Abstract
The ultimate aim of biomedical research is to preserve health and improve patient outcomes. However, by a variety of measures, preservation of kidney health and patient outcomes in kidney disease are suboptimal. Severe acute kidney injury has been treated solely by renal replacement therapy for over 50 years and mortality still hovers at around 50%. Worldwide deaths from chronic kidney disease (CKD) increased by 80% in 20 years––one of the greatest increases among major causes of death. This dramatic data concur with huge advances in the cellular and molecular pathophysiology of kidney disease and its consequences. The gap appears to be the result of sequential roadblocks that impede an adequate flow from basic research to clinical development [translational research type 1 (T1), bench-to-bed and back] and from clinical development to clinical practice and widespread implementation (translational research T2) that supported by healthcare policy-making reaches all levels of society throughout the globe (sometimes called translational research T3). Thus, it is more than 10 years since the introduction of the last new-concept drug for CKD patients, cinacalcet; and 30 years since the introduction of reninangiotensin system (RAS) blockade, the current mainstay to prevent progression of CKD, illustrating the basic science-clinical practice disconnect. Roadblocks from clinical advances to widespread implementation, together with lag time-to-benefit may underlie the 20 years since the description of the antiproteinuric effect of RAS blockade to the observation of decreased age-adjusted incidence of endstage renal disease due to diabetic kidney disease. Only a correct understanding of the roadblocks in translational medicine and a full embracement of a translational research culture will spread the benefits of the biomedical revolution to its ultimate destinatary, the society.
Keywords: acute kidney injury, chronic kidney disease, implementation, registry-based clinical trials, review
Introduction
The ultimate aim of biomedical research, be it basic or clinical, is to preserve health and improve patient outcomes. However, by a variety of measures, patient outcomes in kidney disease are dismal. This implies that either the basic pathophysiology knowledge base is insufficient, or this knowledge base has not translated into preventive or therapeutic clinical interventions or these clinical interventions do exist but have not been extensively implemented or a mixture of all of these factors. In short, there is a real need for more, higher quality and more coordinated translational nephrology. We now review some current key clinical practice issues and discuss what translational research means and how it could be applied to accelerate translation of biomedical advances to solve issues in kidney health.
Current clinical practice issues in nephrology
The ultimate consequence of kidney disease is organ failure, either as acute kidney injury (AKI) or as chronic kidney disease (CKD), and there are serious unmet clinical needs related to both (Table 1).
Table 1.
Some examples of serious unmet needs in nephrology that can only be solved through a translational research approach
| Realm | Diagnosis and prognosis | Prevention | Therapy |
|---|---|---|---|
| AKI | No appropriate gold standard for diagnosis. Current definitions based on insensitive and non-specific parametres | Few resources beyond haemodynamic stability and hydration | No therapy beyond replacement of renal function. |
| CKD | No appropriate diagnostic method that may be used in routine practice to ascertain aetiology of most causes of CKD | High prevalence of CKD of unknown or unclear cause derails prevention efforts | Most conditions causing CKD lack specific pathogenesis-based therapy |
| No objective easy-to-use assay that allows early stratification based on risk of progression of CKD or complications or response to therapy for many conditions | Little understanding of causes underlying CKD hotspots | No regression-inducing therapy | |
| Little evidence to support much needed early interventions that decrease mortality or prevent progression | Non-specific nephroprotection based on 80s drugs. | ||
| No therapeutic approaches to prevent non-specific progression of non-proteinuric kidney disease | Non-specific immune suppression for autoimmune diseases | ||
| Treatment of complications of CKD begins too late: prevention needed | |||
| Implementation and public health | Worldwide most kidney diseases remain undiagnosed | Worldwide most basic preventive efforts for kidney diseases are unavailable or non-applied and this is true even for segments of society within richer countries | Even the few therapeutic options available remain out of reach for most of the world's population |
| Underlying causes of CKD hotspots unclear | Unclear causes of CKD hotspots prevent effective prevention programmes | Unclear causes of CKD hotspots prevent development of specific therapies | |
| Clinical trials | Trials on diagnosis mostly non-existent | Very few trials on prevention | Few trials in therapies compared with other specialties, methodological or design flaws frequent, underpowered in number of participants or follow-up |
| Inadequate or non-specific diagnostic criteria or risk stratification based on biomarkers or pharmacogenomic profiling for participants in clinical trials | |||
| Organ replacement | Non-specific immune suppression for transplantation | ||
| Kidneys cannot be generated in vitro |
AKI
Mortality of severe AKI remains high at around 50% [1]. However, we do not even know the global extent of the problem [2]. Renal replacement therapy by various dialysis or haemofiltration techniques is the only known therapy for established severe AKI, as it was in the fifties [3, 4]. Techniques for renal replacement therapy have evolved, but there has been no conceptual breakthrough in therapy of AKI for >50 years. Clinical trials of drugs for AKI based on animal research have repeatedly failed [1]. Moreover, the recent realization that contrary to conventional belief, AKI does have long-term consequences on renal function has compounded the problem and added another angle to the issue [5]: we need to not only treat the acute episode but to provide therapy that effectively prevents the long-term consequences of AKI.
