Abstract
BACKGROUND
Increases in serum creatinine (ΔSCr) from baseline signify acute kidney injury (AKI) but offer little granular information regarding its characteristics. The 10th Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) suggested that combining AKI biomarkers would provide better precision for AKI course prognostication.
OBJECTIVES
This study investigated the value of combining a functional damage biomarker (plasma cystatin C [pCysC]) with a tubular damage biomarker (urine neutrophil gelatinase-associated lipocalin [uNGAL]), forming a composite biomarker for prediction of discrete characteristics of AKI.
METHODS
Data from 345 children after cardiopulmonary bypass (CPB) were analyzed. Severe AKI was defined as Kidney Disease Global Outcomes Initiative stages 2 to 3 (>100% ΔSCr) within 7 days of CPB. Persistent AKI lasted >2 days. SCr in reversible AKI returned to baseline ≤48 h after CPB. The composite of uNGAL (>200 ng/mg urine Cr = positive [+]) and pCysC (>0.8 mg/l = positive [+]), uNGAL+/pCysC+, measured 2 h after CPB initiation, was compared to ΔSCr increases of ≤50% for correlation with AKI characteristics by using predictive probabilities, likelihood ratios (LR), and area under the curve receiver operating curve (AUC-ROC) values.
RESULTS
Severe AKI occurred in 18% of patients. The composite uNGAL+/pCysC+ demonstrated a greater likelihood than ΔSCr for severe AKI (+LR: 34.2 [13.0:94.0] vs. 3.8 [1.9:7.2]) and persistent AKI (+LR: 15.6 [8.8:27.5] versus 4.5 [2.3:8.8]). In AKI patients, the uNGAL−/pCysC+ composite was superior to ΔSCr for prediction of transient AKI. Biomarker composites carried greater probability for specific outcomes than ΔSCr strata.
CONCLUSIONS
Composites of functional and tubular damage biomarkers are superior to ΔSCr for predicting discrete characteristics of AKI.
Keywords: Acute Dialysis Quality Initiative, acute kidney injury phenotypes, biomarker combinations, cardiac surgery, functional acute kidney injury, pediatric acute kidney injury
Over the past 2 decades, the incidence of acute kidney injury (AKI) has grown exponentially and has been shown to be associated with worse outcomes in hospitalized patients (1–4). Because no singular therapy has demonstrated efficacy for mitigating the negative effects associated with AKI, mainstays of therapy include prevention and supportive care. Unfortunately, this management strategy can be variable, and best practice guidelines have not yet been widely disseminated (5).
Imprecise diagnoses may lead to imprecise therapy. AKI is a syndrome caused by a wide variety of pathophysiologic processes (e.g., sepsis and post-cardiopulmonary bypass [CPB]) and does not manifest equally in all patients. Unfortunately, whereas AKI occurs along a spectrum and is heterogeneous from patient to patient, identification of AKI by serum creatinine (SCr) is binary (AKI or no AKI) and offers no ability to predict the outcome or otherwise define more specific characteristics of the injury. Additionally, the current nomenclature for AKI diagnosis is problematic. Terms such as “pre-renal” and “intrinsic renal” are simplistic and may erroneously imply specific pathophysiology, location of injury, and severity of damage, potentially leading to therapeutic imprecision. For example, fluid resuscitation, often the first course of action in a patient with severe dehydration, can be quite deleterious in a patient with congestive heart failure, even though both have the same AKI classi-fication of “pre-renal” AKI (6). Pre-renal AKI is also often equated with a pathophysiologic description of reversibility or transient AKI, potentially rendering the erroneous conclusion that this type of AKI reflects less damage to the kidney. Intrinsic renal AKI is often considered severe and interchangeable with “acute tubular necrosis,” which may be pathologically inaccurate; available histologic evidence does not consistently match the necrotic shape of renal tubules to a level of renal dysfunction (7). Thus, an approach to AKI using objective, reproducible metrics may be advantageous (8,9).
Use of novel biomarkers indicative of different AKI pathophysiological conditions and carrying different temporal profiles in relation to injury may enhance diagnostic precision (10,11). The 10th Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) recommended testing the efficacy of novel AKI bio-markers in combination with functional biomarkers to more precisely delineate and define AKI characteristics (12). Identification of AKI phenotypes using these biomarkers may be a way to disentangle the AKI syndrome (13,14).
In this study, we tested the performance of different combinations of 2 novel biomarkers, a functional injury marker and a tubular damage marker, to refine the granularity of AKI diagnosis. We compared the abilities of these biomarker combinations to predict creatinine-based discrete AKI outcomes (using Kidney Disease Global Outcomes Initiative [KDIGO] AKI stages 2 to 3) with that of SCr itself to predict outcomes for AKI. Our findings demonstrated that combining biomarkers allows for prediction of temporal and pathophysiologic characteristics of injury, increasing the precision of AKI diagnosis in ways not possible with SCr alone.
METHODS
The Institutional Review Board of Cincinnati Children's Hospital Medical Center approved this study. The study is a retrospective review of children <18 years of age undergoing CPB, enrolled from January 2004 to May 2007 at our institution, initially in a study examining biomarkers and AKI after CPB. Before enrollment, written informed consent was obtained from the legal guardians of all patients, with assent from the patients, when appropriate (15). Patients with pre-existing kidney function insufficiency (defined as a baseline SCr concentration >2× the age-adjusted reference range) were excluded. All patients were admitted to the cardiac intensive care unit (CICU) following surgery.
