Abstract
Recently, we identified neuropathy target esterase (NTE) mutation as the cause of an autosomal recessive motor neuron disease (NTE-MND). Subsequently, we showed that NTE-MND mutations reduced specific activity (SA) and altered inhibitory kinetics of NTE catalytic domain constructs. Recent preliminary results showed that NTE is expressed in cultured human skin fibroblasts, and others have used mutant forms of neuronal proteins expressed in fibroblasts as biomarkers of neurogenetic diseases. Therefore, the present study was carried out to test the hypothesis that NTE in cultured skin fibroblasts from NTE-MND subjects also exhibit altered enzymological properties assessed by SA and IC50 values of mipafox (MIP) and chlorpyrifos oxon (CPO). NTE SA was reduced to 65% of control (wild type NTE from commercially obtained fibroblasts) in homozygous M1012V fibroblasts and 59-61% of control in compound heterozygous R890H/c2946_2947InsCAGC fibroblasts. MIP IC50 values were unaffected by the NTE mutations, but the CPO IC50 increased 4.5-fold in homozygous M1012V fibroblasts. Interestingly, markedly reduced NTE SAs (40-43% of control) were observed in fibroblasts from asymptomatic subjects heterozygous for NTE insertion c2946_2947InsCAGC. This insertion is predicted to produce truncated NTE missing the last 235 residues of its catalytic domain. These observations confirm that NTE-MND mutations reduce NTE SA in vitro. Moreover, to the extent observations made in cultured fibroblasts may be generalized to events in the nervous system, lack of correlation between reduced fibroblast NTE SA and the occurrence of NTE-MND in NTE insertion mutation heterozygotes indicates that reduction of NTE SA alone is insufficient to cause MND.
Keywords: Fibroblast, Motor neuron disease (MND), Mutation, Neuropathy target esterase (NTE), Organophosphorus compound-induced delayed neurotoxicity (OPIDN)
1. Introduction
Neuropathy target esterase (NTE) is a widely expressed endoplasmic reticulum (ER) membrane-associated phospholipase that deacylates intracellular membrane phosphatidylcholine to glycerophosphocholine (Fernández-Murray and McMaster, 2007; Glynn, 2005; Zaccheo et al., 2004). Known initially for its critical role in organophosphorus (OP) compound-induced delayed neuropathy (OPIDN) (Johnson, 1970; Lotti and Moretto, 2005), NTE is increasingly recognized as an important factor in nervous system development and maintenance (Read et al., 2009; Vose et al., 2008). Brain-specific knockout of NTE in mice leads to progressive neurodegeneration (Moser et al. 2004; Akassoglou et al. 2004). Mutation of NTE's Drosophila homologue sws leads to neurodegeneration, motor impairment, and reduced life span in the insect (Kretzschmar et al. 1997; Mühlig-Versen et al. 2005). Homozygous NTE M1012V mutation and compound heterozygous NTE R890H/c2946_2947InsCAGC mutation cause human autosomal recessive motor neuron disease (NTE-MND) (Rainier et al. ,2008). (Hereafter in this paper, NTE mutation c2946_2947InsCAGC will be abbreviated “NTE insertion” [NTEIns])
Using site-directed mutagenesis and a bacterial expression system, we showed that NTE-MND-specific mutations result in reduced specific activity and altered inhibitory kinetics in constructs of the NTE catalytic domain (NEST) (Hein et al., 2010). Moreover, preliminary studies in our laboratory showed that cultured skin fibroblasts expressed NTE. Therefore, we carried out the present study to test the hypothesis that NTE in cultured skin fibroblasts from NTE-MND subjects also exhibits altered enzymological properties. These investigations confirm that disease-specific NTE mutations reduce NTE specific activity in vitro and demonstrate the usefulness of fibroblast cultures for analysis of NTE biochemistry. Moreover, the finding of reduced NTE specific activity in neurologically normal NTE-MND carriers indicates that reduced NTE specific activity alone is insufficient to cause NTE-MND.
2. Methods
2.1. Human subjects
Human subject participation was approved by the University of Michigan Institutional Review Board. NTE-MND subjects were previously described (Rainier et al., 2008; Rainier et al., 2010). Following sterile preparation, drape, and 1% (w/v) sc lidocaine injection, 3 mm superficial skin punch biopsies were obtained.