CKD
CKD does not fare better. According to the Global Burden of Disease 2010 (GBD 2010) study, CKD is among the top three fastest growing main causes of death worldwide: the absolute number of deaths from CKD has increased by 82% in the past 20 years [6]. Age-standardized death rates from CKD increased by 15% in this period while rates for most diseases fell, including other non-transmissible diseases such as major vascular diseases, chronic pulmonary disorders, most forms of cancer and liver cirrhosis [6] (Figure 1). In this regard, despite general advances in patient care, the mortality of chronic kidney failure (CKF) patients remains 10- to 100-fold higher than in the age-matched general population [7]. The higher mortality of CKF patients cannot be pinpointed to a single cause and is evident for both cardiovascular and non-cardiovascular causes [7]. In some cases, nephrology has lagged behind other specialties. Haemodialysis is now the most frequent cause of catheter-related bacteraemia in the USA, after new standards of care have dramatically decreased catheter-related bacteraemia in the intensive care unit [8].
Fig. 1.

Worldwide age-standardized death rates for major non-communicable diseases. Per cent change 1990–2010 according to the Global Burden of Disease 2010 (GBD 2010) study [6]. CKD is among the few causes of death from non-communicable diseases that increased in the past 20 years. CKD, chronic kidney disease; CVD, cardiovascular and circulatory diseases.
Causes of CKD
Among the frequent causes of CKD, therapy for diabetic kidney disease (DKD) is still based on drugs first shown to decrease proteinuria in this condition in the mid-eighties [9]. Major clinical trial after major clinical trial has failed to identify novel therapeutic approaches that can be used in daily clinical practice as add-on to renin-angiotensin system blockade to improve outcomes [10]. Hypertensive kidney disease is the second most frequent cause of CKF in the USA and in at least some European countries [11, 12]. However, its mere existence has been called into question as hypertensive kidney disease is diagnosed mainly in African Americans, and in this population CKD has been linked to a genetic variant in the ApoL1 gene that confers resistance to infection by Trypanosoma brucei [12, 13]. The therapeutic implications of this finding are potentially huge, since it may be interpreted as hypertension being a manifestation rather than a cause of kidney disease. There is similar confusion regarding the prevalence of hypertensive nephropathy in Europe [12]. The adjusted incident rates of RRT per million population for hypertensive nephropathy ranges from 38.8 in Iceland to 4.2 in Finland and 4.2 in Scotland. In European countries that did report the existence of non-hypertensive renal vascular disease as a cause of RRT, the ratio of hypertensive to non-hypertensive vascular disease as a cause of RRT ranged from 0.5 in Croatia and Austria (reflecting a predominance of non-hypertensive vascular causes) to 32 in Norway (reflecting a preponderance of hypertensive vascular causes of CKF) [12]. A pathogenic therapeutic approach cannot be prescribed if the cause of the nephropathy is unclear or even explicitly unknown, which is the case for 2–60% of RRT patients in various European countries [14]. Glomerulonephritis, interstitial nephropathies and cystic diseases are additional major causes of CKF. Current understanding of glomerular disease is still not enough to provide specific pathogenic therapy. Glomerulonephritides are still treated with either non-specific proteinuria-lowering medication or non-specific immune suppressants [15]. Moreover, the cellular and molecular basis for the success of some of these drugs is still unknown and there is debate on whether immune suppression or direct effects on renal cells is key to the mechanism of action [16, 17]. Finally, glomerulonephritides are still classified based on morphological criteria that date back to the seventies. This most probably represents an oversimplification that throws into the same morphological basket different conditions in terms of severity and progression potential that may require very different therapeutic approaches. It may be hypothesized that a molecular classification, similar to that used for some malignancies, may provide insights into pathogenesis and, thus, to susceptibility to individualized therapeutic approaches [18]. Our understanding of primary chronic tubulointerstitial nephropathies is not better, and most lack specific diagnostic criteria and therapy. It is yet unknown whether general management of CKD may result in improved outcomes for autosomal dominant polycystic kidney disease (ADPKD) in terms of preservation of renal function [19, 20], and tolvaptan, which received market authorization in Japan to treat ADPKD based on beneficial effects on surrogate end points, was rejected by the FDA [21].
Consequences of CKD
The situation regarding the pathophysiological consequences of CKD also has some shadows. Most trials aiming at reducing mortality in CKF failed to meet the primary end point [7]. The last new- concept drug to be brought to market was cinacalcet—10 years ago. Current understanding of CKD metabolic bone disease suggests that the process is initiated very early in the course of progressive CKD and that low Klotho, high FGF23 levels and subclinical abnormalities of phosphate balance may contribute to cardiovascular disease before increased serum phosphate levels develop [22]. However, only the current phosphate binders can be used and are recommended once hyperphosphataemia is present [23]. A recent meta-analysis supporting the superiority of some phosphate binders over others with regards to patient survival [24, 25] has not impacted guidelines, and critical voices have been raised. In this regard, there is no consensus on the most basic issues of CKD management, which negatively impacts on implementation. As examples, in 2012–14, various international guidelines recommended or suggested different targets for blood pressure or different indications for statin therapy for CKD patients [26–30]. At the clinical practice level, this originates ‘guidelines wars’ between specialists and confusion for patients and healthcare decision-makers that interferes with implementation. An effort should be made by guideline-issuing bodies to ‘meta-analyze’ guidelines, reach across specialties to agree on some basic tenets and cooperate in shedding light onto disputed concepts.
