Abstract
Over the past four decades, the characterization of memory loss associated with Alzheimer's disease (AD) has been extensively debated. Recent iterations have focused on disordered encoding versus rapid forgetting. To address this issue, we used a behavioral pattern separation task to assess the ability of the hippocampus to create and maintain distinct and orthogonalized visual memory representations in patients with amnestic mild cognitive impairment (aMCI) and mild AD. We specifically used a lag-based continuous recognition paradigm to determine whether patients with aMCI and mild AD fail to encode visual memory representations or whether these patients properly encode representations that are rapidly forgotten. Consistent with the rapid forgetting hypothesis of AD, we found that patients with aMCI demonstrated decreasing pattern separation rates as the lag of interfering objects increased. In contrast, patients with AD demonstrated consistently poor pattern separation rates across three increasingly longer lags. We propose a continuum that reflects underlying hippocampal neuropathology whereby patients with aMCI are able to properly encode information into memory but rapidly lose these memory representations, and patients with AD, who have extensive hippocampal and parahippocampal damage, cannot properly encode information in distinct, orthogonal representations. Our results also revealed that whereas patients with aMCI demonstrated similar behavioral pattern completion rates to healthy older adults, patients with AD showed lower pattern completion rates when we corrected for response bias. Finally, these behavioral pattern separation and pattern completion results are discussed in terms of the dual process model of recognition memory.
Keywords: recognition memory, recollection, familiarity, encoding, hippocampus
INTRODUCTION
While there is no doubt that Alzheimer's disease (AD) causes profound deficits in episodic memory, the exact nature of these deficits has been extensively debated over the last 40 years. Much of the early literature conceptualized the disease as a disorder of memory storage, owing to the fact that cholinesterase inhibitors enhanced storage of information into long-term memory in patients with AD and healthy controls (Drachman, 1977; Davis et al., 1978; Mohs & Davis, 1982). However, most of these studies were pharmacological investigations targeting physiologic mechanisms of memory loss in patients with dementia and were not focused on characterizing memory deficits from a cognitive neuropsychology perspective. As AD became more prevalent in the literature, cognitive and clinical scientists conceptualized the disease as a disorder of memory retrieval, based on the fact that patients performed worse on tests of free recall than on tests of recognition and that patients can, at times, benefit from contextual cueing at retrieval. Much of this work suggested that information was potentially processed and encoded correctly, but memory representations were unable to be retrieved or patients had difficulty retrieving them based on difficulty matching the stored representation with the test cue (Morris et al., 1983; Kopelman, 1989, 1991; Pollmann et al., 1993). Debate again surfaced when nonhuman investigations provided strong evidence that the cholinergic system plays a vital role in the encoding of information into episodic memory (Fibiger, 1991; Hasselmo et al., 1996), consistent with the budding hypothesis that AD was a disorder of memory encoding rather than storage or retrieval (Becker et al., 1987; Gran-holm & Butters, 1988; Money et al., 1992; Kohler, 1994). The advent of neuroimaging helped to support the encoding hypothesis, with the majority of more recent studies demonstrating that patients with AD show decreased activation of medial temporal lobe structures compared to healthy older adults when learning new information (Rombouts et al., 2000; Kato et al., 2001; Sperling et al., 2003).