NTE gene analysis for each subject has been previously reported (Rainier et al., 2008), except subjects 3 and 9 for whom NTE coding sequence was analyzed for this study. Subject 1 was an unaffected individual shown by DNA sequencing not to have pathogenic NTE mutations present in affected siblings. Subjects 2 and 3 (both unaffected) were heterozygous for NTE R890H mutation. Subject 4 (unaffected) was heterozygous for NTE M1012V mutation. Subject 5 (affected) was homozygous for NTE M1012V mutation. Subjects 6 and 7 (both affected) were compound heterozygous for NTE mutations R890H and NTE insertion. Subjects 8 and 9 (both unaffected) were heterozygous for NTE insertion mutation.
2.2. Human skin fibroblast cultures
Human skin fibroblasts from a control subject were obtained from Invitrogen (Carlsbad, CA). Fibroblast cultures from this control sample and from skin punch biopsies were established and maintained using published methods. Briefly, cells were maintained in T75 flasks with minimum essential medium (GIBCO, Invitrogen) containing 20% (w/v) fetal bovine serum (Hyclone, Thermo Fisher Scientific, Waltham, MA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL fungizone (GIBCO, Invitrogen) at 37 °C with 5% (v/v) CO2. Passages 5 through 13 were used for data measurements throughout. Confluent flasks were split 1:2 by volume, and harvested 48 h later for NTE assay. Cells were washed twice with PBS, harvested with 0.25% (w/v) trypsin, and spun 1000 × g for 10 min. The cell pellet was washed twice with buffer containing 50 mM Tris-HCl (pH 8.0 at 25 °C) and 0.1 mM EDTA. The cell pellet was resuspended in buffer containing 100 mM Tris-HCl (pH 8.0 at 25 °C), 1 mM CaCl2, and 0.01% (w/v) Triton-X 100 (Vose et al., 2008) and sonicated three times on ice for 6 sec.
2.3. DNA sequencing
NTE sequencing was performed previously (Rainier et al., 2008) in leukocyte DNA samples from each participant except subjects 3 and 9. Fibroblast DNA from subjects 3 and 9 was extracted and NTE exons 25 and 27 (the location of previously identified NTE mutations in family members) were sequenced as previously described (Rainier et al., 2008). Control fibroblast DNA was extracted and the entire NTE coding sequence analyzed as previously described (Rainier et al., 2008).
2.4. Chemicals for NTE enzymologic analysis
Diethyl-3,4,5-trichloro-2-pyridyl phosphate (chlorpyrifos oxon, CPO; 96% by GC) was furnished by Dow AgroSciences (Indianapolis, IN). Diethyl 4-nitrophenyl phosphate (paraoxon, PO; 98% by HPLC) was purchased from Sigma Aldrich (St. Louis, MO). N, N′-diisopropylphosphorodiamidofluoridate (mipafox, MIP; 99% by HPLC) was synthesized by ChemSyn (Lenexa, KS), and purchased from the Midwest Research Institute (Kansas City, MO). Phenyl valerate (PV) was synthesized and purified as described (Johnson, 1977). All other chemicals were reagent grade or the highest grade commercially available. Aqueous solutions were prepared in deionized distilled water. CPO, MIP, and PO present a potential neurotoxic hazard. Therefore, these chemicals were handled in a chemical fume hood and all items that contacted them were decontaminated by soaking in 1 M NaOH overnight.
