Abstract
Comparative biogerontology has much to contribute to the study of aging. A broad range of aging rates have evolved to meet environmental challenges, and understanding these adaptations can produce valuable insights into aging. The supra Phylum Lophotrochozoa is particularly understudied and has several groups that have intriguing patterns of aging. Members of the Lophotrochozoan phylum Rotifera are particularly useful for aging studies because cohort life tables can be conducted with them easily, and biochemical and genomic tools are available for examining aging mechanisms. This paper reviews a variety of caloric restriction (CR) regimens, small molecule inhibitors, and dietary supplements that extend rotifer lifespan, as well as important interactions between CR and genotype, antioxidant supplements, and TOR and jun-N-terminal kinase (JNK) pathways, and the use of RNAi to identify key genes involved in modulating the aging response. Examples of how rapamycin and JNK inhibitor exposure keeps mortality rates low during the reproductive phase of the life cycle are presented, and the ease of conducting life table experiments to screen natural products from red algae for life extending effects is illustrated. Finally, experimental evolution to produce longer-lived rotifer individuals is demonstrated, and future directions to determine the genetic basis of aging are discussed.
Keywords: comparative biogerontology, caloric restriction, antioxidants, TOR, JNK, aging, evolution
Introduction
There is great diversity in aging rates among species, geographical populations, and mutants within species. This variation is a rich resource to explore loss of function and gain of function in aging processes (Austad 2010). A variety of aging rates have evolved to meet the challenges of specific environments, and understanding these adaptations can produce valuable insights into aging (Deweerdt 2012). An important observation from examining the phylogenetic distribution of aging models is that most animal phyla have not been explored. The supraphylum Lophotrochozoa is particularly understudied (Ungvari & Philipp 2011), despite having several phyla with intriguing properties (Edgecombe et al. 2011), including the great longevity of molluscs, regeneration in planarians, and the asexual-sexual reproduction of rotifers (Austad 2009). The comparative investigation of exceptional animals holds promise for identifying key aging mechanisms that enable some animals to live long lives and resist a variety of environmental stressors.
Investigating variation in aging among more experimentally tractable invertebrate groups could help answer intriguing mysteries of vertebrate aging, such as why humans have the longest lifespan of any primate, and live four times longer than similar-sized mammals such as deer and cougars (Deweerdt 2012). Why do birds tend to live longer than mammals of similar size, and why are bird cells three- to ten-fold more resistant to many of stressors than cells from rodents of the same size (Harper et al. 2007)? How is stress resistance correlated to aging rate (Harper et al. 2011)? A number of long-lived organisms have enhanced protein stability, and this trait most reliably associates with long life in bats, naked mole-rats, and clams (Austad 2010).
Research on model organisms such as mice, roundworms, fruit flies, and yeast has dramatically advanced our understanding of aging over the past 50 years. For all of the explanatory power of genetically tractable models, these organisms are not the only approach to understanding the mechanisms of aging. Questions remain, like how representative are these models of animals in general and humans in particular? A comparative approach can provide new insights beyond those that have come from model organisms. Comparative biogerontology is a powerful alternative method to investigate cellular and molecular mechanisms of aging. By leveraging natural variants produced over evolutionary time, we are likely to be able to identify new pathways that can serve as targets for aging interventions.
Recently, a variety of insights have been produced on the biology of aging using animals from the Lophotrochozoan phylum Rotifera. Rotifers are microinvertebrates with about 1000 cells and a level of complexity similar to nematodes. Their small size, short generation time, and ease of culture, and new genetic tools make them well suited to studies of aging. Snell (2014) has reviewed advances in the biology of aging using rotifers over the past 20 years, emphasizing their unique contributions in understanding aging. In this paper, we summarize the advantages of a comparative approach to studying aging and describe recent advances using a rotifer model. We have employed cohort life table assays to explore the effects on lifespan of a variety of caloric restriction (CR) regimens, small molecule inhibitors, and dietary supplements, and have probed pathways using RNAi. New data from cohort life tables is presented to illustrate how the analysis of age-specific mortality rates can reveal how the fine structure of aging rates changes over the lifespan.