Fortunately there is some light at the end of the tunnel. Since the early 2000s, a stabilization or even decrease in the incidence rates of CKF has been observed in the USA, although the absolute number of incident cases continues to increase [11]. This is true for several nephropathies, including DKD (Figure 2). In addition, recent major advances in nephrology have benefited patients with rare diseases. Success stories include the introduction of eculizumab to treat atypical haemolytic uraemic syndrome (2011) and paroxysmal nocturnal haemoglobinuria (2007) [31]. However, it took 11–15 years from the development of eculizumab to market authorization in Europe [32] and implementation is still in progress in richer countries but has not started in poorer societies, illustrating the uphill road even for successful drugs. However, many drugs did not make it through Phase 3 clinical trials despite promising Phase 2 trials, such as bardoxolone [33]. A careful analysis of the reasons underlying this failure should be performed. In some trials, the reasons for failure are clearer. The TREAT trial of darbepoietin in non-dialysis DKD patents did not make adequate use of well-known biomarkers. Thus, based on the low required transferrin saturation for study entry, the higher use of intravenous iron in placebo-treated patients, the improvement of haemoglobin values over time in placebo patients, and the association of lower iron deposits with impaired erythropoietic responses to darbepoietin, it is now clear that the cause of anaemia in an indeterminate but probably significant number of TREAT participants was iron deficiency [34, 35]. Thus, guidelines today provide advice on erythropoiesis-stimulating agents (ESA) in CKD based in part on a trial in which ESA was used to treat iron deficient anaemia patients. Furthermore, although the trial was not powered to detect differences with such a low number of patients (n = 370, ∼1/10 of the whole study patients), under the nephrology care conditions of Western Europe and Australia, the hazard ratio for the primary composite end point was 0.66 (0.43–1.01) [34]. Other potentially groundbreaking drugs may never make it into the nephrology realm. Ataluren was recently conditionally approved to treat Duchenne's muscular dystrophy in the European Union [36]. This oral drug promotes read-through premature stop codons and holds promise to treat genetic diseases caused by nonsense point mutations [37], among them 5–30% of the main kidney hereditary diseases [38]. However, in recent trials in cystic fibrosis, the incidence of nephrotoxicity was high among ataluren-treated patients (18 versus 1% in placebo), thus providing a barrier for studies in kidney disease [39].
Fig. 2.
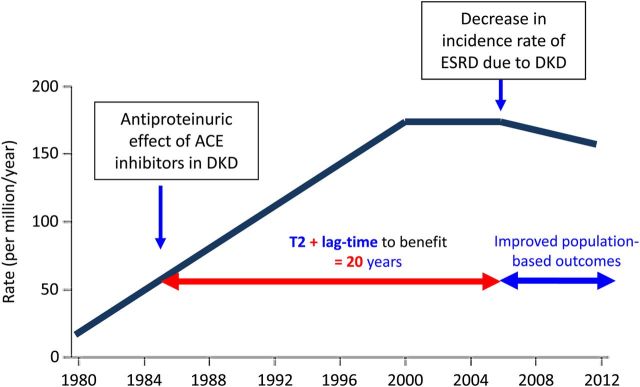
Potential effects of type 2 (T2) translation on improved patient outcomes versus lag time to benefit. Trends in adjusted chronic kidney failure (CKF) due to diabetic kidney disease (DKD) incidence rate, per million/year, in the US population, 1980–2012. The antiproteinuric effect of ACE inhibitors in DKD was described in 1985 [9]. However, it was not until the 2000s that a decrease in the adjusted incidence of CKF due to DKD was observed. One potential contributor to this late decrease is the lag-time to widespread implementation T2 of nephroprotection with RAS-targeting agents from the mid-nineties. [Modified and adapted from United States Renal Data System, 2014 annual data report: An overview of the epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government].
Organ replacement
Kidney replacement is also moving at a slow pace. The last groundbreaking drug to be approved for maintenance immune suppression in kidney transplantation and in widespread use was rapamycin in 2001. Immunosuppression is still non-specific. Finally, we are still not able to generate new functioning kidneys in vitro [40, 41].
Implementation and socioeconomic factors
CKD and CKF are not evenly distributed. CKD hotspots are known in countries, regions or ethnicities [42]. Part of the differences in incidence is explained by their higher frequency in disadvantaged populations throughout the world [43]. Indeed this is the subject of the World Kidney Day 2015 [44], and the causes are multifactorial but insufficient implementation of routine clinical care, standard living conditions and education of the population and authorities is part of the problem.
Thus, nephrology is at present in dire straits, marred by a lack of translation of basic science knowledge into direct benefits for patients and for patients-to-be that results in an ever-growing absolute incidence of AKI and CKF and associated mortality. How to speed up the delivery of solutions is the realm of translational research in general and translational nephrology in particular.
What is translational research?