2.5. NTE specific activity
NTE activity was defined as the portion of PV hydrolase activity that was inhibited by PO but not abolished by MIP. An adaptation of the colorimetric assay of Johnson (1977) as modified by Kayyali et al. (1991) and Kropp and Richardson (2003) was used. A 112.5 μL aliquot of sonicated fibroblasts was preincubated for 20 min at 37 °C with either 12.5 μL of 400 μM paraoxon to inhibit background esterase activity or 12.5 μL of 400 μM paraoxon plus 500 μM mipafox to inhibit background and NTE activity. Following this preincubation, 50 μL aliquots of the preincubation mixture were mixed with 100 μL of a substrate solution containing 2.71 mM PV/0.03% (w/v) N,N′-dimethylformamide in 0.03% (w/v) Triton X-100, and the reaction was allowed to proceed for 20 min at 37 °C. Production of phenol was stopped by adding 100 μL of 5.0 mg/mL sodium dodecyl sulfate/1.2 mM 4-aminoantipyrine followed by 50 μL of 12.1 mM K3Fe(CN)6, and color was allowed to develop and stabilize for 10 min. Endpoint absorbance was measured at 486 nm using a SpectraMax 340 microplate reader (Molecular Devices, Sunnydale, CA). Protein concentration was determined colorimetrically using the Bio-Rad Protein Assay Dye Reagent (Hercules, CA), and dilutions of bovine serum albumin (New England BioLabs, Ipswich, MA) were used as standards. In this paper, 1 unit of NTE specific activity = 1 nmol phenol produced/min/mg protein.
2.6. NTE IC50 determinations
NTE inhibition was carried out by a modification of the procedure of Davis and Richardson (1987). CPO or MIP (10 μL in incubation buffer with 0.1% v/v acetone) were added to 40 μL aliquots of preincubation mixtures at the end of the first preincubation interval, and inhibition was allowed to continue for 20 min at 37 °C before addition of substrate. Residual NTE activity was then measured as described above. The concentration of acetone used to solubilize the inhibitors did not affect enzyme activity. For each experiment, 5 concentrations of inhibitor were used over two log-orders of concentration. IC50 values were determined by fitting plots of percent inhibition vs log concentration to a sigmoid curve using GraphPad Prism 5.03 for Windows (GraphPad Software, Inc., San Diego, CA).
2.7. Statistical analysis
Data are presented as mean ± SEM, with the number of separate determinations indicated in each case. Significance of differences between test means and the control mean was determined by one-way analysis of variance (ANOVA) followed by Dunnett's test. In addition, in order to discuss comparisons of means within test groups, we examined all pairwise comparisons within the groups of NTE activity, MIP IC50, and CPO IC50 using one-way ANOVA with Tukey's post-test. For all analyses, the maximum p-value for significance was 0.05. Statistical analysis was carried out using GraphPad Prism 5.03 for Windows (GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Clinical examinations
All subjects were interviewed and examined (by J.K.F.). Clinical features of NTE-MND affected subjects have been described previously (Rainier et al., 2008; Rainier et al., 2010). Parents and unaffected siblings of NTE-MND subjects were neurologically asymptomatic and had normal neurologic examinations.
3.2. NTE sequencing
Sequence analysis of each NTE exon from the control fibroblast DNA did not identify any sequence variations (data not shown). DNA sequence analysis of the affected subjects and obligate (parental) carriers of NTE mutation and one unaffected sibling has been previously reported (Rainier et al., 2008). NTE sequencing analysis was performed on skin fibroblast DNA from subjects 3 and 9 who had two NTE-MND-affected siblings (compound heterozygotes for NTE mutation R890H/NTE insertion). One unaffected sibling (subject 3) carried the R890H mutation and the other unaffected sibling (subject 9) carried the NTE insertion (data not shown).
3.3. NTE specific activity
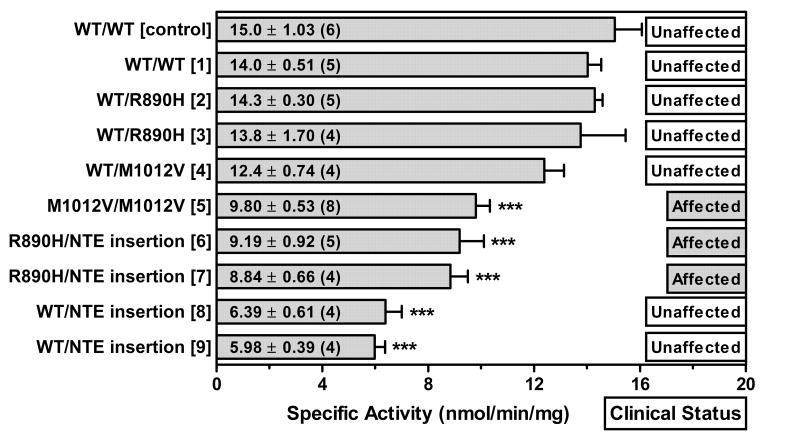
Fig. 1 shows NTE specific activity in fibroblasts with the indicated NTE genotypes. NTE activity in commercially obtained, control fibroblasts (15 units) was similar to that of the unaffected subject without pathogenic NTE mutation (subject 1, 14 units). The 3 clinically affected subjects had moderate, statistically significant decreases in specific activity (59-65% of control). Interestingly, two unaffected subjects heterozygous for NTE insertion mutation (Fig. 1, subjects 8 and 9) had even further reductions in NTE specific activity (40–43% of control). The NTE activity (12.4–14.3 units) in fibroblasts from three other unaffected NTE mutation carriers (subjects 2 and 3, heterozygous for R890H; and subject 4, heterozygous for M1012V) did not differ from the control.