Comparative biology of lifespan extension by CR
We are making use of the intrinsic advantages of monogonont rotifers to understand the gene regulatory networks involved in producing lifespan extension through CR. In the first phase of this work, we measured the lifespan and fecundity of three different reproductive modes (asexual, sexual, and asexual from dormant resting eggs) of the monogonont rotifer Brachionus manjavacas under six food concentrations (chronic caloric restriction, CCR) ranging from ad libitum (AL) feeding to starvation and to alternate day feeding and starvation (intermittent fasting, IF). The response to CR varied with reproductive mode and degree or type of CR, with differences in lifespan, fecundity, and the trade-off between the two (Gribble & Mark Welch 2013). More recently, we extended earlier work demonstrating a maternal effect of CR on lifespan of offspring (Kaneko et al. 2011) by documenting that maternal CR can partially rescue the deleterious effects of maternal age on the lifespan of offspring (Gribble et al. 2014). To understand the intraspecific evolutionary conservation of lifespan extension by CR, we tested the effects of CCR and IF in 12 isolates from a group of closely related species including B. manjavacas (Gribble et al. forthcoming). Lifespan under AL feeding varied among isolates and predicted the lifespan response to CCR: longer lived isolates under AL were less likely to have a significant increase in lifespan under CCR. Lack of trade-off between lifespan and fecundity under CCR, and differences in lifespan and fecundity under CCR and IF, even when average food intake was similar, suggest (a) that longevity changes are not always directly determined by energy intake and (b) that CCR and IF regimens extend lifespan through diverse genetic mechanisms.
Lifespan extension by small molecule inhibitors and dietary supplements
Working with a variety of antioxidants, Snell et al. (2012) showed that exposing rotifers to certain combinations of antioxidant supplements can produce up to about 20% lifespan extension, but most antioxidants had no effect. Supplementation of rotifer diets with most antioxidants produced no lifespan extension. However, 12% of the 60 two-way antioxidant pairings tested yielded significant lifespan extension. Interactions among pairs of the antioxidants trolox, N-acetyl cysteine, L-carnosine, and EUK-8 were the most efficacious at extending lifespan.
A significant interaction between inhibitors of the TOR kinase signalling pathway (rapamycin) and the jun-N-terminal kinase pathway (JNK) was observed (Snell et al. 2014). Exposure to 1 μM of either inhibitor extended mean rotifer lifespan about 35%, but when both pathways were simultaneously inhibited, 65% greater lifespan extension was recorded. Exposure to a combination of rapamycin and JNK inhibitors also conveyed greater protection to starvation, UV, and osmotic stress than either inhibitor alone. These observations lead us to conclude that nutrient sensing is a central regulator of aging rate in rotifers. Complex interactions among pathways adjust metabolism and aging rate to environmental circumstances. There are strong interactions between genotype and environment that determine which interventions accelerate or retard aging and by how much.
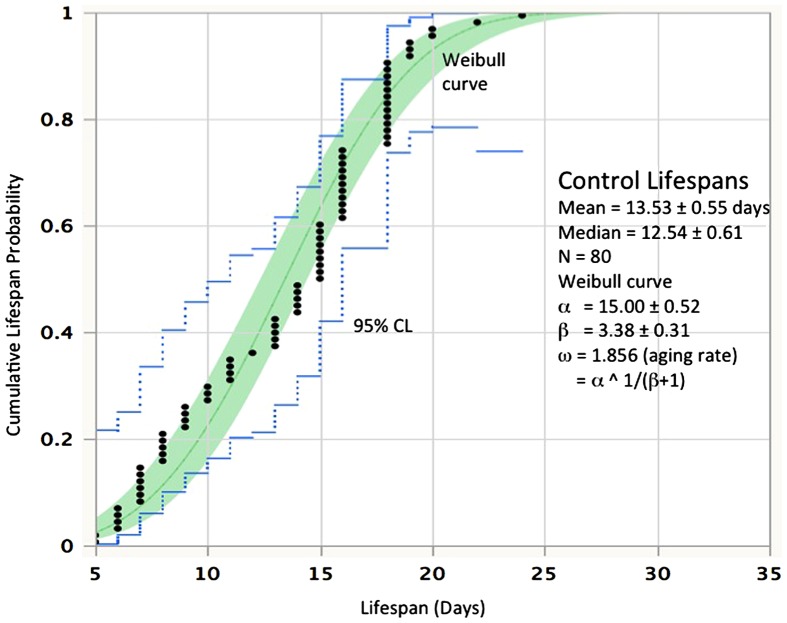
An example of the data from rotifer cohort life tables is presented in Figure 1 (see Snell et al. 2014 for methods) to illustrate how age-specific mortality rates can change over the lifespan and how mortality rates are altered by interventions. The cumulative probabilities of living a lifespan from 5–24 days are plotted with 95% confidence limits. These data fit a Weibull distribution, with a mean lifespan of 13.5 days ± 0.55 and a median lifespan of 12.5 days ± 0.61. Parameters α and β from the Weibull equation can be used to estimate aging rate ω = α (1/(β + 1)) (Ricklefs 2008). Other treatments in this experiment were exposed to either 1 μM rapamycin or 1 μM JNK inhibitor (data not shown). These treatments extended rotifer mean and median lifespan by 35%. Interestingly, despite the sizeable changes in mean lifespan with rapamycin and JNK inhibitor treatment, there was little change in overall aging rate. The aging rate (ω) in the control was 1.86, compared to 1.93 and 1.88 in the rapamycin and JNK inhibitor treatments, respectively; the treatments differed from control by only 1–4%.