For the last decade, cognitive neuroscientists have focused on understanding the intricacies of the encoding deficit in patients with AD, from the molecular to the psychological level, in an attempt to exploit areas of intact functioning and perhaps provide targets for behavioral or pharmacological intervention. In parallel, animal models of memory impairment in AD, and its precursor amnestic mild cognitive impairment (aMCI), provide evidence that AD is a disorder of rapid forgetting (Ridley & Baker, 1991; see McDonald & Overmier, 1998 for review). Some early human studies provided supportive evidence that patients with AD demonstrate more rapid rates of forgetting than healthy older adults and patients with other neurologic disorders (Hart et al., 1988; Salmon et al., 1989; Carlesimo et al., 1993; Reed et al., 1998). However, there were problems cited with the methods used to evaluate rates of forgetting, including failing to equate levels of initial learning between groups. To address these concerns, Christensen et al. (1998) used experimental manipulation to equate levels of initial learning and reported that rates of forgetting did not differ between patients with AD and controls on tests of picture recognition, word recognition, design recognition, or word stem completion (Christensen et al., 1998). More recently, a study focusing on “real-world” memory for the events of 9/11, found that patients with aMCI and mild to moderate AD demonstrated poor encoding of the initial events, but forgetting curves were generally parallel between groups at later time points (Budson et al., 2007). Since this time, there has been little experimental investigation or support for rapid forgetting in patients with aMCI or AD, but clinicians continue to conceptualize and discuss the disease as a disorder of rapid forgetting, primarily based on anecdotal and functional evidence provided by family and caregivers.
There may be a large degree of overlap between the concepts of disordered encoding and rapid forgetting. One might assume that if information is not properly encoded, the resulting memory representation will be rapidly or easily forgotten. However, limitations in experimental design have hindered our ability to address this issue. One way to determine whether patients with aMCI and mild AD suffer from an encoding deficit or whether they have intact encoding but rapid forgetting is to target specific abilities of the hippocampus and observe how they change over time in patients with aMCI and mild AD. In the current study, we elected to target performance on a behavioral task thought to assess the hippocampus's ability to keep memory representations distinct and resistant to confusion (see Yassa & Stark, 2011 and Hunsaker & Kesner, 2013 for reviews). The hippocampus is involved in forming new memories, tagging and storing memories as individual representations, retrieving memories using only partial or degraded cues, and flexibly applying stored memory representations to novel situations (Yassa & Stark, 2011). One of the more vital functions of the hippocampus is pattern completion, a process that recovers a complete, stored representation based on a partial, degraded, or noisy retrieval cue (Bakker et al., 2008). In contrast, the hippocampus is also responsible for maintaining distinct, orthogonal representations for similar patterns of activation to avoid interference from long-term memory and new incoming stimuli. This process, known as pattern separation, is critical for storing individual representations of highly similar sensory inputs and preventing interfering representations from overwriting previously stored information (Yassa & Stark, 2011). The consequences of faulty pattern separation could be devastating, especially for patients with aMCI and AD, leading to confusion over falsely taking a certain medication, or perhaps leading to confusion over landmarks or the identity of relatives.
Computational modeling and rodent work has suggested that the dentate gyrus and its connections to hippocampal sub-region CA3 are responsible for pattern separation (Hunsaker & Kesner, 2013). The perforant path/dentate granule neuron system acts as a competitive learning system used to eliminate shared or redundant features from sensory inputs to produce more orthogonal and distinct representations (Marr, 1971, Treves & Rolls, 1994). These inputs are then transferred to the CA3 autoassociation subregion via the mossy fibers to allow episodic memories to be formed and stored for brief periods of time before consolidation (Treves & Rolls, 1994). Therefore, at encoding, dentate gyrus and CA3 are responsible for the pattern separation and tagging of incoming information so that it can be stored for future recognition of specific items (Hunsaker & Kesner, 2013). Less is known about pattern completion, and recent debate has surrounded whether pattern completion is simply the opposing end of a unitary pattern separation/pattern completion process or a distinct process (see Guzowski et al., 2004). Computational models suggest that synaptic transmission and plasticity in the recurrent CA3 network play a role in pattern completion (Nakashiba et al., 2012), which has been proposed to occur primarily at retrieval when complete recollection occurs based only on a partial or degraded cue (Hunsaker & Kesner, 2013).