Figure 1.
Specific activity (nmol phenol produced/min/mg protein) of NTE in control fibroblasts (WT/WT [control]) and in subject fibroblasts with NTE genotype indicated. Subject number is shown in square brackets. Clinical status of subjects is indicated as affected or unaffected with NTE-MND. Data are mean values ± SEM; number of separate determinations shown in parentheses. *** Significantly different from WT/WT [control], p < 0.001.
3.4. NTE inhibition studies
As shown in Fig. 2, all of the MIP IC50 values were in a comparable range of 7.2 to 11.4 μM, and none of the values differed statistically from that of the WT/WT control.
Figure 2.
Mipafox (MIP) 20-min IC50 values (μM) at 37 °C, pH 8.0 against NTE in control fibroblasts (WT/WT [control]) and in subject fibroblasts with the NTE genotype indicated. Subject number is shown in square brackets. Clinical status of subjects is indicated as affected or unaffected with NTE-MND. Data are mean values ± SEM; number of separate determinations shown in parentheses. None of the values was significantly different from that of WT/WT [control] (p > 0.05).
Fig. 3 depicts the distribution of IC50 values for CPO. Fibroblasts homozygous for the M1012V mutation had a significantly elevated value that was 4.5 times that of the control. Although fibroblasts heterozygous for this mutation (M1012V) had a CPO IC50 value that was over twice that of the control value, this was not statistically significant.
Figure 3.
Chlorpyrifos oxon (CPO) 20-min IC50 values (μM) at 37 °C, pH 8.0 against NTE in control fibroblasts (WT/WT [control]) and in subject fibroblasts with the NTE genotype indicated. Subject number is shown in square brackets. Clinical status of subjects is indicated as affected or unaffected with NTE-MND. Data are mean values ± SEM; number of separate determinations shown in parentheses. ***Significantly different from WT/WT [control], p < 0.001.
4. Discussion
NTE activity has been measured in cultured cells from a variety of sources. These include human neuroblastoma (SH-SY5Y and variants) (Ehrich et al., 1994; Hong et al, 2003; Massicotte et al., 2005; Nostrandt and Ehrich, 1992; Pope et al., 1995; Veronesi and Ehrich, 1993); mouse neuroblastoma (NB41A3) (Veronesi and Ehrich, 1993); human cervical adenocarcinoma (HeLa); rat adrenal pheochromocytoma (PC-12) and rat glioma (C6) (Li and Casida, 1997; 1998); bovine and porcine adrenal medullary chromaffin cells (Romero et al., 2006); mouse primary cortical and cerebellar granule cells and astrocytes (Read et al., 2007); mouse inner medullary collecting duct cells (Gallazzini et al., 2006); and, most recently, mouse embryonic stem cells (Pamies et al., 2010).
To our knowledge, our data represent the first demonstration of NTE activity in cultured human skin fibroblasts. Our findings indicate that analysis of NTE in cultured skin fibroblasts provides a useful resource to study the biochemistry of NTE-related neurodegeneration and by extension its pathogenesis.
