Abstract
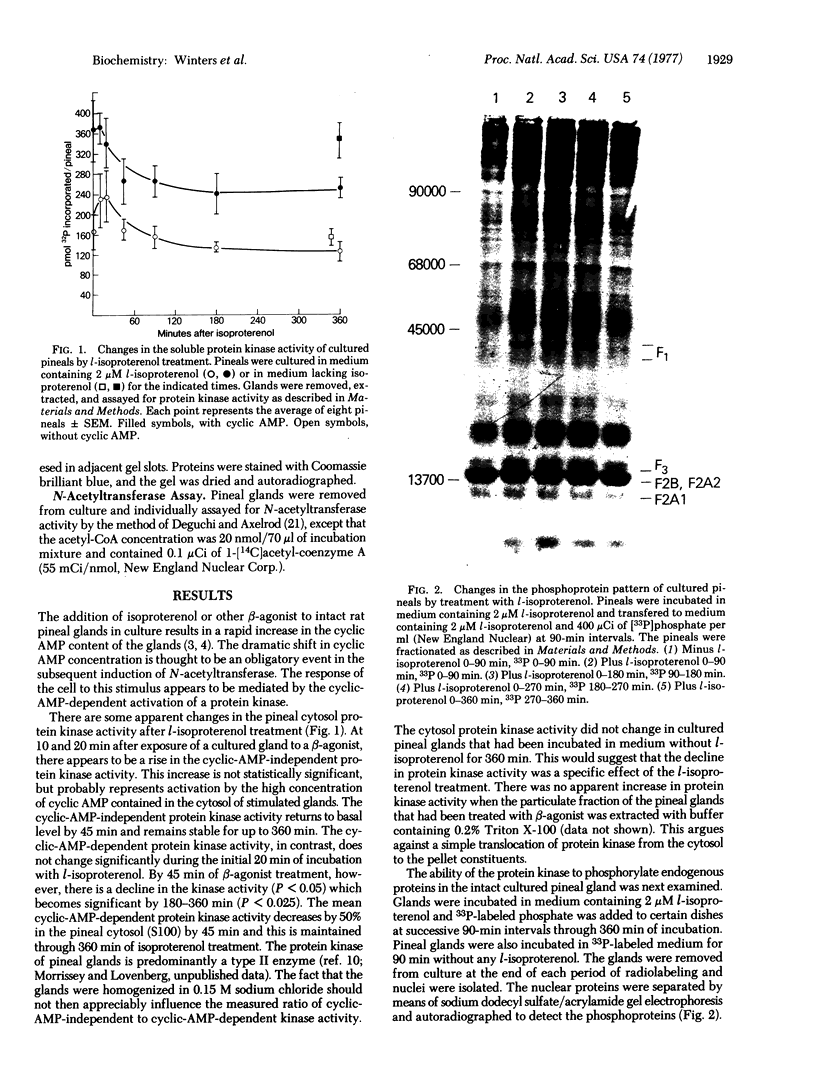
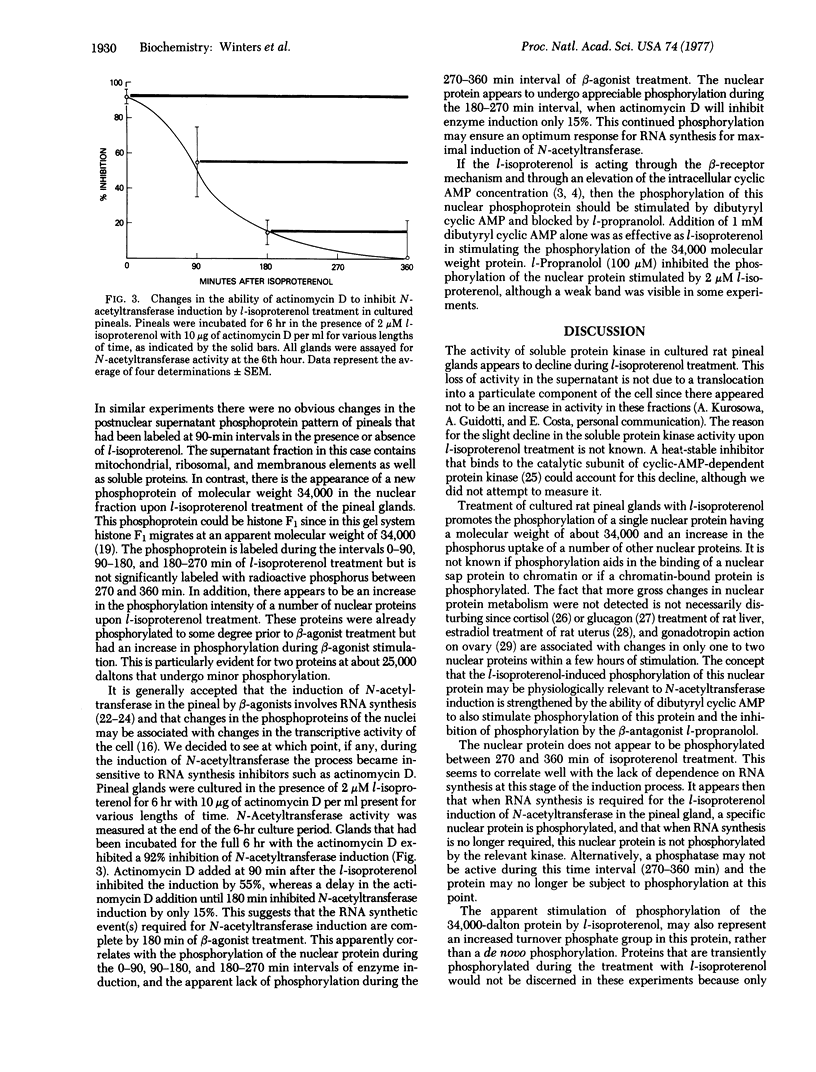
The activity of soluble protein kinase (ATP:protein phosphotransferase,EC 2.7.1.37) and pattern of nuclear protein phosphorylation was monitored in cultured rat pineal glands during the induction of serotonin N-acetyltransferase (acetyl-CoA:serotonin N-acetyltransferase;EC 2.3.1.5)by l-isoproterenol. A nuclear protein appears to be phosphorylated during the early stages of enzyme induction but is not phosphorylated at later stages of induction. This correlates well with the need for RNA synthesis associated with the induction process. The nuclear protein was also phosphorylated when the pineal glands were treated with dibutyryl 3':5'-cyclic AMP. The soluble protein kinase activity appeared to decline during mid-to-late stages of enzyme induction, but there was no concomitant increase in the particulate protein kinase activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstein M., Axelrod J. Pineal gland: 24-hour rhythm in norepinephrine turnover. Science. 1974 Apr 12;184(4133):163–165. doi: 10.1126/science.184.4133.163. [DOI] [PubMed] [Google Scholar]
- Chuang D. M., Costa E. Trans-synaptic regulation of ribonucleic acid biosynthesis in rat adrenal medulla. Mol Pharmacol. 1976 May;12(3):514–518. [PubMed] [Google Scholar]
- Chuang D. M., Hollenbeck R., Costa E. Enhanced template activity in chromatin from adrenal medulla after phosphorylation of chromosomal proteins. Science. 1976 Jul 2;193(4247):60–62. doi: 10.1126/science.180597. [DOI] [PubMed] [Google Scholar]
- Cohen M. E., Hamilton T. H. Effect of extradiol-17beta on the synthesis of specific uterine nonhistone chromosomal proteins. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4346–4350. doi: 10.1073/pnas.72.11.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Induction and superinduction of serotonin N-acetyltransferase by adrenergic drugs and denervation in rat pineal organ. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2208–2211. doi: 10.1073/pnas.69.