Patient data were collected from the pre-operative period to CICU post-operative day 7 (POD7). Demographic data included hospital admission date, CICU admission date, CICU discharge date, hospital discharge date, prematurity; and sex, race, age, height, and weight. Procedural cardiac data collected included CPB history and CPB time; surgical complexity score using Risk Adjustment Congenital Heart Surgery Score 1 (RACHS-1) (16); and surgical procedure type. Post-operative CICU outcome data included length of stay, death, and provision of renal replacement therapy. Indices specific for kidney function included baseline SCr, first post-operative SCr, and SCr from POD1 and 2. The first post-operative SCr level was measured on arrival at the CICU, after surgery.
Data for this study included serum and urine samples. Samples were stored in aliquots at −80°C until measurement (17). Urinary biomarkers used in this study were measured 2 h after initiation of CPB, and included urinary creatinine and neutrophil gelatinase-associated lipocalin (uNGAL), measured by an enzyme-linked immunoassay kit (product no. 036, AntibodyShop, Grusbakken, Denmark) that specifically detects human NGAL (15). The concentration of serum cystatin C, also measured 2 h after initiation of CPB, was quantified by nephelometry using a standardized clinical laboratory platform (BN ProS-pec, Dade-Behring, Newark, Delaware), according to the manufacturer's recommendations (18). For the original study, biomarkers were collected at 2, 6, 12, 24, and 48 h after initiation of CPB. Measurement of SCr during CPB is not standard of care and was not routinely performed.
The primary outcome variable was development of severe AKI, defined as KDIGO stage 2 or 3 on any POD, using creatinine-based KDIGO stage definitions, where stage 1 = 1.5 to 1.99× baseline SCr; stage 2 = 2 to 2.99× baseline SCr; and stage 3 = ≥3× baseline SCr (5). Duration of severe AKI was classified into 1 to 2 days or >2 days (indicating persistent AKI). Among patients who developed severe AKI, the return of SCr to baseline at <48 h was defined as transient (reversible) AKI. To derive decision trees, we used classification and regression tree (CART) analysis. The CART algorithm can operate using supervised decision rules (i.e., a priori defined cutoff values) or unsupervised decision rules, deriving decision nodes that maximally discriminate between AKI and no AKI (or other outcomes of interest) (19). The trees were built using Salford Predictive Modeler version 6.6 software (Salford Systems, San Diego, California). Performance of the tree was reported using diagnostic test statistics with 95% confidence intervals computed using MedCalc for statistical computation (20). Areas under the receiver operating characteristic curve (AUC-ROC) were derived.
Biomarker composites were created for comparison of changes in creatinine concentration for prediction of outcomes. For this primary analysis, measurements of biomarkers at 2 h into CPB procedure were used. Using this time point for biomarker analysis theoretically offered the most meaningful yield for early prediction of AKI versus changes in SCr (median time was 5 to 6 h after CPB initiation in our cohort). The composites combined a functional injury marker, plasma cystatin C (pCysC), with the tubular damage biomarker (uNGAL). Four unique composites were created using combinations denoting positivity or negativity of each biomarker relative to reference cutoffs for injury (Figure 1). Urinary NGAL was normalized to urinary creatinine (uNGAL/uCr). A cutoff of 200 ng/mg was used to denote uNGAL/uCr positivity (derived from reference values of 100 ng/ml for uNGAL and 0.5 mg/ml of uCr) and 0.8 mg/l for pCysC positivity (21–23). The notation for a composite is “uNGAL/pCysC,” with the inclusion of “+” or “−” sign for each biomarker, depending on its value relative to cutoff values. Biomarker composites were compared to changes in SCr from baseline (pre-operative) to first post-operative check (ΔSCr). The primary cutoff value used to denote a positive ΔSCr was ≥50%, equivalent to 1.5 times baseline (≥KDIGO stage 1).
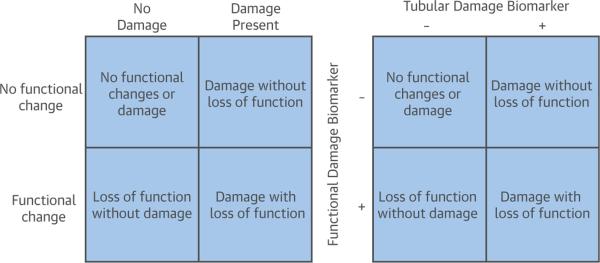
FIGURE 1. Side-by-Side 2 × 2 Tables Show 4 Different AKI Phenotypes Based on Pathophysiology of Injury Versus Translation When Using Combinations of Biomarkers to Detect the Corresponding Injuries.
Transient AKI, formerly known as “pre-renal” AKI, falls into the category of loss of function without damage (bottom left of each table). Damage with loss of function is a combination of functional and tubular injury. Adapted with permission from Endre et al. (6). AKI = acute kidney injury.
