transplant renal artery stenosis: experience and comparative results between surgery and angioplasty. Transpl Int. 1990;3:137–140. doi: 10.1007/BF00355459. [DOI] [PubMed] [Google Scholar]
- 66.Chew LL, Tan BS, Kumar K, et al. Percutaneous transluminal angioplasty of transplant renal artery stenosis. Ann Acad Med Singapore. 2014;43:39–43. [PubMed] [Google Scholar]
- 67.Merkus JW, Huysmans FT, Hoitsma AJ, et al. Renal allograft artery stenosis: results of medical treatment and intervention. A retrospective analysis. Transpl Int. 1993;6:111–115. doi: 10.1007/BF00336655. [DOI] [PubMed] [Google Scholar]
- 68.Seratnahaei A, Shah A, Bodiwala K, et al. Management of transplant renal artery stenosis. Angiology. 2011;62:219–224. doi: 10.1177/0003319710377076. [DOI] [PubMed] [Google Scholar]
- 69.Shames BD, Odorico JS, D'Alessandro AM, et al. Surgical repair of transplant renal artery stenosis with preserved cadaveric iliac artery grafts. Ann Surg. 2003;237:116–122. doi: 10.1097/00000658-200301000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orion KC, Abularrage CJ. Renal artery aneurysms: movement toward endovascular repair. Semin Vasc Surg. 2013;26:226–232. doi: 10.1053/j.semvascsurg.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Yang M, Song L, et al. Endovascular treatment of renal artery aneurysms and renal arteriovenous fistulas. J Vasc Surg. 2013;57:765–770. doi: 10.1016/j.jvs.2012.09.042. [DOI] [PubMed] [Google Scholar]