In a slight, but important variation, behavioral studies in humans posit that pattern completion occurs when the hippo-campus overwrites or integrates incoming information into a previously stored representation (Yassa & Stark, 2011; Hunsaker & Kesner, 2013 for reviews). Overall, these studies conceptualize behavioral pattern completion as a generalization process in which a subject incorrectly endorses a perceptually similar test lure as an “old” studied item. Human neuroimaging work suggests that the CA1 and subiculum subregions of hippocampus demonstrate BOLD activation during behavioral pattern completion (Kirwan & Stark, 2007; Bakker et al., 2008; Lacy et al., 2011). This behavioral framework posits that when dentate gyrus and its connections to CA3 are unable to effectively pattern separate, CA3 and its connections to CA1 must integrate the incoming input into previously stored representations or overwrite the previous representation (Yassa et al., 2010). In other words, when information enters the hippocampal episodic memory system, if dentate gyrus processes that information as repetitive (i.e., no need to orthogonalize), CA3 must work to retrieve a stored representation based on the cue. If the cue is noisy, partial in nature, or degraded, CA3 and its connections to CA1 must work to integrate the pattern with previous information, resulting in behavioral pattern completion. Under this assumption, pattern completion and pattern separation complement one another (i.e., impaired pattern separation results in “enhanced” pattern completion) or potentially pattern separation and pattern completion comprise two ends of a unitary process that are dynamically at odds with one another (Yassa & Stark, 2011; Hunsaker & Kesner, 2013).
There has been very little work investigating behavioral pattern separation and pattern completion in aMCI or AD. One recent neuroimaging study using a continuous recognition paradigm had patients with aMCI and healthy older adults view repeated items, perceptually similar lure items, and novel items, with random intervening lags between studied items and test cues. Yassa et al. (2010) found that patients with aMCI had impaired behavioral pattern separation (BPS) rates compared to healthy older controls. That is, patients were unable to effectively discriminate between a studied item and a perceptually similar lure item in a manner similar to healthy older adults. The authors also reported that aMCI patients demonstrated BOLD hyperactivity in the dentate gyrus/CA3 subfields of the hippocampus and BOLD hypoactivity in the entorhinal cortex during pattern separation. Additionally, patients had smaller entorhinal and dentate gyrus/CA3 volumes (bilaterally) compared to healthy older adults, suggesting that this region and its connections could be the locus of pattern separation deficits in patients with aMCI. These findings are highly similar with others reporting BOLD hyperactivity in hippocampus as a potential mechanism of compensation (Putcha et al., 2011). Yassa et al. (2010) also reported that patients with aMCI demonstrated a slightly higher pattern completion rate than healthy older adults, suggesting a potential mechanism for the increased false recognition often seen in patients with AD (Budson et al., 2006; Ally, 2012). The results of Yassa and colleagues (2010) were generally supported by a recent study of pattern separation in patients with bilateral hippocampal damage. Kirwan et al. (2012) found that although standard accuracy measures of recognition memory did not differ between the lesion group and a group of age and gender-matched controls, behavioral pattern separation rates were significantly impaired in the lesion group only, suggesting that medial temporal lobe regions may not be required to accomplish standard single item recognition memory decisions, but more complex visual discrimination may require areas such as perirhinal cortex, parahippocampal regions, and hippocampus proper (Saksida & Bussey, 2010).
The pathophysiology of aMCI and AD tends to affect hippo-campal subregions in a mildly variable, but predictable, pattern. Our best effort to characterize the course of pathology in AD throughout the different stages (even pre-aMCI) has been through neuropathologic work (Braak & Braak, 1991; Hyman, 1997; Newell et al., 1999). Recent high-resolution MRI protocols have been fairly successful at subregion volumetric analysis throughout the various stages of pre-clinical and clinical AD, but it is important to note that there have been inconsistencies in the literature. An initial high-resolution T2-weighted volumetric study of hippocampal subregions in healthy older controls, patients with aMCI, and patients with AD revealed that early in the course of the disease, there were no statistical differences in the total volume of the subiculum, CA1, CA3, dentate gyrus, and total hippocampal volume in patients with MCI compared to controls (Mueller et al., 2010). The only noted volumetric difference between these two groups was the CA1 to CA2 transition zone. In contrast, patients with AD demonstrated statistically smaller volumes for all subregions, with the exception of CA3 and dentate gyrus, which appear to remain only modestly affected until later in the disease course (Apostolova et al., 2010; Mueller et al., 2010). A more recent semi-automatic segmenta tion study using T2-weighted MRI reported significantly smaller overall hippocampal volumes, as well as smaller CA1, CA4/dentate gyrus, head, and tail volumes in patients with aMCI compared to healthy older adults (Pluta et al., 2012). The discrepancy in these findings likely has to do with the need for higher neuroimaging resolution and individual variation. Perhaps tasks that tax the pattern separation and pattern completion systems can provide information to help understand aberrant hippo-campal subregion function (Small et al., 2011), as well as how memory breaks down in patients with aMCI and mild AD.