Many of the current practical issues in nephrology can be traced to deficiencies in translational research. The essence of translational research is to apply biomedical advances to benefit the society in the form of improved patient outcomes or preservation of health. There is ample consensus on the importance of translational research. New journals have been named after the concept (Science Translational Medicine, Translational Research, Journal of Translational Medicine and others). National and international funding bodies such as the United States National Institutes of Health and Clinical and Translational Science Award (CTSA) program, the European Union Innovative Medicines Initiative (IMI) or the Horizon 2020 programme, the European Clinical Research Infrastructures Network (ECRIN) and its national subsidiaries, the German Centres for Health Research, the Spanish Instituto de Salud Carlos III Acción Estratégica en Salud and Institutos de Investigación Sanitaria (e.g. http://www.fjd.es/iis_fjd/) and even the recent ERA-EDTA research programme call on biomarkers emphasize translational research. However, despite the agreement on the general concept of translational research, there is some disagreement as to nomenclature and the detailed definition of its phases or even whether phases should be recognized at all [45–51] (Figure 3).
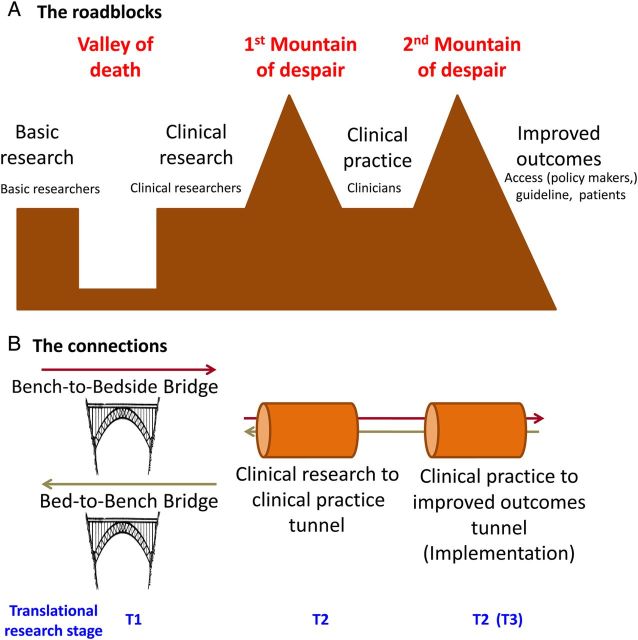
Fig. 3.
The ‘valley of death’ and the ‘mountains of despair’. The term valley of death has been previously used to describe the chasm between biomedical researchers and the patients who need their discoveries [51]. Here the valley illustrates the disconnection between basic researchers and clinical researchers. Both bench-to-bedside and bed-to-bench bridges are necessary to bridge the gap. However, success in clinical research does not automatically result in improved patient outcomes or preserved health. Two mountains of despair lag between clinical knowledge and patients. The first one involves regulatory and business model issues that may delay marketing of a successful solution. The second one involves education of stakeholders (doctors, healthcare professionals, healthcare decision-makers, patients) and policies that allow widespread implementation of the solution across socioeconomic and geographical barriers. Tunnels are required to sort out these obstacles. The image of a mountain to depict difficulties in implementing technologies that have shown some measure of success in humans was previously used to illustrate the plight of novel biomarkers [52].
The Institute of Medicine described two roadblocks in translational research [46]. Type 1 (T1) translation was described as ‘the transfer of new understandings of disease mechanisms gained in the laboratory into the development of new methods for diagnosis, therapy and prevention and their first testing in humans.’ This fits into the traditional bench-to-bedside concept. However, we should not forget that translational research is a two-way road, and bed-to-bench research is needed to guide research priorities and questions. The very descriptive term ‘valley of death’ was previously used to describe the chasm between biomedical researchers and the patients who need their discoveries [51]. T2 translation was defined as ‘the translation of results from clinical studies into everyday clinical practice and health decision-making’. This gap between technology that appears to work in humans and marketing-authorization and eventual widespread use has been likened to a mountain that the higher you climb the more previously occult higher peaks you discover that have to be climbed [52]. We have termed these the ‘mountains of despair’ (Figure 3). In this regard, sometimes drugs with successful results in Phase 2 trials fail on decisive Phase 3 trials or only prove effective on surrogate markers or raise safety flags, and the reasons for failure may remain unclear (drug not useful for that indication?, wrong dose-range?, wrong follow-up?, wrong study subjects?, wrong sample size assumptions?), but drug development is abandoned because of a variety of potential reasons that may not be directly related to the chances of success of novel trials (no funding?), to the despair of physicians and patients alike. In this regard, the excess rigidity and consumption of resources of clinical trials has been criticized [53]. As summarized by Woolf, T1 needs basic and clinical scientists to unravel molecular and cell biology problems in order to design and test novel diagnostic and therapeutic approaches in humans [45]. The end result may be a novel diagnostic test or therapeutic intervention. T2 picks up the new test or intervention and deals with implementation to really improve health in the public realm. T2 thus requires population-based interventions, practice-based research networks, clinical epidemiology and evidence synthesis, communication theory, behavioural science, public policy, financing, organizational theory, system redesign, informatics and qualitative research [45]. Within T2, some authors recognize a T3 practice-based research, which is required for distilled knowledge (e.g. systematic reviews, guidelines) to be implemented in practice [45, 49].