Figure 1.
The distribution of lifespans in untreated controls at 22 °C. Lifespan is in days, probability is the cumulative frequency of lifespan. Black dots represent the lifespans of individual rotifers from a cohort of 80. The continuous line is the Weibull fit to the data and the dotted lines define the 95% confidence limits.
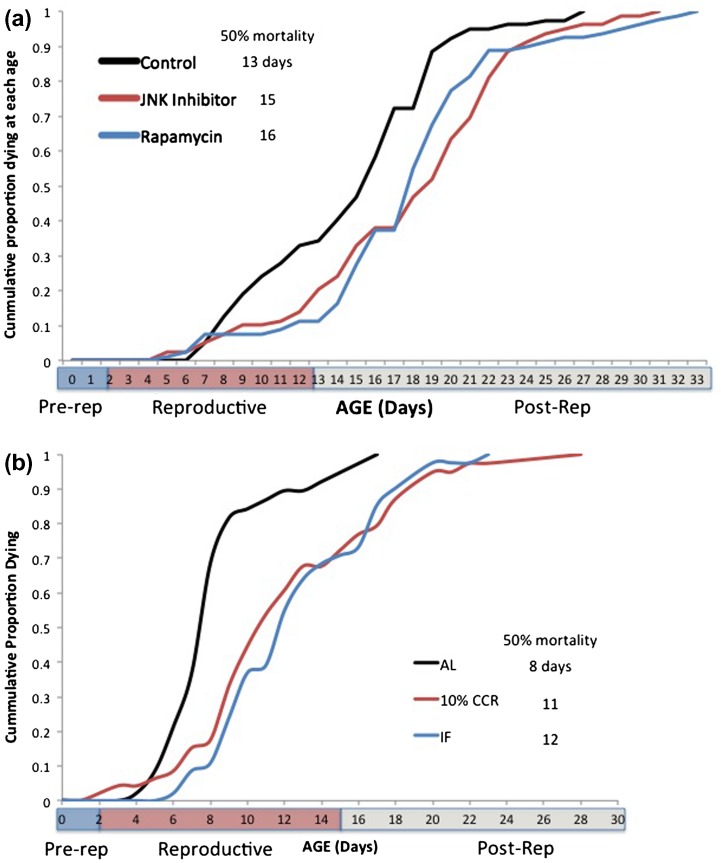
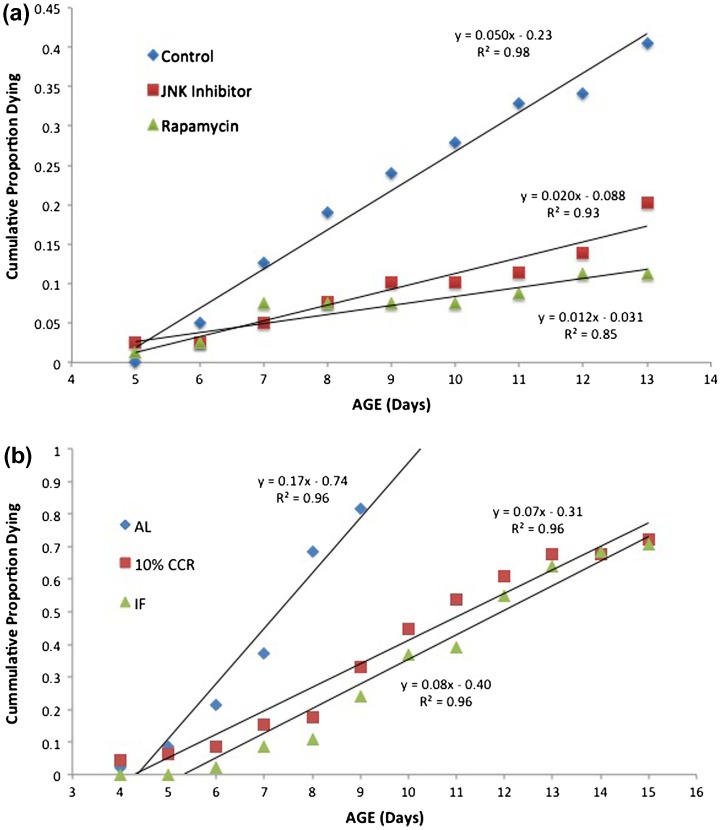
Closer examination of aging rates, however, reveals significant differences. Life tables yield age-specific mortality data, which can be plotted as cumulative mortality (Figure 2). When the reproductive phases of the life cycle are superimposed, it is clear that the control treatment suffers more mortality in the reproductive phase than the rapamycin and JNK inhibitor treatments. This is further demonstrated by the time when 50% mortality is reached, which is day 13 in the control, compared to days 16 and 15 in the rapamycin and JNK inhibitor treatments, respectively. In the control, mortality begins to increase sharply after day 6, but this sharp increase does not occur until day 13 in the rapamycin and JNK inhibitor treatments. Deeper analysis of the 5–13-day age classes can be seen in Figure 3. Change in mortality rates is linear over this portion of the curves, with R 2 ranging from 0.85 to 0.98. The slopes of the rapamycin and JNK inhibitor treatments are significantly smaller than the control (t = 7.67, 8.10, df = 14, p < 0.001). Somehow, rapamycin and JNK inhibitor treatment prevents increases in aging rate in the critical reproductive phase of the life cycle. This result motivates more experiments to reveal the mechanism of protection afforded by rapamycin and JNK inhibitor.
Figure 2.
Cumulative age-specific mortality in Brachionus manjavacas. (a) The rapamycin and JNK inhibitor treatments received 1 µM continuous exposure. (b) AL – ad libitum feeding, CCR – chronic caloric restriction, IF – intermittent fasting. 50% mortality is the age at which cumulative mortality reaches 50%. Pre-rep – pre-reproductive, post-rep – post-reproductive phase of the life cycle.
Figure 3.