The main goal of the current study was to determine whether patients with aMCI and mild AD properly encode representations that are rapidly forgotten, or whether patients fail to properly encode the representations. We specifically used a lag-based continuous recognition task to understand whether patients’ performance on a behavioral pattern separation task varied as a function of the time and interference provided by a pre-determined set of intervals between the studied item and retrieval cue. In accordance with the rapid forgetting hypothesis of AD, we predicted that patients with aMCI and mild AD would show decreasing behavioral pattern separation rates and increasing pattern completion rates as the lag increased between studied and cue/test pictures. For example, we would expect that patients with aMCI and mild AD show relatively normal pattern separation rates at an intervening lag of four items between the target and cue items, but impaired pattern separation rate at higher lags (i.e., 12 items, 40 items). This pattern of results would suggest that visual representations are properly encoded but degrade rapidly over time, supporting the rapid forgetting hypothesis. In contrast, if patients showed poor pattern separation rates across the three lags, this would lend support to the disorder of encoding hypothesis. Secondarily, we hypothesized that as pattern separation rates decrease over lag, pattern completion rates would increase over lag for patients with aMCI and mild AD. Under this assumption, degraded visual representations should lead to false recognition of lure items as “old” and subsequently leading to increased behavioral pattern completion rates as the lag between study and cue items increases. Patients with aMCI and mild AD commonly show elevated false recognition rates on tasks using highly perceptual overlap between study and test items (Budson et al., 2003; Newsome et al., 2012), which could result from the over-writing or re-writing of a memory representation by the cue item. Incidentally, if this hypothesis were borne out, it would provide supportive evidence that pattern separation and pattern completion comprise two ends of a unitary process that are dynamically at odds with one another rather than two separate processes (see Hunsaker & Kesner, 2013 for review).
MATERIALS AND METHODS
Participants
Participants for this study included 16 patients diagnosed with probable mild AD (eight female), 16 patients diagnosed with aMCI (seven female), and 16 healthy older adults (eight female). All patients with aMCI and mild AD were evaluated by a neurologist and neuropsychologist in the Memory Disorders Clinic in the Department of Neurology at Vanderbilt University Medical Center. To help confirm diagnosis, patients underwent neuroimaging, blood work, and were presented at a case consensus conference before enrolling in the study. Neuro-imaging took place at several different Vanderbilt and outside locations, which resulted in the use of different scanner platforms and imaging sequences. Therefore, structural imaging was not used as part of the experimental design. Patients with aMCI or their caregiver reported a subjective memory complaint, patients demonstrated abnormal memory performance for their age as evidence by performing more than 1.5 standard deviations below the healthy control group on either the free recall or recognition portions of the CERAD World List Memory Test (Morris et al. 1989) but did not report functional impairment in activities of daily living according to the care-giver. Patients with probable mild AD met criteria described by the National Institute on Aging—Alzheimer's Association (McKhann et al., 2011), and were in the mild range of the disease based on Mini Mental Status Exam (MMSE; Folstein et al., 1975) score. Patients with AD were excluded if they scored below 21 on the MMSE. Patients with aMCI were excluded if they scored below 26 on the MMSE. Healthy older adults were excluded if they scored 1.5 standard deviations below the standardized normative means on any aspect of neuropsychological testing or if they scored below 28 on the MMSE. Healthy older adults were recruited from the Nashville community via Vanderbilt IRB-approved e-mail and internet advertisements. Participants were excluded if they had a history of alcohol or drug abuse, stroke or other focal brain injury, or if they were currently diagnosed with clinical depression or another neurodegenerative condition. All participants were tested for normal or corrected-to-normal color vision.