Part of the growing gap between basic science and the clinic has been attributed to the decreasing role of MDs or PhD/MDs in leading biomedical research efforts when compared with PhDs: the gap in number of NIH grants led by a PhD or an MD has increased 10-fold, from around 500 to around 5000 projects [51]. In this regard, one of the missions of the new CKJ is to propagate the values of translational nephrology, to increase awareness of the advances and unmet needs in each of these areas of knowledge among major stakeholders (basic researchers, clinical researchers, practicing clinicians, epidemiologists and other kidney-related specialists), so as to build bridges over the valley of death and tunnels that bypass the mountains of despair (Figure 3).
Translational nephrology: translational research in nephrology
Translational research in nephrology should address the unmet needs regarding preservation of kidney health and improvement of outcomes for patients with kidney disease as enumerated in the initial section of the manuscript. This often entails multidisciplinary and multicentre collaborations. In addition to a shift towards an overall focus on translation in biomedical research, structures have been created to specifically foster translation. Currently there are a number of such initiatives ongoing in Europe that emphasize translational nephrology. These include the French F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists) network (http://www.fcrin.org/en/support-tools/ini-crct-cardio-renal-diseases) and the Spanish REDINREN (Red de Investigación Renal) network (http://redinren.org/) [54, 55].
Two large ongoing European Union-funded research projects focus on translational nephrology. PRIORITY aims at the early identification of type 2 diabetes patients at risk for subsequent development of DKD by using a urinary peptidomics panel [56] and testing the clinical use of such risk stratification to select patients for preventive approaches to DKD. Thus, at-risk patients will be randomized to placebo or spironolactone to prevent DKD (http://www.eu-priority.org). EURenOmics is using high-throughput technologies to find new genes causing or predisposing to kidney diseases, characterize molecular signatures unique to individual disease entities, identify prognostic biomarkers and screen for potential drug candidates (http://www.eurenomics.eu/).
On a broader perspective, translational issues in AKI and CKD were recently reviewed [57, 58]. Overall, the following strategies need to be implemented to foster translational nephrology (Table 2).
Table 2.
Strategies for translational nephrology
| Development of novel diagnostic, risk stratification and individualization tools to personalize therapeutic approaches |
| Development of novel preventive and therapeutic approaches based on aetiologic and pathophysiological insights |
| Optimize clinical trial design and supportive structures |
| Optimize the scope and role of registries |
| Emphasize type 2 translation leading to widespread implementation and accessibility of proven medical approaches |
Development of novel diagnostic, risk stratification and individualization tools to personalize therapeutic approaches
A new sensitive and specific gold-standard definition of AKI is required that together with novel biomarkers contributes to staging and stratifying patients according to aetiology/pathophysiology and prognosis and need for intervention [57]. This would facilitate recognition of AKI and allow for homogenization of patients recruited into clinical trials and eventually to the implementation of the precise therapeutic agent.
Accurate, sensitive, specific and non-invasive diagnostics tests should be developed that allow the identification of the aetiology of CKD in the high proportion of patients that reach CKF with an unknown or unclear aetiology. This may require the study of patients at the earlier stages of kidney disease. The creation of a cohort of young adults without prior known kidney disease with sequential clinical and analytical follow-up and sequential biobanking may provide the materials that allow the identification of early markers of kidney disease.
A molecular or pathophysiological classification of kidney diseases and specifically glomerulonephritis is needed that completes the currently available morphological or clinicopathological ones. This should identify specific molecular signatures and targets and predict progression and response to therapy in order to guide the indication and monitoring of specific therapeutic approaches. Systems biology approaches applied to blood, urine or kidney tissue in humans or experimental systems and hold promise for such classification or identification of components of the classification [59–61]. The recent identification of the autoantigen of membranous nephropathy as the M-type phospholipase A2 receptor (PLA2R) and the development of clinical assays is a step in the right direction that requires validation of its precise role in the assessment and therapeutic decision-making, integration into guidelines and extensive implementation [62, 63].
Imaging techniques should advance in order to allow repetitive, non-invasive monitoring of kidney inflammation and fibrosis and assessment of diverse kidney functions in a dynamic manner that allows characterization of active versus chronic lesions and progressing versus stable chronic lesions.
Development of novel preventive and therapeutic approaches based on aetiologic and pathophysiological insights
Nephrology needs novel therapeutic approaches for unmet needs, and these can only be developed by a precise and detailed understanding of pathophysiological events, the evaluation of preclinical models relevant to the human situation and the improved design of lower cost clinical trials.
The importance of representative animal models cannot be overemphasized [64]. Continuing with the membranous nephropathy example, the availability for over 50 years of a rat model, Heymann nephritis, allowed the identification of molecular mediators of podocyte injury. However, there was no clinical translation. The identification of PLA2R as the human autoantigen and the characterization of the major PLA2R epitopes will allow the development of more relevant animal models as well as novel and more specific therapeutic approaches, including antibody inhibition therapy and immunoadsorption of circulating autoantibodies [65, 66]. In this regard, the array of potential therapeutic tools is continuously expanding from the realm of small molecules to therapeutic proteins, therapeutic cells and other molecular biology tools such as antisense oligonucleotides, gene therapy and others. The microRNA-122 (miR-122) targeting the drug miravirsen was recently successfully tested in human HCV infection, although it might be nephrotoxic [67, 68].