NTE specific activities from control fibroblasts (15 units) and fibroblasts from subject 1 without NTE pathogenic mutation (14 units) are comparable to values reported for human neuroblastoma cell lines SK-N-SH (7.2 units), SY-5Y (7.6 units), and differentiated SH-SY5Y (21 units) (Ehrich and Veronesi 1995; Nostrandt and Ehrich, 1992). Moreover, the 20-min IC50 values for MIP against the NTE activity in fibroblasts from the control subject and the unaffected subject with normal NTE sequence (8.2 and 9.5 μM, respectively) are within the range of values previously reported for hen or human brain NTE or NEST (median 7.3 μM; interquartile range, 4.4-10.8 μM) (Hein et al., 2010). Similarly, the 20-min IC50 values for CPO against the NTE activity in control fibroblasts of 0.16 μM and those from subject 1 (0.19 μM) are within the range of previously reported values for hen or human NTE or NEST (median, 0.20 μM; interquartile range, 0.13–0.27 μM) (Hein et al., 2010). Taken together, these biochemical parameters serve to establish the suitability of cultured skin fibroblasts for NTE studies.
Our studies demonstrate conclusively that NTE-MND subjects bearing homozygous or compound heterozygous disease-specific NTE mutations have reduced NTE specific activity in cultured skin fibroblasts. These results confirm prior studies that demonstrated reduced activity when disease-specific NTE mutations were introduced into NEST constructs (Hein et al., 2010). There was no significant difference in NTE specific activity between fibroblasts from the NTE-MND subject who was homozygous for the M1012V mutation (Fig. 1, subject 5) and the two NTE-MND subjects who were compound heterozygous for mutations R890H and NTE insertion (Fig. 1, subjects 6 and 7).
Interestingly, we observed greater reductions in fibroblast NTE activity in two unaffected subjects who were heterozygous for NTE insertion mutation (Fig. 1, subjects 8 and 9) compared to affected subjects (either homozygous for NTE M1012V or compound heterozygous for NTE R890H and NTE insertion). These unaffected subjects were asymptomatic and had normal neurologic examinations. As these unaffected NTE insertion heterozygotes were three (sibling) and seven (parent) decades older than the age-of-symptom onset (early childhood), it is considered quite likely that they will remain neurologically unaffected.
These findings indicate that reduced NTE specific activity, as measured by phenyl valerate hydrolysis, is not sufficient to cause progressive neurodegeneration. This conclusion must be tempered by consideration of the fact that the analysis was performed on non-neuronal cells (fibroblasts) and the assumption that NTE specific activity in neurons would be similar.
Our studies also indicate that some but not all disease-specific NTE mutations altered the enzymological characteristics of NTE. Whereas fibroblasts with homozygous M1012V mutation, compound heterozygous R890H/NTE insertion mutations, and heterozygous NTE insertion mutation displayed reduced PV hydrolase activity, only fibroblasts homozygous for M1012V mutation exhibited significant increase in the CPO IC50 (all pairwise comparisons, p < 0.01 for comparison with subject 4 and p < 0.001 for comparisons with all other subjects). This result is consistent with our recent findings (Hein et al., 2010) in NEST constructs, where the M1012V mutation produced changes in inhibitory constants as well as specific activity, but the R890H mutation produced changes only in specific activity. The fact that the heterozygosity for the NTE insertion mutation reduced the specific activity to 40-43% of control is consistent with abolishing the activity contributed by one allele without any other effect on the enzymological characteristics that were investigated.
Our results underscore the importance of examining mechanisms other than reduction of NTE specific activity by which NTE disturbance (both through organophosphorylation and by genetic mutation) may be pathogenic. For example, recent work has shown that the Drosophila NTE homologue SWS binds to cAMP protein kinase (PKA) catalytic subunit C3 and acts similarly to the PKA R1 regulatory subunit to inhibit PKA-C3 (Bettencourt da Cruz et al., 2008). Furthermore, neurodegeneration in sws Drosophila mutants is exacerbated by increased PKA-C3 expression. Whereas expression of wild-type sws cDNA rescued neurodegeneration, expression of sws cDNA bearing mutations that disturbed PKA-C3 interaction had significantly reduced rescue ability. From these observations, the authors proposed that disruption of SWS regulation of PKA-C3 leads to neurodegeneration (Bettencourt da Cruz et al., 2008). Finding that mammalian NTE has similar PKA regulatory properties as its SWS homologue would expand the possible mechanisms (including altered phosphorylation of target proteins and/or cAMP-regulated gene expression) by which NTE disturbance leads to neurodegeneration.