Comparison of mortality rates in the reproductive phase. (a) The rapamycin and JNK inhibitor treatments received 1 µM continuous exposure. (b) AL – ad libitum feeding, CCR – chronic caloric restriction, IF – intermittent fasting.
Rotifers are well suited for screening large numbers of small molecules for lifespan extending effects. Because of the complex interactions among pathways described above, it is especially important to screen for lifespan extending effects with whole animal assays. Rotifers are one of the easiest and fastest animals on which life table experiments can be performed. We have exploited this feature in a screen of natural products from marine red algae that are capable of extending rotifer lifespan (Snare et al. 2013). Rotifer lifespan increased 9–14% upon exposure to 3 of 200 natural products extracted from red algae. Bioassay guided fractionation indicated that lipids were responsible for the primary activity, but through a mechanism independent of their antioxidant activity.
Experimental evolution of aging
Experimental evolution of longevity was demonstrated in rotifers by Smith and Snell (2014). Over 84 generations (one year), asexual females evolved a 26% increase in lifespan and a 23% decrease in the rate of aging when grown in an environment with constant food and population density compared with a highly dynamic, ephemeral environment. Greater longevity was selected for in this low-hazard environment, and lifespan and senescence rate was shown to be evolutionarily adjustable, with selection working primarily on the length of the reproductive lifespan.
The advent of next-generation sequencing technology and recent advances in rotifer genomic resources now make it possible to identify and manipulate genes involved in modulating aging in rotifers. It is fairly easy to conduct experimental evolution experiments with rotifers and then use RNA-Seq analysis to identify specific genes that have evolved different expression patterns. RNA-Seq is also being used to measure differential expression across the genome in response to different CR regimens. RNAi allows specific targeted gene expression knock-down, as we have demonstrated for TOR and JNK (Snell et al. 2014).