The bed-to-bench interaction should intervene at every step of clinical development. As an example, the introduction of enzyme replacement therapy (ERT) for Fabry disease was expected to solve the enzymatic defect and prevent progression of nephropathy. However, ERT failed to prevent progression once nephropathy was present [69], raising the need to better understand the molecular mediators of tissue injury. A glycolipid not very responsive to ERT, lyso-Gb3, was found to promote fibrosis, a hallmark of the disease, in podocytes, and this was prevented by vitamin D receptor activators [70, 71]. Back to the clinic, paricalcitol was later reported to reduce proteinuria in Fabry nephropathy [72].
Optimize clinical trial design and supportive structures
Clinical trials are one of Achilles heels of translational nephrology. In other areas of medicine there is growing concern over excess complexity, expense and time required for recruitment of study participants, as well as inadequate representativeness of enrollees for the general patient population [73]. In addition, the number of randomized controlled trials (RCT) published in nephrology from 1966 to 2002 was 50 to 90% lower than in every other medical specialties and no secular increase was observed in the last 5 years of the study [74]. The glomerulonephritis field was particularly poor in trials. Overall, the quality of RCT reporting in nephrology was low and had not improved for 30 years. It was proposed to emphasize using standard guidelines and checklists for trial reporting, improving reporting of loss to analysis, better describing the methods and improving the design of multicentre, larger and simpler trials [74, 75]. A 2014 systematic review of Clinicaltrials.gov disclosed that only <3% of trials were classified under nephrology [76]. Most nephrology trials were for treatment (75%) or prevention (16%), with very few diagnostic, screening, or health services research studies. Nephrology trials were more likely than cardiology trials to be smaller (64 versus 48% enrolling ≤100 patients), Phases I–II, unblinded and to include a drug intervention. The higher representation of Phase I/II trials may be interpreted positively as a sign of interest to search for new drugs for kidney disease. Thus, an effort should be made to increase the scope, quality and quantity of clinical trials within nephrology. In this regard, national or international collaborative structures, especially if linked to basic researchers like the above-mentioned F-CRIN INI-CRCT and REDINREN hold promise. REDINREN through the Spanish Society of Nephrology workgroup GLOSEN is providing logistical support for the ERA-EDTA-funded STARMEN trial of conventional immune suppression versus rituximab-tacrolimus in membranous nephropathy while at the same time addressing the molecular basis of the benefit provided by tacrolimus. The Australasian Kidney Trials Network was formed in 2005 to improve RCT quality and quantity and is an example to follow [77].
A major issue in nephrology RCTs is the definition of adequate surrogate end points. The emphasis on hard end points results in a catch 22 situation: since in many kidney diseases the natural history is measured in decades, advanced cases must be enrolled in order to get sufficient events. However, advanced cases may be less responsive to therapy or require different therapeutic approaches and trials fail. In this regard, the qualification of novel biomarkers that may be used as surrogate end points also requires long follow-up times. This stalls research and deprives patients of biomedical advances. Voices have risen for a more practical approach [52]. In this regard, part of the solution may be to optimize the scope and role of registries.
Optimize the scope and role of registries
Advances in electronic databases and big data management and analysis have renewed and expanded the role of registries in clinical research. In the rare diseases, field registries may be the only source of new large-scale clinical knowledge [69]. In addition, novel roles have been envisioned. Large-scale observational registries are still ill-fitted to provide hard comparative effectiveness data, owing to the absence of randomization. However, registry-based randomized trials may complement the strengths and address the weaknesses of registries and trials by lowering costs, facilitating recruitment and providing real-world populations created from consecutively enrolled registry patients [78, 79]. Registry-based biobanks may also provide crucial information. For example, the ERA-EDTA Registry may identify CKD hotspots within Europe and the availability of big data and a Registry-linked biobank may allow developing and testing hypotheses regarding contributing factors, either genetic, environmental or dependent on socioeconomic or organizational factors. Advances in analytical tools that require ever lower sample sizes for analysis and implementation of dried blood spot testing may facilitate the effort.
Emphasize type 2 translation
Research should also focus on identifying factors and strategies that lead to adoption, maintenance, and sustainability of science-based interventions in practice to provide high-quality care and better health outcomes once interventions are known to be beneficial. Essential elements are multidisciplinary team care, health information technology and stakeholder engagement leading to patient safety and transitions; delivery of high-quality, evidence-based care and elimination of disparities within and between societies [58].
In summary, there is a growing gap between advances in basic research in the nephrology field and the development of clinical advances that may be used to preserve kidney health or improve the outcomes of kidney disease patients, and there is a further gap between clinical advances and their implementation in the community. CKJ will devote a series of CKJ reviews to address the unmet needs, the contributors to these gaps and potential solutions to speed up translation into clinical practice and implementation of biomedical science advances. The series will explore the barriers and facilitators to translational research in general and in Nephrology in Europe, Translational Research in Diagnosis, Translational Research in Prognosis, Translational Research in Therapy, and Translational Research in Prevention and Public Health. With these series we expect to provide the CKJ readership with a balanced view of the current state-of-the-art as well as with thought-provoking commentary that will help promote the values and aims of translational medicine in the nephrology community.
Conflict of interest statement
None declared.