It is also possible that NTE-mediated motor neuron degeneration is unrelated to either reduction in its catalytic activity or its putative role in cAMP-dependent protein kinase regulation. In particular, protein misfolding and consequent ER stress is emerging as a mechanism common to many neurodegenerative disorders (Luo and Le, 2010), including Alzheimer's disease (Salminen et al., 2009), amyotrophic lateral sclerosis (Johnson et al., 2009; Kerman et al., 2010), Parkinson's disease (Bandopadhyay and de Belleroche, 2010), and polyglutamine-expansion disorders (Williams and Paulson, 2008). Moreover, axonal degeneration is increasingly being recognized as an early event in neurodegenerative diseases previously classified as primary neuronopathies (Vickers et al., 2009). Furthermore, NTE is localized in the ER and either its inhibition by neuropathic OP compounds or disruption of its encoding gene results in intraaxonal membranous inclusions and axonal degeneration (Glynn, 2006; Read et al., 2010). Thus, it is possible that NTE disturbance by irreversible organophosphorylation and/or pathogenic mutation leads to progressive axonal degeneration via protein misfolding and initiation of the unfolded protein response. Examining these mechanisms will provide insight into NTE-mediated pathogenesis and treatment possibilities.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NINDS R01NS053917), the Department of Veterans Affairs (Merit Review Awards), the Spastic Paraplegia Foundation, and the generous support from the Paul and Lois Katzman Family Fund (to J.K.F.); the National Ataxia Foundation (to S.R.); and postdoctoral support (to N.D.H.) from the University of Michigan Institute for Clinical and Health Research (supported by NIH award UL1RR024986). We gratefully acknowledge the participation of patients and their families, without whom this research would not have been possible.
Footnotes
Conflict of Interest: JKF and SRR jointly hold patent #7384748 B2 that applies to the use of neuropathy target esterase (NTE) gene analysis for diagnosis of neurologic disease; however, the patent has not been licensed and no royalties have been obtained. RJR is a co-inventor on a patent application #12/121,389 on a nanostructured biosensor containing neuropathy target esterase activity that has potential applications for detection of neuropathic organophosphorus compounds; however, the patent has not been issued and no income has been received.
References
- Akassoglou K, Malester B, Xu J, Tessarollo L, Rosenbluth J, Chao MV. Brain-specific deletion of neuropathy target esterase/swisscheese results in neurodegeneration. Proc Natl Acad Sci USA. 2004;101:5075–5080. doi: 10.1073/pnas.0401030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson's disease: emerging role of molecular chaperones. Trends Mol Med. 2010;16:27–36. doi: 10.1016/j.molmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Bettencourt da Cruz A, Wentzell J, Kretzschmar D. Swiss cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3. J Neurosci. 2008;28:10885–10892. doi: 10.1523/JNEUROSCI.3015-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Richardson RJ. Neurotoxic esterase: characterization of the solubilized enzyme and the conditions for its solubilization from chicken brain microsomal membranes with ionic, zwitterionic, or nonionic detergents. Biochem Pharmacol. 1987;36:1393–1399. doi: 10.1016/0006-2952(87)90104-3. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–281. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M, Correll L, Veronesi B. Neuropathy target esterase inhibition by organophosphorus esters in human neuroblastoma cells. Neurotoxicology. 1994;15:309–13. [PubMed] [Google Scholar]
- Ehrich M, Veronesi B. Esterase comparison in neuroblastoma cells of human and rodent origin. Clin Exp Pharmacol Physiol. 1995;22:385–386. doi: 10.1111/j.1440-1681.1995.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Correll L, Veronesi B. Acetylcholinesterase and neuropathy target esterase inhibitions in neuroblastoma cells to distinguish organophosphorus compounds causing acute and delayed neurotoxicity. Fundam Appl Toxicol. 1997;38:55–63. doi: 10.1006/faat.1997.2330. [DOI] [PubMed] [Google Scholar]