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Sensitive assay for serotonin N-acetyltransferase activity in rat pineal. Anal Biochem. 1972 Nov;50(1):174–179. doi: 10.1016/0003-2697(72)90496-4. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Supersensitivity and subsensitivity of the beta-adrenergic receptor in pineal gland regulated by catecholamine transmitter. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2411–2414. doi: 10.1073/pnas.70.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T. Role of the beta adrenergic receptor in the elevation of adenosine cyclic 3',5'-monophosphate and induction of serotonin N-acetyltransferase in rat pineal glands. Mol Pharmacol. 1973 Mar;9(2):184–190. [PubMed] [Google Scholar]
- Enea V., Allfrey V. G. Absorption, accretion and endogenous faecal excretion of calcium in the newborn infant. Nature. 1973 Mar 23;242(5395):265–267. doi: 10.1038/242265b0. [DOI] [PubMed] [Google Scholar]
- Fontana J. A., Lovenberg W. A cyclic AMP-dependent protein kinase of the bovine pineal gland. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2787–2790. doi: 10.1073/pnas.68.11.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J. A., Lovenberg W. Pineal protein kinase: effect of enzymic phosphorylation on actinomycin D binding by, and template activity of, chromatin. Proc Natl Acad Sci U S A. 1973 Mar;70(3):755–758. doi: 10.1073/pnas.70.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Kurosawa A., Chuang D. M., Costa E. Protein kinase activation as an early event in the trans-synaptic induction of tyrosine 3-monooxygenase in adrenal medulla. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1152–1156. doi: 10.1073/pnas.72.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Mechanism of action of gonadotropin. IV. Cyclic adenosine monophosphate-dependent translocation of ovarian cytoplasmic cyclic adenosine monophosphate-binding protein and protein kinase to nuclear acceptor sites. Endocrinology. 1974 Jan;94(1):168–183. doi: 10.1210/endo-94-1-168. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Lee S., DeAngelo A. B. Translocation of cytoplasmic protein kinase and cyclic adenosine monophosphate-binding protein to intracellular acceptor sites. Adv Cyclic Nucleotide Res. 1975;5:281–306. [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa A., Guidotti A., Costa E. Induction of tyrosine 3-monooxygenase elicited by carbamylcholine in intact and denervated adrenal medulla: role of protein kinase activation and translocation. Mol Pharmacol. 1976 May;12(3):420–432. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Axelrod J. Regulation of sensitivity to beta-adrenergic stimulation in induction of pineal N-acetyltransferase. Proc Natl Acad Sci U S A. 1975 May;72(5):1661–1665. doi: 10.1073/pnas.72.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Axelrod J. Beta-adrenergic stimulation of pineal N-acetyltransferase: adenosine 3':5'-cyclic monophosphate stimulates both RNA and protein synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2107–2111. doi: 10.1073/pnas.72.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton K. R., Allfrey V. G. Selective synthesis of a nuclear acidic protein in liver cells stimulated by cortisol. Nature. 1970 Oct 10;228(5267):132–134. doi: 10.1038/228132a0. [DOI] [PubMed] [Google Scholar]
- Smith K. B. Reduced histone levels induced by "reeler" and "weaver" mutations in the mouse cerebellum. Biochem Biophys Res Commun. 1976 Apr 19;69(4):868–877. doi: 10.1016/0006-291x(76)90454-x. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Strada S. J., Klein D. C., Weller J., Weiss B. Effect of norepinephrine on the concentration of adenosine 3',5'-monophosphate of rat pineal gland in organ culture. Endocrinology. 1972 Jun;90(6):1470–1475. doi: 10.1210/endo-90-6-1470. [DOI] [PubMed] [Google Scholar]
- Taylor A. N., Wilson R. W. Electrophysiological evidence for the action of light on the pineal gland in the rat. Experientia. 1970 Mar 15;26(3):267–269. doi: 10.1007/BF01900087. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Zatz M., O'Dea R. F. Regulation of protein kinase in rat pineal: increased Vmax in supersensitive glands. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):427–439. [PubMed] [Google Scholar]