STATISTICAL ANALYSIS
Data are described using medians, interquartile ranges (IQR), frequencies, and percents. Outcome comparisons used the Mann-Whitney U test, chi-square test, or Fisher exact test, as appropriate. Descriptive statistics and comparisons were analyzed using SigmaStat version 12.3 software (San Antonio, Texas). Likelihood ratios (LR) were derived using standard calculation formats, where +LR = (sensitivity/[1 − specificity]); −LR = ([1 − sensitivity]/specificity), by MedCalc. A signi-ficant elevation in LR was denoted by a 95% confidence interval (CI) that did not cross 1. Significance of the differences among LRs was determined by lack of overlap between 95% CI. CART decision tree derivation was performed both with and without restrictions (i.e., supervised and unsupervised) for ΔSCr cutoff values.
RESULTS
AKI HAS A HIGH PREVALENCE AND IS ASSOCIATED WITH INCREASED MORBIDITY
Severe AKI occurred in 18% (62 of 345) of patients. Only younger age was associated with AKI; demographics of patients with or without AKI were otherwise similar. Surgical complexity scores did not differ between patients with versus those without severe AKI. Patients with severe AKI had higher mortality rates, longer CPB times, and longer lengths of CICU stay (Table 1).
TABLE 1.
Demographics
| Total | No AKI | AKI | |
|---|---|---|---|
| n (%) | 345 | 283 (82) | 62 (18) |
| Age, yrs | 1.05 (0.43-4.44) | 1.75 (0.46-5.1) | 0.52 (0.32-0.85)* |
| Male | 184 (53) | 156 (55) | 28 (45) |
| History of CPB | 148 (43) | 130 (46) | 18 (29) |
| RACHS-1 class | 2 (2-3) | 2(2-3) | 2(2-3) |
| 6: 6 | 6: 5 (1.7) | 6: 1 (1.6) | |
| 5: 5 | 5: 3 (1.1) | 5: 2 (3.2) | |
| 4: 11 | 4: 10 (3.5) | 4: 1 (1.6) | |
| 3: 127 | 3: 106 (37.5) | 3: 21 (33.8) | |
| 2: 162 | 2: 125 (44.2) | 2: 37 (59.7) | |
| 1: 34 | 1: 34 (12.0) | 1: 0 | |
| CPB time, min | 101 (74-145) | 95 (71-134) | 135 (89-192)* |
| ICU length of stay, days | 5 (4-10) | 5 (4-8) | 11 (6-21)* |
| Mortality | 6 (1.7) | 4 (1.4) | 2 (3.2) |
Values are n, n (%), or median (IQR). Patients studied were separated by the development of acute kidney injury (AKI) within the first 7 days after cardiopulmonary bypass (CPB).
p < 0.001 for AKI versus no AKI.
ICU = intensive care unit; IQR = interquartile range; RACHS-1 = Risk Adjustment for Congenital Heart Surgery-1 score.
DOUBLE-POSITIVE BIOMARKER COMPOSITE (UNGAL+/PCYSC+) DEMONSTRATES SIGNIFICANTLY SUPERIOR PREDICTIVE PERFORMANCE FOR SEVERE AKI
Dual positivity in the biomarker composite (uNGAL >200 ng/mg and pCysC >0.8 mg/l) was more predictive of severe AKI than positivity of ΔSCr (change ≥50%) (Table 2). The uNGAL+/pCys− composite biomarker also demonstrated greater prediction of severe AKI than did ΔSCr.
TABLE 2.
Prediction of Severe AKI by Biomarker Composites
| Marker | Sensitivity | Specificity | PPV | NPV | + LR | –LR |
|---|---|---|---|---|---|---|
| ASCr+ | 22 (13-35) | 94 (91-96) | 45 (27-64) | 85 (80-89) | 3.8 (1.9-7.2) | 0.8 (0.7-0.9) |
| uNGAL– pCysC– |
5(1-14) | 46 (41-52) | 2 (0-6) | 69 (62-76) | 0.1 (0-0.3) | 2.0 (1.8-2.3) |
| uNGAL– pCysC+ |
23 (13-35) | 57 (50-63) | 10 (6-17) | 77 (71-82) | 0.5 (0.3-0.8) | 1.4 (1.2-1.6) |
| UNGAL+ pCysC– |
24 (14-37) | 98 (96-99) | 75 (51-91) | 75 (51-91) | 13.7 (5-36) | 0.8 (0.7-0.9) |
| UNGAL+ pCysC+ |
48 (36-61) | 99 (96-100) | 88 (73-97) | 90 (86-93) | 34.2 (13-94) | 0.5 (0.4-0.7) |
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are % (95% CI). Biomarker composites of urinary neutrophil gelatinase-associated lipocalin (uNGAL) and plasma cystatin C (pCysC) in relation to their respective cutoff values for positivity (+) >200 ng/mg and 0.8 mg/l demonstrate significantly improved prediction of severe acute kidney injury (AKI) compared to the change in serum creatinine (ΔSCr) from pre-operative to first post-operative value (cutoff value for positivity of ≥50% increase). uNGAL is normalized to urinary creatinine.
LR = likelihood ratio.
DOUBLE-POSITIVE BIOMARKER COMPOSITE (UNGAL+/PCYSC+) DEMONSTRATES SUPERIOR PREDICTIVE PERFORMANCE FOR PERSISTENT AKI
The dual-positive uNGAL+/pCysC+ composite was a better predictor of persistent AKI than ΔSCr ≥50% (Table 3). The uNGAL+/pCyspCys− composite also trended toward better prediction of persistent AKI than ΔSCr ≥50%.