Acknowledgements
FIS PS09/00447, PI13/00047, ISCIII-RETIC REDinREN RD12/0021, Comunidad de Madrid S2010/BMD-2378, CYTED IBERERC. Programa Intensificación Actividad Investigadora (ISCIII) to AO.
References
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Benigni A, Finkelstein FO, et al. Kidney failure: aims for the next 10 years and barriers to success. Lancet. 2013;382:353–362. doi: 10.1016/S0140-6736(13)60438-9. [DOI] [PubMed] [Google Scholar]
- 3.Bracey DW. Acute renal failure; two cases treated by decapsulation and peritoneal dialysis. Br J Surg. 1951;38:482–488. doi: 10.1002/bjs.18003815210. [DOI] [PubMed] [Google Scholar]
- 4.Fine J, Seligman A, Frank HA. Peritoneal dialysis for acute renal failure. NY Med. 1949;5:16–20. [PubMed] [Google Scholar]
- 5.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 6.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz A, Covic A, Fliser D, et al. Board of the EURECA-m Working Group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 8.James MT, Conley J, Tonelli M, et al. Meta-analysis: antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med. 2008;148:596–605. doi: 10.7326/0003-4819-148-8-200804150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Taguma Y, Kitamoto Y, Futaki G, et al. Effect of captopril on heavy proteinuria in azotemic diabetics. N Engl J Med. 1985;313:1617–1620. doi: 10.1056/NEJM198512263132601. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, et al. Therapeutic approaches to diabetic nephropathy-beyond the RAS. Nat Rev Nephrol. 2014;10:325–346. doi: 10.1038/nrneph.2014.74. [DOI] [PubMed] [Google Scholar]
- 11.Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. United States Renal Data System, 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 12.ERA-EDTA Registry. Annual Report 2012. http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2012.pdf. accessed 7 December 2014.
- 13.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipkowitz MS, Freedman BI, Langefeld CD, et al. SK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 16.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieson PW. Proteinuria and immunity—an overstated relationship? N Engl J Med. 2008;359:2492–2494. doi: 10.1056/NEJMcibr0806881. [DOI] [PubMed] [Google Scholar]
- 18.Queirós AC, Villamor N, Clot G, et al. A B-cell epigenetic signature defines three biologic subgroups of chronic lymphocytic leukemia with clinical impact. Leukemia. 2014 doi: 10.1038/leu.2014.252. doi:10.1038/leu.2014.252 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 19.Spithoven EM, Kramer A, Meijer E, et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86:1244–1252. doi: 10.1038/ki.2014.120. [DOI] [PubMed] [Google Scholar]
- 20.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evenepoel P, Rodriguez M, Ketteler M. Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Semin Nephrol. 2014;34:151–163. doi: 10.1016/j.semnephrol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD–MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD–MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 24.Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268–1277. doi: 10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz A, Sanchez-Niño MD. The demise of calcium-based phosphate binders. Lancet. 2013;382:1232–1234. doi: 10.1016/S0140-6736(13)61165-4. [DOI] [PubMed] [Google Scholar]
- 26.Stone NJ, Robinson J, Lichtenstein AH. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Gallegos-Villalobos A, Portolés J, Ortiz A. Application of the new cholesterol guidelines. N Engl J Med. 2014;371:77–78. doi: 10.1056/NEJMc1405680. [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Wanner C Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid management in chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med. 2014;160:182. doi: 10.7326/M13-2453. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 30.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 31. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000791/human_med_001055.jsp&mid=WC0b01ac058001d124. accessed 7 December 2014.
- 32.Thomas TC, Rollins SA, Rother RP, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 33.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 35.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363:1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 36.Ryan NJ. Ataluren: first global approval. Drugs. 2014;74:1709–1714. doi: 10.1007/s40265-014-0287-4. [DOI] [PubMed] [Google Scholar]
- 37.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 38.Torra R, Oliveira JP, Ortiz A. UGA hopping: a sport for nephrologists too? Nephrol Dial Transplant. 2010;25:2391–2395. doi: 10.1093/ndt/gfq301. [DOI] [PubMed] [Google Scholar]
- 39.Kerem E, Konstan MW, De Boeck K, et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2:539–547. doi: 10.1016/S2213-2600(14)70100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Sancho-Martinez I, Nivet E, et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc. 2014;9:2693–2704. doi: 10.1038/nprot.2014.182. [DOI] [PubMed] [Google Scholar]
- 41.Xia Y, Nivet E, Sancho-Martinez I, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 42.Martín-Cleary C, Ortiz A. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin Kidney J. 2014;7:519–523. doi: 10.1093/ckj/sfu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendy E, Hoy WE. Kidney disease in Aboriginal Australians: a perspective from the Northern Territory. Clin Kidney J. 2014;7:524–530. doi: 10.1093/ckj/sfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Garcia G, Jha V on behalf of the World Kidney Day Steering Committee. Chronic kidney disease (CKD) in disadvantaged populations. Clin Kidney J. 2015;8:3–6. doi: 10.1093/ckj/sfu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 46.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 47.Graham ID, Tetroe J. Nomenclature in translational research. JAMA. 2008;299:2148–2150. doi: 10.1001/jama.299.18.2149-a. [DOI] [PubMed] [Google Scholar]
