Abstract
There is a strong rationale for renal denervation (RDN) of the native kidneys in kidney transplant recipients with treatment-resistant hypertension. We present a patient with a stable graft function, who underwent RDN for posttransplant therapy-resistant hypertension (24-h ambulatory blood pressure measurement (ABPM) 143/89 mmHg, while compliantly using five different antihypertensive agents). After RDN, BP measurements and orthostatic complaints required withdrawal of two antihypertensive agents and halving a third. At 6 months, ABPM was 134/84 mmHg and allograft function remained unchanged. This case calls for designing well-designed prospective studies on RDN in kidney transplant recipients.
Keywords: kidney transplantation, renal denervation
Background
Hypertension in kidney transplant recipients has a prevalence of 75–90% [1] and is a major modifiable cardiovascular risk factor [2]. Moreover, hypertension jeopardizes graft function. However, in 8–24% of kidney transplant recipients, hypertension treatment targets are not reached despite intense medical treatment [1]. This condition has been coined ‘post-renal transplant therapy-resistant hypertension’ [3].
The last resort therapy for ‘post-renal transplant therapy-resistant hypertension’ is a bilateral nephrectomy of the native kidneys. This procedure results in clinically significant decreases in blood pressure (BP) [4]. However, the benefits of improved BP control usually do not balance the high peri-operative morbidity risks.
In recent years, the observations on bilateral native nephrectomy as antihypertensive treatment founded the rationale for catheter-based renal denervation (RDN) in non-renal transplant patients [5]. Subsequently, RDN has been elaborated for treatment of resistant hypertension. The proof-of-concept Symplicity HTN-1 study (2010) [5, 6] and the Symplicity HTN-2 trial (randomized controlled against medical therapy alone, 2012) [7], reported BP effects similar to that of bilateral native nephrectomies.
Against this background we set out to assess the feasibility of RDN of the native kidneys of patient with post-renal transplant therapy-resistant hypertension (Netherlands Trial Registry 3866) by performing an uncontrolled clinical study in 20 consecutive patients. This study was approved by the local medical ethics committee. Shortly after RDN in our first included patient, the randomized sham-intervention controlled Symplicity HTN-3 trial [8] showed no benefit of RDN compared with sham intervention in general treatment-resistant hypertensive patients. This finding took away the rationale for our study design and therefore we discontinued the study. Here, we report on our single patient receiving RDN.
Case report
We studied a 50-year-old Caucasian male patient who had received a renal allograft from a non-heart beating donor in 2004. His original renal disease was IgA nephropathy. Mean 24-h ambulatory BP measurement (ABPM) (WatchBP O3, Microlife, Inc.) was 143/89 mmHg while compliantly using nifedipine 60 mg 1dd, doxazosine 4 mg 1dd, metoprolol 200 mg 1dd, aliskiren 150 mg 1dd and furosemide 80 mg 1dd. In the past years he had been using an increasing number of antihypertensive agents with for blood pressure beyond targets. These induced inconvenient side effects. The renal graft function had been stable over a period of >3 years, with a creatinine level of 208 µmol/L and a measured creatinine clearance of 37 mL/min while using mycophenolate mofetil 2000 mg and prednisolone 10 mg. Left ventricular hypertrophy was diagnosed using the ECG voltage criteria for left ventricular hypertrophy. No cardiac ultrasound was available.
On abdominal ultrasound both native kidneys appeared atrophied (length of the right kidney was 5.6 cm and the left 5.0 cm). The right renal native artery was 5.8 mm in diameter, sufficient for access of the ablation catheter. No flow was visualized in the left native renal artery and therefore we decided to perform an angiography peri-procedurally to further assess its accessibility.
The patient provided oral and written informed consent. The renal denervation procedure was performed via the left femoral artery under sedation (propofol 14 mg and alfentanil 300 mcg) and with an intravenous hydration protocol of 2000 mL sodium chloride 0.9% peri-procedurally. The angiography revealed that both native renal arteries were accessible, but no filling of parenchymal intrarenal vessels could be visualized in either kidney. RDN was performed using the Symplicity Catheter System (Medtronic), in a spiral pattern with 4 radiofrequency ablation pulses to the right and 7 pulses to the left native renal artery (Figure 1). To prevent ‘over-heating’ in the left artery, shorter denervation pulses were applied. No peri-procedural complications occurred. A validated questionnaire on antihypertensive medication compliance with Hill Bone to High Blood Pressure Therapy Scale [9] was taken before the intervention and at every follow-up visit.