- Fernández-Murray JP, McMaster CR. Phosphatidylcholine synthesis and its catabolism by yeast neuropathy target esterase 1. Biochim Biophys Acta. 2007;1771:331–336. doi: 10.1016/j.bbalip.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Gallazzini M, Ferraris JD, Kunin M, Morris RG, Burg MB. Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc Natl Acad Sci U S A. 2006;103:15260–15265. doi: 10.1073/pnas.0607133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn P. Neuropathy target esterase and phospholipid deacylation. Biochim Biophys Acta. 2005;1736:87–93. doi: 10.1016/j.bbalip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Glynn P. A mechanism for organophosphate-induced delayed neuropathy. Toxicol Lett. 2006;162:94–97. doi: 10.1016/j.toxlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hein ND, Stuckey JA, Rainier SR, Fink JK, Richardson RJ. Constructs of human neuropathy target esterase catalytic domain containing mutations related to motor neuron disease have altered enzymatic properties. Toxicol Lett. 2010;196:67–73. doi: 10.1016/j.toxlet.2010.03.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MS, Hong SJ, Barhoumi R, Burghardt RC, Donnelly KC, Wild JR, Venkatraj V, Tiffany-Castiglioni E. Neurotoxicity induced in differentiated SK-N-SH-SY5Y human neuroblastoma cells by organophosphorus compounds. Toxicol Appl Pharmacol. 2003;186:110–118. doi: 10.1016/s0041-008x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Organophosphorus and other inhibitors of brain ‘neurotoxic esterase’ and the development of delayed neurotoxicity in hens. Biochem J. 1970;120:523–531. doi: 10.1042/bj1200523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. Improved assay of neurotoxic esterase for screening organophosphates for delayed neurotoxicity potential. Arch Toxicol. 1977;37:113–115. doi: 10.1007/BF00293860. [DOI] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJI, Ford SR, Seehra K, Malave VB, Baenziger NL. Alzheimer's disease skin fibroblasts selectively express a bradykinin signaling pathway mediating tau protein Ser phosphorylation. FASEB J. 2003;17:2319–2321. doi: 10.1096/fj.02-1147fje. [DOI] [PubMed] [Google Scholar]
- Kayyali US, Moore TB, Randall JC, Richardson RJ. Neurotoxic esterase (NTE) assay: optimized conditions based on detergent-induced shifts in the phenol/4-aminoantipyrine chromophore spectrum. J Anal Toxicol. 1991;15:86–89. doi: 10.1093/jat/15.2.86. [DOI] [PubMed] [Google Scholar]
- Kerman A, Liu HN, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The Swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci. 1997;17:7425–7432. doi: 10.1523/JNEUROSCI.17-19-07425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp TJ, Richardson RJ. Relative inhibitory potencies of chlorpyrifos oxon, chlorpyrifos methyl oxon, and mipafox for acetylcholinesterase versus neuropathy target esterase. J Toxicol Environ Health, Part A. 2003;66:1145–1157. doi: 10.1080/15287390306360. [DOI] [PubMed] [Google Scholar]
- Li W, Casida JE. Actions of two highly potent organophosphorus neuropathy target esterase inhibitors in mammalian cell lines. Toxicol Lett. 1997;92:123–130. doi: 10.1016/s0378-4274(97)00047-7. [DOI] [PubMed] [Google Scholar]
- Li W, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- Luo GR, Le WD. Collective roles of molecular chaperones in protein degradation pathways associated with neurodegenerative diseases. Curr Pharm Biotechnol. 2010;11:180–187. doi: 10.2174/138920110790909740. [DOI] [PubMed] [Google Scholar]
- Massicotte C, Knight K, Van der Schyf CJ, Jortner BS, Ehrich M. Effects of organophosphorus compounds on ATP production and mitochondrial integrity in cultured cells. Neurotox Res. 2005;7:203–217. doi: 10.1007/BF03036450. [DOI] [PubMed] [Google Scholar]