- 48.Fiscella K, Bennett NM, Szilagyi PG. Nomenclature in translational research. JAMA. 2008;299:2148–2149. doi: 10.1001/jama.299.18.2148-b. [DOI] [PubMed] [Google Scholar]
- 49.Westfall JM, Mold J, Fagnan L. Practice-based research—‘blue highways’ on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 50.Trochim W, Kane C, Graham MJ, et al. Evaluating translational research: a process marker model. Clin Transl Sci. 2011;4:153–162. doi: 10.1111/j.1752-8062.2011.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler D. Translational research: crossing the valley of death. Nature. 2008;453:840–842. doi: 10.1038/453840a. [DOI] [PubMed] [Google Scholar]
- 52.Mischak H, Ioannidis JP, Argiles A, et al. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest. 2012;42:1027–1036. doi: 10.1111/j.1365-2362.2012.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477:526–528. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- 54.Calleros L, Cortés MA, Luengo A, et al. Start-up of a clinical sample processing, storage and management platform: organisation and development of the REDinREN Biobank. Nefrologia. 2012;32:28–34. doi: 10.3265/Nefrologia.pre2011.Oct.11121. [DOI] [PubMed] [Google Scholar]
- 55.Cortés MA, Irrazábal E, García-Jerez A, et al. Impact of implementing ISO 9001:2008 standard on the Spanish Renal Research Network biobank sample transfer process. Nefrologia. 2014;34:552–560. doi: 10.3265/Nefrologia.pre2014.Apr.12292. [DOI] [PubMed] [Google Scholar]
- 56.Siwy J, Schanstra JP, Argiles A, et al. Multicentre prospective validation of a urinary peptidome-based classifier for the diagnosis of type 2 diabetic nephropathy. Nephrol Dial Transplant. 2014;29:1563–1570. doi: 10.1093/ndt/gfu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarjou A, Sanders PW, Mehta RL, et al. Enabling innovative translational research in acute kidney injury. Clin Transl Sci. 2012;5:93–101. doi: 10.1111/j.1752-8062.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuttle KR, Tuot DS, Corbett CL, et al. Type 2 translational research for CKD. Clin J Am Soc Nephrol. 2013;8:1829–1838. doi: 10.2215/CJN.00130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martini S, Nair V, Keller BJ, et al. European Renal cDNA Bank; C-PROBE Cohort; CKDGen Consortium. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol. 2014;25:2559–2572. doi: 10.1681/ASN.2013080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ledo N, Ko YA, Park AS, et al. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014010028. pii: ASN.2014010028 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He JC, Chuang PY, Ma'ayan A, et al. Systems biology of kidney diseases. Kidney Int. 2012;81:22–39. doi: 10.1038/ki.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seitz-Polski B, Payré C, Ambrosetti D, et al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol Dial Transplant. 2014;29:2334–2342. doi: 10.1093/ndt/gfu252. [DOI] [PubMed] [Google Scholar]
- 64.Sanz AB, Sanchez-Niño MD, Martín-Cleary C, et al. Progress in the development of animal models of acute kidney injury and its impact on drug discovery. Expert Opin Drug Discov. 2013;8:879–895. doi: 10.1517/17460441.2013.793667. [DOI] [PubMed] [Google Scholar]
- 65.Fresquet M, Jowitt TA, Gummadova J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014050502. pii: ASN.2014050502 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kao L, Lam V, Waldman M, et al. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013121315. pii: ASN.2013121315 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Niño MD, Ortiz A. HCV infection and miravirsen. N Engl J Med. 2013;369:877–878. doi: 10.1056/NEJMc1307787. [DOI] [PubMed] [Google Scholar]
- 69.Warnock DG, Ortiz A, Mauer M, et al. Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transplant. 2012;27:1042–1049. doi: 10.1093/ndt/gfr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Niño MD, Sanz AB, Carrasco S, et al. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 2011;26:1797–1802. doi: 10.1093/ndt/gfq306. [DOI] [PubMed] [Google Scholar]
- 71.Weidemann F, Sanchez-Niño MD, Politei J, et al. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8:116. doi: 10.1186/1750-1172-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pisani A, Sabbatini M, Duro G, et al. Antiproteinuric effect of add-on paricalcitol in Fabry disease patients: a prospective observational study. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu273. doi:10.1093/ndt/gfu273. Epub ahead of print August 20, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Antman EM, Harrington RA. Transforming clinical trials in cardiovascular disease: mission critical for health and economic well-being. JAMA. 2012;308:1743–1744. doi: 10.1001/jama.2012.14841. [DOI] [PubMed] [Google Scholar]
- 74.Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004;15:411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- 75.Deo A, Schmid CH, Earley A, et al. Loss to analysis in randomized controlled trials in CKD. Am J Kidney Dis. 2011;58:349–355. doi: 10.1053/j.ajkd.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis. 2014;63:771–780. doi: 10.1053/j.ajkd.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morrish AT, Hawley CM, Johnson DW, et al. Establishing a clinical trials network in nephrology: experience of the Australasian Kidney Trials Network. Kidney Int. 2014;85:23–30. doi: 10.1038/ki.2013.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lauer MS, D'Agostino RB., Sr The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–1581. doi: 10.1056/NEJMp1310102. [DOI] [PubMed] [Google Scholar]
- 79.Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]