TABLE 3.
Prediction of AKI >2 Days by Biomarker Composites
| Marker | Sensitivity | Specificity | PPV | NPV | + LR | –LR |
|---|---|---|---|---|---|---|
| Δ SCr+ | 31 (15-51) | 93 (90-96) | 29 (14-48) | 93 (90-96) | 4.5 (2.3-8.8) | 0.7 (0.6-0.9) |
| uNGAL– pCysC– |
7 (1-23) | 52 (46-58) | 1.3 (0.2-4.6) | 86 (80-90) | 0.1 (0-0.6) | 1.8 (1.5-2.0) |
| uNGAL– pCysC+ |
0(0-16) | 57 (51-62) | 0 (0-3) | 89 (83-93) | 0 (NC) | 1.8 (1.6-1.9) |
| UNGAL+ pCysC– |
24 (10-44) | 96 (93-98) | 35 (15-59) | 93 (90-96) | 5.9 (2.5-13.5) | 0.8 (0.6-1.0) |
| UNGAL+ pCysC+ |
69 (49-85) | 96 (93-98) | 59 (41-75) | 97 (95-99) | 15.6 (8.8-27.5) | 0.3 (0.2-0.6) |
Sensitivity, specificity, PPV, and NPV are % (95% CI). Biomarker composites of uNGAL and pCysC in relation to their respective cutoff values for positivity (+) >200 ng/mg and 0.8 mg/l demonstrate significantly improved prediction of the duration of severe AKI compared to the ΔSCr from pre-operative to first post-operative value (cutoff value for positivity of ≥50% increase). uNGAL is normalized to urinary creatinine.
Abbreviations as in Table 2.
IN PATIENTS WHO DEVELOPED AKI, A BIOMARKER COMPOSITE OF FUNCTIONAL DAMAGE POSITIVITY BUT TUBULAR DAMAGE NEGATIVITY (UNGAL−/PCYS+) IDENTIFIES TRANSIENT INJURY
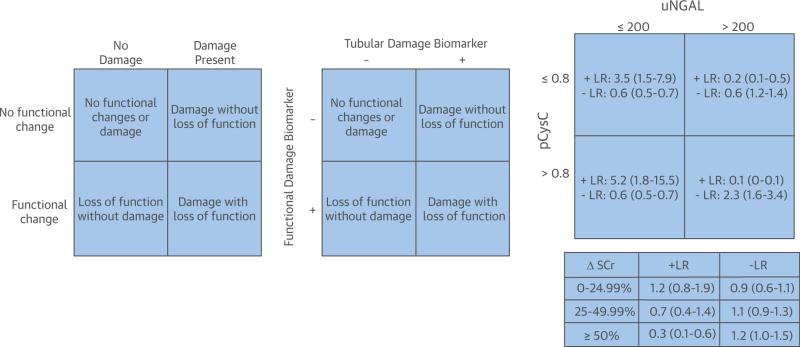
In the 62 patients who developed AKI post-operatively, 29 experienced AKI at >2 days. The uNGAL−/pCys+ composite identified patients with transient AKI with 100% specificity and 100% positive predictive value (PPV) (Table 4). Analysis of ΔSCr at multiple thresholds did not demonstrate a statistically significant ability to distinguish patients with transient AKI from patients who developed AKI. Additionally, the uNGAL+/pCysC+ composite demonstrated a significantly negative LR for transient AKI. When the composites were tested relative to the entire cohort of 345 patients, the +LR for uNGAL−/pCysC+ to predict transient AKI was 5.2 (1.8 to 15.5) versus 0.7 (0.4 to 14.4) and 0.3 (0.1 to 0.6) for ΔSCr of 25 to 49.9% and ≥50%, respectively (Figure 2).
TABLE 4.
Prediction of Transient AKI by Biomarker Composites
| Marker | Sensitivity | Specificity | PPV | NPV | + LR | –LR |
|---|---|---|---|---|---|---|
| ΔSCr 0%-24% | 42 (25-61) | 59 (39-77) | 54 (33-74) | 47 (30-65) | 1.0 (0.6-1.9) | 1.0 (0.6-1.5) |
| ΔSCr 25%-49% | 24 (11-42) | 83 (64-94) | 62 (32-86) | 49 (34-64) | 1.4 (0.5-3.8) | 0.9 (0.7-1.2) |
| ΔSCr ≥50% | 15 (5-32) | 69 (49-85) | 36 (13-65) | 42 (28-57) | 0.5 (0.2-1.3) | 1.2 (0.9-1.6) |
| uNGAL– pCysC– |
3(0-16) | 93 (77-99) | 33 (5-88) | 46 (33-59) | 0.4 (0-4.6) | 1.0 (0.9-1.2) |
| uNGAL– pCysC+ |
42 (25-61) | 100 (88-100) | 100 (77-100) | 60 (45-74) | NC | 0.6 (0.4-0.8) |
| UNGAL+ pCysC– |
24 (11-42) | 76 (56-90) | 53 (27-79) | 47 (32-62) | 1.0 (0.4-2.4) | 1.0 (0.8-1.3) |
| UNGAL+ pCysC+ |
30 (16-49) | 31 (15-51) | 33 (17-53) | 28 (14-47) | 0.4 (0.3-0.8) | 2.3 (1.3-4.0) |
Sensitivity, specificity, PPV, and NPV are % (95% CI). For patients who developed AKI (62 of 345), biomarker composites of uNGAL and pCysC in relation to their respective cutoff values for positivity ≥200 ng/mg and 0.8 mg/l (+) demonstrate the ability to use composite biomarker panels to identify transient injury. Prediction was significantly superior to that afforded by any ΔSCr from pre-operative to first post-operative value. uNGAL is normalized to urinary creatinine.