- Moser M, Li Y, Vaupel K, Kretzschmar D, Kluge R, Glynn P, Buettner R. Placental failure and impaired vasculogenesis result in embryonic lethality for neuropathy target esterase-deficient mice. Mol Cell Biol. 2004:1667–1679. doi: 10.1128/MCB.24.4.1667-1679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlig-Versen M, Bettencourt da Cruz A, Tschape JA, Moser M, Buttner R, Athenstaedt K, Kretzschmar D. Loss of swiss cheese/neuropathy target esterase activity causes disruption of phosphatidylcholine homeostasis and neuronal and glial death in adult Drosophila. J Neurosci. 2005;25:2865–2873. doi: 10.1523/JNEUROSCI.5097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostrandt AC, Ehrich M. Development of a model cell culture system in which to study early effects of neuropathy-inducing organophosphorus esters. Toxicol Lett. 1992;60:107–114. doi: 10.1016/0378-4274(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Pamies D, Reig JA, Vilanova E, Sogorb MA. Expression of neuropathy target esterase in mouse embryonic stem cells during differentiation. Arch Toxicol. 2010 doi: 10.1007/s00204-010-0518-8. in press. [DOI] [PubMed] [Google Scholar]
- Pope C, di Lorenzo K, Ehrich M. Possible involvement of a neurotrophic factor during the early stages of organophosphate-induced delayed neurotoxicity. Toxicol Lett. 1995;75:111–117. doi: 10.1016/0378-4274(94)03167-6. [DOI] [PubMed] [Google Scholar]
- Rainier S, Bui M, Mark E, Thomas D, Tokarz D, Ming L, Delaney C, Richardson RJ, Albers JW, Matsunami N, Stevens J, Coon H, Leppert M, Fink JK. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. 2008;82:780–785. doi: 10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainier S, Albers JW, Dyck PJ, Eldevik OP, Richardson RJ, Fink JK. Motor neuron disease due to neuropathy target esterase gene mutation: clinical features of the index family. Muscle & Nerve. 2010 doi: 10.1002/mus.21777. in press. [DOI] [PubMed] [Google Scholar]
- Read DJ, Langford L, Barbour HR, Forshaw PJ, Glynn P. Phospholipase B activity and organophosphorus compound toxicity in cultured neural cells. Toxicol Appl Pharmacol. 2007;219:190–195. doi: 10.1016/j.taap.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Read DJ, Li Y, Chao MV, Cavanagh JB, Glynn P. Neuropathy target esterase is required for adult vertebrate axon maintenance. J Neurosci. 2009;29:11594–11600. doi: 10.1523/JNEUROSCI.3007-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DJ, Li Y, Chao MV, Cavanagh JB, Glynn P. Organophosphates induce distal axonal damage, but not brain oedema, by inactivating neuropathy target esterase. Toxicol Appl Pharmacol. 2010;245:108–115. doi: 10.1016/j.taap.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Romero D, Quesada E, Sogorb MA, García-Fernández AJ, Vilanova E, Carrera V. Comparison of chromaffin cells from several animal sources for their use as an in vitro model to study the mechanism of organophosphorous toxicity. Toxicol Lett. 2006;165:221–229. doi: 10.1016/j.toxlet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi B, Ehrich M. Differential cytotoxic sensitivity in mouse and human cell lines exposed to organophosphate insecticides. Toxicol Appl Pharmacol. 1993;120:240–246. doi: 10.1006/taap.1993.1108. [DOI] [PubMed] [Google Scholar]
- Vickers JC, King AE, Woodhouse A, Kirkcaldie MT, Staal JA, McCormack GH, Blizzard CA, Musgrove REJ, Mitew S, Liu Y, Chuckkowree JA, Bibari O, Dickson TC. Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res Bull. 2009;80:217–223. doi: 10.1016/j.brainresbull.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vose SC, Fujioka K, Gulevich AG, Lin AY, Holland NT, Casida JE. Cellular function of neuropathy target esterase in lysophosphatidylcholine action. Toxicol Appl Pharmacol. 2008;232:376–383. doi: 10.1016/j.taap.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Hemming ML, Allen DM, Quistad GB, Casida JE, Barlow C. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nat Genet. 2003;33:477–485. doi: 10.1038/ng1131. [DOI] [PubMed] [Google Scholar]
- Wijeyesakere SJ, Richardson RJ. Neuropathy target esterase. In: Krieger R, editor. Hayes' Handbook of Pesticide Toxicology. 3rd. Chapter 67. Elsevier/Academic Press; San Diego: 2010. pp. 1435–1478. [Google Scholar]
- Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccheo O, Dinsdale D, Meacock PA, Glynn P. Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J Biol Chem. 2004;279:24024–24033. doi: 10.1074/jbc.M400830200. [DOI] [PubMed] [Google Scholar]