Fig. 1.
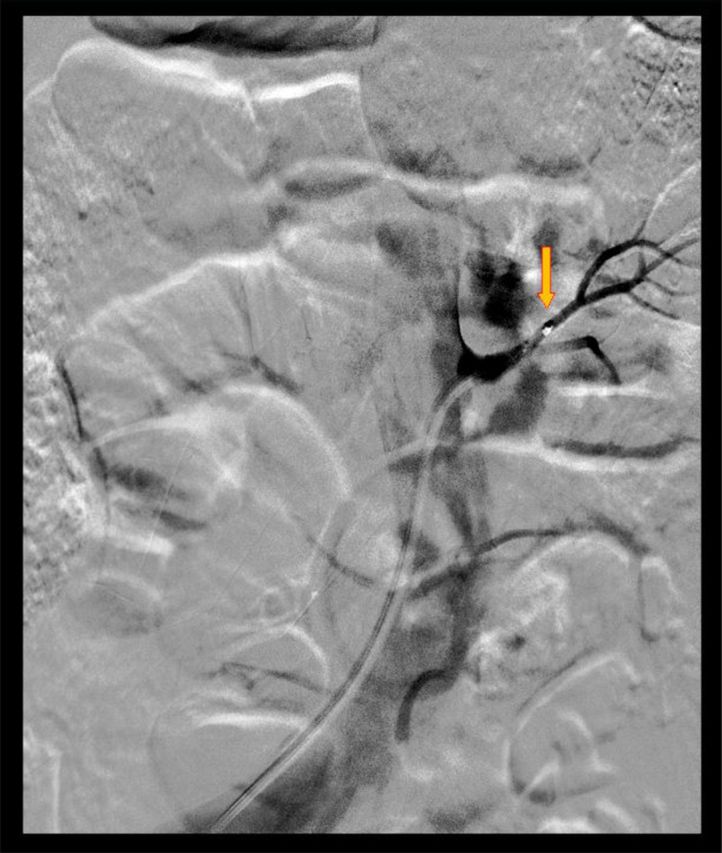
Renal denervation of the native left kidney. The arrow points the tip of the radiofrequency ablation catheter. Filling of the renal arteries with contrast-agent is shown; however no filling of intrarenal parenchymal vessels is visualized.
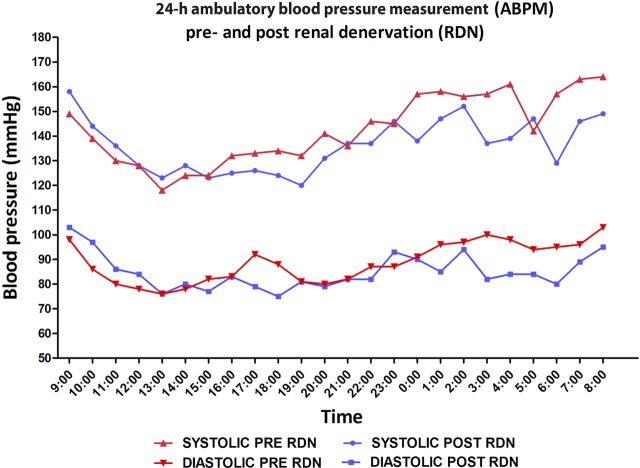
Two weeks after the procedure, office BP measurement fell from 139/93 to 127/82 mmHg. Moreover, the patient developed orthostatic complaints that allowed stepwise withdrawal of nifedipine and doxazosine and reduction of metoprolol by 50%. At 6 months, ABPM showed a mean of 134/84 mmHg (−9/−5 mmHg), while on treatment with metoprolol 100 mg 1dd, furosemide 80 mg 1dd and aliskiren 150 mg 1dd (Figure 2). The absent dipping pattern, i.e. the nocturnal BP drop, was not reversed after RDN. The plasma creatinine levels up to 6 months after the procedure remained roughly unchanged (208 µmol/L pre-RDN to 185 µmol/L post-RDN) nor the creatinine clearance changed (37 mL/min pre-RDN to 41 mL/min post-RDN) (Figure 1). Also, proteinuria did not alter (0.04–0.05 g/L) and medication compliance remained equal as assessed by the Hill-Bone Compliance scale. An ECG performed 18 months after RDN revealed a decrease in voltages compared with an ECG before the RDN, indicating a possible reduction of left ventricular mass.