Abbreviations as in Table 2.
FIGURE 2. Transposition of Table of Likelihood Ratios Next to 2 × 2 Tables From Figure 1 Allows Visualization of the Predictive Power of Composites of Biomarkers Representative of Pathophysiologic Types of Injury.
Urinary neutrophil gelatinase-associated lipocalin (uNGAL) was used as a tubular damage marker, and plasma cystatin C (pCysC) was used as a functional damage marker. Prediction of transient acute kidney injury (denoted as a loss of function without damage) between the biomarker composites (bottom left of the far right table) should be compared to the post-operative percent of creatinine changes (ΔSCr; bottom right table). Data are for the entire cohort of 345 patients with 95% confidence intervals listed for the positive (+) and negative (−) likelihood ratios.
USE OF BIOMARKER COMPOSITES YIELDS A SIGNIFICANTLY HIGHER PREDICTIVE DISCRIMINATION BETWEEN SEVERITY AND PERSISTENCE OF AKI THAN ΔSCR
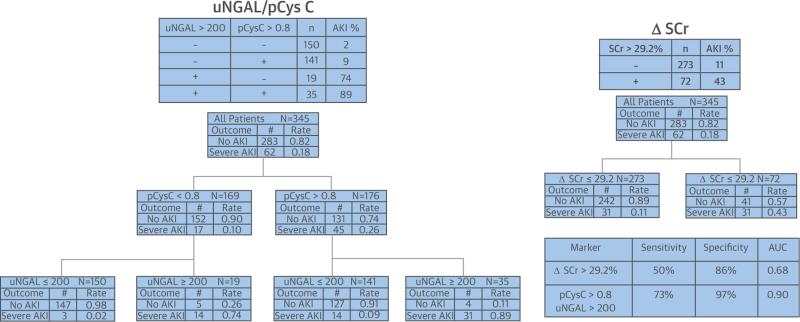
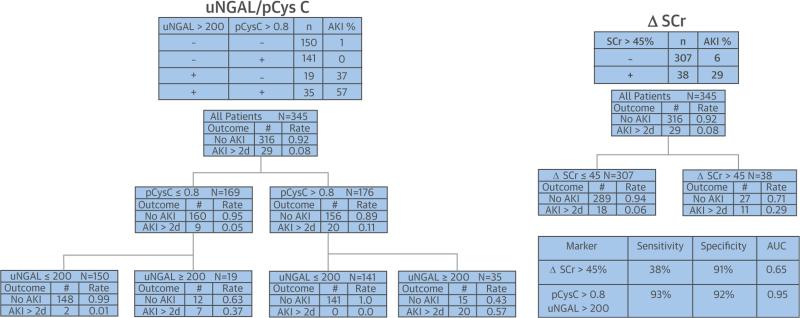
Use of CART analysis demonstrated the superior predictive precision of biomarker composites for AKI severity (Figure 3) and AKI persistence (Figure 4) compared to ΔSCr. The probabilities of severe AKI and persistent AKI in the terminal nodes, using supervised decision rules for pCysC (>0.8 mg/l) and uNGAL (>200 ng/mg), are approximately twice the probabilities in the terminal nodes, using the CART-derived optimal unsupervised cutoff value for ΔSCr (89% vs. 43% for AKI KDIGO stages 2 to 3 and 57% vs. 29% for persistent AKI, respectively). Additionally, the AUC-ROC of each outcome was higher for the composites than for even the most optimal ΔSCr derived by CART (29.2% for AKI severity and 45% for AKI persistence). CART identified optimal unsupervised cutoff values for pCysC of 0.9 mg/l and 1.2 mg/l, leading to AUC values of 0.63 and 0.59 for discrimination of severe AKI and persistent AKI, respectively. Similarly, optimal unsupervised cutoff values for uNGAL of 61 ng/ml and 130 ng/ml led to AUC values of 0.90 and 0.89 for severe AKI and persistent AKI, respectively.
FIGURE 3. Decision Trees Created by CART Analysis Demonstrate Differences in Discrimination for Severe AKI by ΔSCr and Composites of uNGAL and pCysC.
The terminal node of the supervised decision rules for uNGAL (ng/mg urinary creatinine) and pCysC (mg/l) shows 89% probability of severe AKI versus 43% for the unsupervised rule for ΔSCr (>29.2%). CART analysis also demonstrated a higher sensitivity, specificity, and AUC-ROC for the biomarker composite than the creatinine change. No AKI is defined as no injury or KDIGO stage 1; severe AKI is KDIGO stage 2 or within 7 days of surgery. AKI = acute kidney injury; AUC-ROC = areas under the receiver operating characteristic curve; CART = classification and regression tree; KDIGO = Kidney Disease Global Outcomes Initiative; other abbreviations as in Figure 2.