The Behavioral Science Committee Division of the IRB at Vanderbilt University, Nashville, TN, approved this study. All participants completed an informed consent form before participating. Participants were paid $10/hour for participating in this study. Healthy older adults completed a neuropsychological battery, which included the MMSE (Folstein et al., 1975), CERAD Word List (Morris et al., 1989), Trail Making Test Parts A and B (Adjunct General's Office, 1944), Verbal Fluency (Monsch et al., 1992), and the 15-item Boston Naming Test (Mack et al., 1992). All patients in the aMCI and AD groups completed a more comprehensive neuropsychological battery, which included the aforementioned tests, during their visit to the Memory Disorders Clinic.
Stimuli and Procedure
Stimuli were those used in previous studies of behavioral pattern separation (Kirwan & Stark, 2007; Bakker et al., 2008; Toner et al., 2009; Yassa et al., 2010, 2011; Stark et al., 2013; Kim & Yassa, 2013) and have been well characterized in previous work (see Lacy et al., 2011). Two sets of stimuli each consisting of 192 color picture pairs were used to create four counterbalanced lists to control for study status so that all items had an equal opportunity to be correctly judged as “old,” “similar,” or “new.” Participants completed two experimental blocks, in which they saw a total of 232 pictures across the two blocks. Each block consisted of a total of 116 pictures: 18 identical pairs, 18 similar pairs, and 44 unrelated novel items. The items were presented in a continuous recognition sequence with a pseudo-randomized lag manipulation. Over the two experimental blocks, participants were presented with intervening lags of 4, 12, and 40 items, resulting in 12 items per item condition (repeated, lure, novel). See Figure 1 below for a schematic of the behavioral design.
FIGURE 1.
An example schematic of the experimental procedure. The trial sequence moves from left to right, with an example of a lure item at Lag 4 and a repeated item at Lag 12. The gray boxes signify lure pairs, the black boxes signify repeated pairs, and the white boxes signify novel items. The correct answer to each example picture appears below the item. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Participants were instructed that they would see a sequence of pictures continuously presented on the computer screen, one at a time. They were informed that some of the pictures could be judged as “old” (repeated), some of the pictures could be judged as “similar” to previously seen pictures but would not be exactly the same (lure), and some pictures could be judged as “new” (novel). As an example, the experimenter then showed the participant an example of what a repeated (to which they would respond “Old”), lure (to which they would respond “Similar”), and novel (to which they would respond “New”) picture might look like. Participants started the first block when they were able to correctly verbalize a clear understanding of the procedure and instructions. Pictures were shown on the screen for 2500 ms and were immediately followed by a response screen, which displayed the three response options of: “Old,” “Similar,” or “New.” Participants were given as much time as they needed to make a response. A 500-ms inter-stimulus interval was presented before each picture. After participants completed the first block, they were given a short break of approximately 10 minutes. Before they began the second block, participants were explicitly told that none of the pictures from the first block would appear in the second block. Because participants would complete the same basic task as they did in the first block (except with new pictures), the same instructions were repeated before the second block commenced. There was no repetition or overlap between the pictures in the first and second blocks. For the sake of clarity, Table 1 summarizes the terminology and formulas used to derive experimental measures.
TABLE 1.
Terminology and Formulas Used to Examine Pattern Separation and Pattern Completion
| Response |
|||
|---|---|---|---|
| Item Type | “New” | “Similar” | “Old” |
| Novel | Correct rejection rate | Similar bias rate | False alarm rate |
| Lure | Incorrect | Pattern separation rate | Pattern completion rate |
| Repeated | Miss Rate | Incorrect | Hit rate |
All rates express as a percentage.