Future directions
It is increasingly clear that aging is regulated by complex networks of parallel pathways. This produces complex interactions, so that single compounds will not likely be effective at lifespan extension (Hopkins 2008). Rather, multi-target drugs and combinatorial therapies are likely to be more efficacious (de Magalhaes et al. 2012). We expect that the greatest extension of longevity will likely be achieved by simultaneously inhibiting multiple pathways. Consequently, an important line of investigation is to identify positive interactions among pathways. Rotifers have proven especially useful in beginning to identify such relationships. In addition to the experiments described above, we have exciting new results demonstrating interactions between the hexokinase inhibitor 2-deoxyglucose and rapamycin, and the dietary supplement glycerol. These can be used to shift metabolism away from glycolysis and mitigate the pro-aging effects of glucose. Moreover, co-administration of these compounds lowers their efficacious doses and reduces their undesirable side effects.
A second recurring theme that we have observed is that the response to interventions depends upon genetic background. The lifespan and fecundity response to CR is strongly dependent upon genotype, and different CR regimens elicit different lifespan outcomes even within the same isolate. Comparing the differential responses of genotypes to CR is likely to produce useful insights into the mechanisms of CR lifespan extension. Using RNA-Seq to identify specific genes involved in these pathways also seems like a productive experimental approach. These genes could then become targets of inhibitors or supplements that diminish or enhance their activity, thereby producing lifespan extension.
Acknowledgments
We express our appreciation for the expert technical assistance provided by Cody Zipperer, Stephanie Teat, Brett Rabeneck, and Corey Rennolds.
Funding
We are grateful for the support of the National Institute of Aging, [grant number R01 AG037960-02] for this work and for a Postdoctoral Fellowship from the Ellison Medical Foundation/American Federation for Aging Research to K. Gribble.
References
- Austad SN. Is there a role for new invertebrate models for aging research? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Methusaleh’s zoo: how nature provides us with clues for extending human health span. Journal of Comparative Pathology. 2010;142:S10–S21. doi: 10.1016/j.jcpa.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweerdt S. Comparative biology: looking for a master switch. Nature. 2012;492:S10–S11. doi: 10.1038/492S10a. [DOI] [PubMed] [Google Scholar]
- Edgecombe GD, Giribet G, Dunn CW, Hejnol A, Kristensen RM, Neves RC, Rouse GW, Worsaae K, Sørensen MV. Higher-level metazoan relationships: recent progress and remaining questions. Organisms Diversity & Evolution. 2011;11:151–172. doi: 10.1007/s13127-011-0044-4. [DOI] [Google Scholar]
- Gribble KE, Mark Welch DB. Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera) The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68:349–358. doi: 10.1093/gerona/gls170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Kaido O, Jarvis G, Mark Welch DB. Patterns of intraspecific variability in the response to caloric restriction. Experimental Gerontology. 2014;51:28–37. doi: 10.1016/j.exger.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Jarvis G, Bock M, Mark Welch DB. Maternal caloric restriction partially rescues the deleterious effects of advanced maternal age on offspring. Aging Cell. Forthcoming doi: 10.1111/acel.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/ace.2007.6.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. Journal of Experimental Biology. 2011;214:1902–1910. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biology. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Functional Ecology. 2011;25:209–216. doi: 10.1111/j.1365-2435.2010.01773.x. [DOI] [Google Scholar]
- de Magalhaes JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-environment interactions that modulate aging: powerful targets for drug discovery. Pharmacological Reviews. 2012;64:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. The evolution of senescence from a comparative perspective. Functional Ecology. 2008;22:379–392. doi: 10.1111/fec.2008.22.issue-3. [DOI] [Google Scholar]
- Smith HA, Snell TW. Differential evolution of lifespan and fecundity between asexual and sexual females in a benign environment. International Review of Hydrobiology. 2014;99:1–8. doi: 10.1002/iroh.201301711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snare DJ, Fields AM, Snell TW, Kubanek J. Lifespan extension of rotifers by treatment with red algal extracts. Experimental Gerontology. 2013;48:1420–1427. doi: 10.1016/j.exger.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW. Rotifers as models for the biology of aging. International Review of Hydrobiology. 2014;99:1–13. doi: 10.1002/iroh.201301707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Fields AM, Johnson RK. Antioxidants can extend lifespan of Brachionus manjavacas (Rotifera), but only in a few combinations. Biogerontology. 2012;13:261–275. doi: 10.1007/s10522-012-9371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Johnson RK, Rabeneck B, Zipperer C, Teat S. Joint inhibition of TOR and JNK pathways interacts to extend the lifespan of Brachionus manjavacas (Rotifera) Experimental Gerontology. 2014;52:55–69. doi: 10.1016/j.exger.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Philipp EER. Comparative gerontology–from mussels to man. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A:295–297. doi: 10.1093/gerona/glq198. [DOI] [PubMed] [Google Scholar]