FIGURE 4. Decision Trees Created by CART Analysis Demonstrate Differences in Discrimination for Persistence of AKI by ΔSCr and Composites of uNGAL and pCysC.
The terminal node of the supervised decision rules for uNGAL and pCysC shows 57% probability of severe AKI versus 29% for the unsupervised rule for ΔSCr (>45%). CART analysis also demonstrated a higher sensitivity, specificity, and AUC-ROC for the biomarker composite compared to the creatinine change. Abbreviations as in Figures 1 and 3.
DISCUSSION
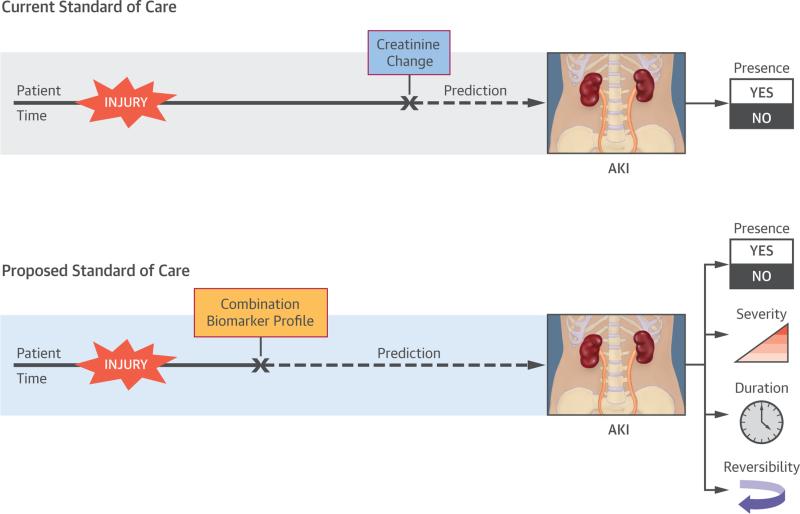
In this study, we operationalized an objective of the ADQI workshop, combining novel AKI biomarkers representative of different types of renal injury to improve the precision of AKI course prognostication (6,9). We demonstrated that a composite of bio-markers, including a marker indicative of functional glomerular damage (cystatin C) and a marker of tubular damage (NGAL), yields greater predictive precision for discrete SCr-based AKI outcomes than ΔSCr analysis by itself. By combining novel biomarkers, both temporal and pathophysiologic information about AKI after CPB can be predicted (Central Illustration).
CENTRAL ILLUSTRATION.
Combining Biomarker Profiles Increases the Predictive Precision of Acute Kidney Injury Diagnosis
Prediction of AKI severity and duration is more robust using biomarker composites than using changes in creatinine concentration. Severe AKI prevalence in our cohort was 18% and was associated with the well-established risk factors of younger age and longer CPB times. In addition, severe AKI was associated with longer ICU stay. Although ΔSCr predicted severe AKI with a reasonably high specificity and predictive value, the +LR of severe AKI, using a composite of functional and tubular injury, was ~10 stronger (LR of 34.2 vs. 3.8, respectively). Additionally, the PPV was 2× higher (88% vs. 45%, respectively), and the negative predictive value (NPV) was 90% for the double-positive composite. Conversely, the −LR of uNGAL−/pCyspCys− was negative, indicating that kidney injury was unlikely. Finally, the likelihood of severe AKI differed among the 4 possible biomarker composites, suggesting that pathophysiological differences in AKI exist. The prediction of persistent AKI was similarly more robust using the biomarker composites than the ΔSCr. The population of patients with persistent AKI (8.4% [29 of 345]) had an associated CPB time of 174 min (IQR: 125 to 224 min) and length of stay of 19 days (IQR: 11 to 28 days). Using the uNGAL+/pCysC+ composite for prediction of persistent AKI, the +LR was ~3.5 that using ΔSCr (15.6 vs. 4.5, respectively). Once again, the PPV was nearly 2× higher (59% vs. 29%, respectively). The NPV for uNGAL+/pCysC+ was 96%.
Biomarker measurements occurred notably before the first post-operative SCr measurement. In most cases, biomarkers were measured 2 to 3 h after CPB initiation, whereas the first post-operative Cr measurement was obtained 5 to 6 h after CPB initiation. Assessment of kidney health at 2 h into CPB procedure carries an obvious benefit for optimal postoperative or intraoperative management. Even if uNGAL is measured 6 h after CPB initiation (at around the same time as most of the first post-operative SCr measurements) and is combined with a 2-h measurement of pCysC, the prediction of both severe AKI and AKI lasting more than 2 days is superior to that afforded by ΔSCr (Online Appendix 1).
For instance, optimal fluid management after CPB generally balances tight regulation of inputs and outputs on the basis of fluid delivery, post-operative diuresis, and medication-induced diuresis (diuretic agents) or mechanical fluid removal (peritoneal dialysis). Using biomarker composites, a patient with known functional and tubular damage, carrying a high likelihood of severe and persistent AKI, may be more expeditiously directed toward a certain modality of post-operative fluid management, such as fluid restriction and/or early use of renal replacement therapy (e.g., peritoneal dialysis or continuous renal replacement therapy). Although a ΔSCr of >50% from baseline to first post-operative laboratory value is modestly predictive of severe AKI or persistent AKI, changes less than 50% but greater than 0% carry no statistically significant predictive power for either AKI characteristic.