Bias corrected pattern separation score (BPS) = (pattern separation rate – similar bias rate).
Bias corrected pattern completion score (BPC) = (pattern completion rate – false alarm rate).
RESULTS
Demographic and Neuropsychological Data
It should be noted that one healthy older adult, two patients with aMCI, and four patients with AD were removed from the final dataset due to neuropsychological or experimental performance well outside of the expected range. Specifically, the healthy older adult had neuropsychological scores that suggested mild-to-moderate impairment in executive functioning, one patient with aMCI and one patient with AD responded “similar” to greater than 70% of all items (including novel items), one patient with aMCI and two patients with AD responded “old” to greater than 90% of all items, and one patient with AD became frustrated and did not continue after responding “new” to all items in Block 1. After these subjects were removed, there remained no group differences in age [F(2, 39) < 1], education [F(2, 39) = 1.07, P = 0.353], or gender [χ2(2, N = 39) = 1.27, P = 0.773]. Demographic and neuropsychological data can be seen in Table 2.
TABLE 2.
Demographic and Neuropsychological Data Means and (Standard Deviations) for All Three Groups
| OC | aMCI | AD | |
|---|---|---|---|
| Age | 70.5 (6.9) | 72.9 (7.7) | 72.0 (6.4) |
| Years of education | 15.4 (2.1) | 15.8 (3.0) | 14.7 (2.7) |
| MMSE | 29.43 (0.8) | 28.33 (1.1) | 24.20 (2.24) |
| CERAD Immed Recall | 22.57 (2.3) | 16.88 (5.1) | 12.50 (2.9) |
| CERAD Delayed Recall | 7.79 (1.4) | 4.25 (1.8) | 0.30 (0.7) |
| CERAD Recognition | 9.93 (0.3) | 8.18 (1.7) | 4.20 (2.7) |
| Trails A | 30.93 (10.2) | 32.13 (12.2) | 52.10 (10.6) |
| Trails B | 76.71 (24.4) | 101.26 (48.2) | 167.74 (81.2) |
| FAS | 45.57 (11.1) | 43.88 (9.9) | 30.44 (10.4) |
| CAT | 50.21 (10.0) | 39.75 (8.0) | 24.66 (7.7) |
| BNT (15) | 14.86 (0.5) | 13.93 (0.9) | 11.13 (3.0) |
Pattern Separation, Pattern Completion, and Related Data
In the following section, we provide a standard analysis of behavioral pattern separation and pattern completion rates, as well as recently derived indices of pattern separation and pattern completion to account for differences in response tendencies, or response bias, in patients with aMCI and mild AD. These patients tend to respond “similar” or “old” significantly more than healthy individuals, demonstrating a liberal response bias (see Budson et al., 2006 and Ally, 2012 for reviews). Therefore, previous aging and memory-disordered population studies of pattern separation have used a bias-corrected behavioral pattern separation (BPS) score (Toner et al., 2009; Yassa et al., 2010; Yassa et al., 2011; Stark et al, 2013). Similarly, we created a bias-corrected behavioral pattern completion (BPC) score for the current study to control for baseline false alarm rates in these patient populations. For definitions of key terms and formulas used to generate the BPS and BPC scores, see Table 1. All behavioral data can be seen in Table 3 and we have supplied a detailed figure of all responses made by each group at each lag in Supporting Information A.
TABLE 3.