Fig. 2.
Results of the 24-h ambulatory blood pressure measurement (ABPM). The ABPM after RDN was recorded while the patient had discontinued two antihypertensive agents and one drug dosage was reduced to 50%.
Discussion
This is the first reported case of catheter-based renal denervation in a kidney transplant recipient. The case does not prove the efficacy of renal denervation in posttransplant hypertension per se. However, it illustrates that renal denervation of the native kidneys is feasible. The rationale to apply renal denervation to non-functioning native kidneys seems stronger than the rationale to apply RDN in so called ‘treatment-resistant hypertension’ in patients without kidney disease [10]. For kidney transplant recipients it can serve as an alternative to native nephrectomy and the (native) kidney function is not set at risk. Also, if RDN is successful the total daily drug dose might be decreased and it might increase adherence in this population with a high burden of daily drug intake. There is only one case description that reports on blood pressure reduction after renal denervation in a patient with ESRD on dialysis [11].
However, the lesson from the Symplicity HTN-1,-2,-3 saga is that the effectiveness of renal denervation can only be reliably studied in a sham-intervention controlled study design [12]. Therefore we discontinued our uncontrolled open label study. Also in our patient we cannot rule out that the blood pressure effects are due to increased medication compliance, such as has been suggested to explain the blood pressure changes as observed in Symplicity HTN-1 and -2. This case provides an argument for performing a sham-intervention controlled trial on RDN in kidney transplant recipients. RDN could be an attractive alternative for bilateral nephrectomy for treatment of post-renal transplant therapy-resistant hypertension.
Conflict of interest statement
C.T.P.K. is supported by grants IP-11.40 and KJPB12.29 from the Dutch Kidney Foundation and ZONMW Clinical Fellowship (40007039712461). These grants are gratefully acknowledged.
References
- 1.Dobrowolski LC, Bemelman FJ, van Donselaar-van der Pant KAMI, et al. Treatment efficacy of hypertension in Dutch kidney transplant recipients. Neth J Med. 2014;72:258–263. [PubMed] [Google Scholar]
- 2.Mange KC, Cizman B, Joffe M. Arterial hypertension and renal allograft survival. J Am Med Assoc. 2000;283:633–638. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 3.Arias M, Fernandez-Fresnedo G, Gago M, et al. Clinical characteristics of resistant hypertension in renal transplant patients. Nephrol Dial Transplant. 2012;27(Suppl 4):iv36–iv38. doi: 10.1093/ndt/gfs481. [DOI] [PubMed] [Google Scholar]
- 4.Curtis JJ, Diethelm AG, Luke RG, et al. Benefits of removal of native kidneys in hypertension after renal transplantation. Lancet. 1985;2:739–742. doi: 10.1016/s0140-6736(85)90627-0. [DOI] [PubMed] [Google Scholar]
- 5.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 6.Krum H, Schlaich MP, Böhm M, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2013;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 7.Esler M, Krum H, Sobotka PA, et al. Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 9.Krousel-Wood M, Munter P, Jannu A, et al. Reliability of a medication adherence measure in an outpatient setting. Am J Med Sci. 2005;330:128–133. doi: 10.1097/00000441-200509000-00006. [DOI] [PubMed] [Google Scholar]
- 10.de Beus E, de Jager R, Joles JA, et al. Sympathetic activation secondary to chronic kidney disease: therapeutic target for renal denervation? J Hypertens. 2014;32:1751–1761. doi: 10.1097/HJH.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 11.Di Daniele N, De Francesco M, Violo L, et al. Renal sympathetic nerve ablation for the treatment of difficult-to-control or refractory hypertension in a haemodialysis patient. Nephrol Dial Transplant. 2012;27:1689–1690. doi: 10.1093/ndt/gfs044. [DOI] [PubMed] [Google Scholar]
- 12.Persu A, Jin Y, Lengel JP, et al. Con: renal denervation for all resistant hypertensive patients: the Emperor's new clothes. Nephrol Dial Transplant. 2014;29:1116–1119. doi: 10.1093/ndt/gfu098. [DOI] [PMC free article] [PubMed] [Google Scholar]