Identification of AKI pathophysiology is possible using biomarker composites. In the subset of patients who developed AKI KDIGO stages 2 to 3 (62 of 345 patients), the absence of tubular damage was highly predictive of transient, reversible AKI. Conversely, the presence of tubular damage was highly predictive of AKI with persistent injury and delayed return to pre-operative health. Analysis of the different cutoff values for SCr demonstrated no statistically significant ability of ΔSCr to identify transient AKI at any level. A practitioner caring for a child with a doubling of creatinine on POD1 would be informed to a greater degree by measurement of a tubular damage-negative/ functional damage-positive composite biomarker at 2 h into CPB than a first post-operative ΔSCr assay with 50% to 100% higher concentrations than baseline. The diagnosis of pre-renal AKI may be presumptively (and potentially inaccurately) applied in the context of the latter, whereas the diagnosis of functional change AKI would be more precisely (and accurately) applied in the context of the former.
Decision tree analysis highlighted the probability for severe and persistent AKI in stratified populations. Comparisons of the most optimal ΔSCr cutoff value, determined by unsupervised CART analysis, to the a priori-determined uNGAL and pCysC cutoff values demonstrated that the probabilities for each outcome were notably higher in the terminal nodes for the biomarker composites. Of the 35 patients with uNGAL+/pCysC+ composites, 89% developed KDIGO stages 2 to 3 within 7 postoperative days, and 57% experienced persistent AKI (Figures 3 and 4). Interestingly, although individually robust, uNGAL+ terminal nodes that were pCysC− carried a lower probability of each outcome (74% of patients for severe AKI and 37% for persistent AKI) versus dual positivity. By comparison, less than one-half of the 72 patients (43%) who had a ΔSCr of >29.2% developed severe AKI, and less than one third of the 38 patients (29%) who had a ΔSCr of >45% experienced persistent AKI. The cutoff values of 29.2% for AKI severity and 45% for AKI persistence were the optimal values derived from CART for prediction of the outcomes of interest. The discrimination afforded by the biomarker composites for severe and persistent AKI, denoted by CART-derived AUC values, was superior to that afforded by the ΔSCr AUC values. Discrepancies between incremental increases in the AUC after the stepwise addition of each biomarker to the model and those of the composite model can be attributed to the nature of the cutoff determination. The supervised decision rule offered higher AUC values than the unsupervised individual decision rules, but in discrimination for both severity and duration of AKI, the composite biomarker AUC values were higher than those for individual bio-markers or ΔSCr AUC values.
This study operationalized the directives of the consensus statements calling for the use of biomarker combinations to increase the precision of AKI diagnosis. Strengths of our study include being the first to directly study the directives of the ADQI classifications; use of a large sample size; robust differences in outcome measurements; and the interpretation of the findings, carrying the potential to affect management of a child following CPB. Our primary outcome metrics were chosen on the basis of their clinical relevance and to create a reference more easily followed than the previous nomenclature (pre-renal versus intrinsic AKI are anecdotally separated on the basis of severity and duration of ≤48 h) (6). Separation of patients into 1 of 4 biomarker composite classifications lends itself directly to targeted therapy and interventions (e.g., testing the efficacy of diuretic therapy vs. peritoneal dialysis in fluid balance in patients with uNGAL+/pCysC+ composite). Previous data suggest a high progression rate of “mild AKI” (i.e., KDIGO stage 1 or RIFLE [Risk, Injury, Failure, Loss, End-Stage AKI] R) to “severe AKI” (i.e., KDIGO stages 2–3 or RIFLE I-F). Our data show that combining novel biomarkers predicts creatinine-based outcomes with greater precision than measuring changes of creatinine itself (10).
STUDY LIMITATIONS
Our study has several limitations. We were limited by the single-center and retrospective nature of our data collection. Our population was all post-CPB and relatively homogeneous; newer methodology and analyses are required to extrapolate the findings for study in heterogeneous patient populations (24). Validation in a prospective population outside of our institution would verify our model and strengthen our findings. We defined AKI using the creatinine-based KDIGO definition, not by urine output, and we did not correct SCr for degree of fluid overload. We did not analyze biomarkers other than those listed here and readily admit that other novel biomarkers may carry greater or lesser predictive precision (10,25). For instance, incorporation of tubular cell cycle arrest marker tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein-7 (IGF-BP7) may further enrich the precision of using biomarker composites by increasing the granularity of AKI phenotypes (26). Also, not having earlier creatinine measurements is a limitation of the study; however, the routine use of modified ultrafiltration before removal from CPB at our institution limits the potential of creatinine dilution and underestimation of creatinine changes in the first post-operative measurement. Additionally, we demonstrated that biomarker composites created from measurements at different times (Online Appendix 1) were consistently superior to initial changes in creatinine. We normalized uNGAL to urinary creatinine in our analysis, but separate analysis of uncorrected uNGAL also demonstrated the performance of the biomarker composites was superior to that of ΔSCr alone (Online Appendix 2). By highlighting the ability to identify transient AKI using biomarker composites, we assumed that tubular damage is more indicative of lasting kidney injury than damage denoted by a functional marker such as pCysC. When SCr is substituted for pCysC in the composite, prediction of AKI severity and duration is more robust than with ΔSCr alone but less robust than with the composite of uNGAL and pCysC (Online Appendix 3).
CONCLUSIONS
Our findings open the door to the use of novel AKI biomarkers for improving the precision of AKI diagnosis. Future study is needed to investigate whether biomarker composites can be matched to unique injuries. For instance, sepsis-associated AKI may have a biomarker profile that is distinctly different from that of nephrotoxin-associated AKI. However, our work suggests that using novel AKI biomarkers, representative of different locations of injury within the nephron and carrying different temporal pro-files in relation to injury, may render the ability to identify AKI phenotypes, on the basis of timing, severity, and pathophysiology. Ideally, these pheno-types would lead directly to targeted therapeutic interventions, ultimately attempting to disentangle the AKI syndrome and ushering in the era of personalized AKI medicine (14,27).
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Conventional indicators of AKI, such as a rise in SCr concentration, are delayed and not specific to the type of injury involved. Characterization of these conditions as “pre-renal” or “intrinsic renal disease” is imprecise and insufficient to guide management. Combinations of novel biomarkers that react earlier than SCr after AKI may correlate with specific sites of injury to the nephron in ways that could improve classification and with implications for clinical management.
TRANSLATIONAL OUTLOOK: Correlative clinical studies are needed to integrate biomarker profiles for classification of AKI into treatment algorithms applicable to the early management of patients with renal injury, including pharmacotherapy targeted toward specific AKI subtypes on the basis of the predominance of glomerular or tubular injury.
Acknowledgments
This research was supported by National Institutes of Health grants R01-HL08676, R01-HL085757, and R01-DK069749. Dr. Devarajan has reported that he has licensing agreements with Abbott Diagnostics and Alere, Inc., to develop NGAL as a biomarker of kidney injury; and is a consultant for Biosite.
A BBREVIATIONS AND ACRONYMS
- AKI
acute kidney injury
- AUC
area under the curve
- CART
classification and regression tree analysis
- CPB
cardiopulmonary bypass
- LR
likelihood ratio
- pCysC
plasma cystatin C
- uNGAL
urinary neutrophil gelatinase-associated lipocalin
- uNGAL+/pCysC+
biomarker composite designating both >200 ng/mg uNGAL and >0.8 mg/l pCysC
- ΔSCr
change in serum creatinine from baseline
Footnotes
All other authors have reported they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental tables, please see the online version of this article.
REFERENCES
- 1.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–9. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 2.Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Kellum JA, Katz NM, et al. Epidemiology of acute kidney injury. Contrib Nephrol. 2010;165:1–8. doi: 10.1159/000313737. [DOI] [PubMed] [Google Scholar]
- 4.Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: an update on diagnosis and treatment. Pediatr Clin North Am. 2013;60:669–88. doi: 10.1016/j.pcl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 6.Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 7.Rosen S, Stillman IE. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol. 2008;19:871–5. doi: 10.1681/ASN.2007080913. [DOI] [PubMed] [Google Scholar]
- 8.Cruz DN, Bagshaw SM, Maisel A, et al. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi: 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA, Bouchard J, Waikar SS, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the Tenth Consensus Conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:5–12. doi: 10.1159/000349962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–9. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–8. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Kellum JA. Acute kidney injury in 2011: biomarkers are transforming our understanding of AKI. Nat Rev Nephrol. 2012;8:68–70. doi: 10.1038/nrneph.2011.216. [DOI] [PubMed] [Google Scholar]
- 14.Chawla LS. Disentanglement of the acute kidney injury syndrome. Curr Opin Crit Care. 2012;18:579–84. doi: 10.1097/MCC.0b013e328358e59c. [DOI] [PubMed] [Google Scholar]
- 15.Krawczeski CD, Woo JG, Wang Y, et al. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–15. e1. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123:110–8. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 17.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krawczeski CD, Vandevoorde RG, Kathman T, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–7. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis RJ. An introduction to classification and regression tree (CART) analysis.. Paper presented at: Annual Meeting of the Society for Academic Emergency Medicine; San Francisco, California. May 2000. [Google Scholar]
- 20.MedCalc Software [October 14, 2014];Diagnostic test evaluation version 14.8.1. 1993–2014. Available at: http://www.medcalc.org/calc/diagnostic_test.php.
- 21.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl. 2014;25:582–8. doi: 10.4103/1319-2442.132194. [DOI] [PubMed] [Google Scholar]
- 23.Wald R, Liangos O, Perianayagam MC, et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1373–9. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu RK, Wang Y, Wong HR, et al. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9:654–62. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwiatkowski DM, Goldstein SL, Krawczeski CD. Biomarkers of acute kidney injury in pediatric cardiac patients. Biomark Med. 2012;6:273–82. doi: 10.2217/bmm.12.27. [DOI] [PubMed] [Google Scholar]
- 26.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest bio-markers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devarajan P. The future of pediatric acute kidney injury management—biomarkers. Semin Nephrol. 2008;28:493–8. doi: 10.1016/j.semnephrol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.