Behavioral Data Means and (Standard Deviations) for All Three Groups
| OC | aMCI | AD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hit Rate | 0.89 (0.11) | 0.71 (0.21) | 0.50 (0.23) | ||||||
| Correct Rejection Rate | 0.98 (0.02) | 0.90 (0.10) | 0.68 (0.19) | ||||||
| Baseline FA rate | 0.01 (0.01) | 0.02 (0.03) | 0.12 (0.11) | ||||||
| Accuracy (Pr) | 0.88 (0.08) | 0.69 (0.21) | 0.38 (0.16) | ||||||
| Similar Bias Rate | 0.02 (0.02) | 0.07 (0.08) | 0.20 (0.12) | ||||||
| Lag |
Lag |
Lag |
|||||||
| 4 | 12 | 40 | 4 | 12 | 40 | 4 | 12 | 40 | |
| BPS score | 0.48 (0.18) | 0.51 (0.15) | 0.49 (0.14) | 0.46 (0.14) | 0.30 (0.23) | 0.17 (0.20) | 0.13 (0.21) | 0.14 (0.18) | 0.15 (0.18) |
| BPC score | 0.39 (0.13) | 0.39 (0.17) | 0.42 (0.13) | 0.33 (0.12) | 0.45 (0.23) | 0.47 (0.24) | 0.31 (0.21) | 0.29 (0.22) | 0.20 (0.22) |
Novel Items
The initial analysis was performed on novel items only. A repeated measures ANOVA with the factors of Group (OC, aMCI, AD) and Response Type (old, similar, new) was performed to examine responses for all three groups (lag is not included in this analysis, as novel items appeared pseudo-randomly throughout the continuous recognition paradigm). The ANOVA revealed a marginal effect of Group [F(2, 37) = 3.12, P = 0.056), an effect of Response Type [F(2, 74) = 688.24, P < 0.001], and an interaction of Group and Response Type [F(4, 74) = 20.23, P < 0.001]. Follow-up independent samples t-tests revealed that healthy older adults demonstrated a higher correct rejection rate (rate at which subjects correctly classified a novel item as “new”) than patients with aMCI [t(26) = 2.61, P = 0.015] and patients with AD [t(24) = 5.99, P < 0.001]. Additionally, patients with aMCI demonstrated a higher correct rejection rate than the patients with AD [t(24) = 4.06, P < 0.001]. Post-hoc t-tests also revealed that patients with AD demonstrated higher baseline false alarms rates (rate at which subjects incorrectly classify a novel item as “old”) than patients with aMCI [t(26) = 3.18, P = 0.004] and healthy older adults [t(24) = 3.91, P = 0.001]. Additionally, patients with aMCI demonstrated a higher baseline false alarm rate than healthy older adults [t(26) = 2.69, P = 0.012]. Finally, to understand baseline similar bias rates (see Toner et al., 2009; Yassa et al., 2010, 2011; Stark et al, 2013), responses were compared between groups when responding “similar” to novel items. In other words, this calculates a baseline tendency to respond “similar”, which will then be subtracted from the pattern separation rate to provide a behavioral bias-corrected pattern separation (BPS) rate. Here, patients with AD demonstrated more “similar” responses to novel items than patients with aMCI [t(26) = 3.53, P = 0.002] and healthy older adults [t(24) = 6.12, P < 0.001]. Similarly, patients with aMCI demonstrated more “similar” responses to novel items than healthy older adults [t(26) = 2.53, P < 0.018]
Repeated and Lure Items
To examine differences in hit rate (rate at which subjects correctly classified a repeated item as “old”) between the three groups, we performed a repeated measures ANOVA with the factors of Group (OC, aMCI, AD) and Lag (4, 12, 40). The ANOVA revealed effects of Group [F(2, 37) = 17.18, P < 0.001] and Lag [F(2, 74) = 3.21, P = 0.046], but no interaction of Group and Lag [F(4, 72) = 1.28, P = 0.287]. Follow-up independent samples t-tests revealed that healthy older adults demonstrated higher hit rates than patients with aMCI [t(26) = 3.08, P = 0.005] and patients with AD [t(24) = 6.80, P < 0.001]. Similarly, patients with aMCI showed higher hit rates than patients with AD [t(24) = 2.65, P = 0.014]. Post-hoc t-tests following up the effect of Lag revealed that all Groups showed higher hit rates at Lag 12 compared to Lag 40 [t(39) = 2.80, P = 0.008].
Next, we examined whether pattern separation rates across lags differed between the three groups using a repeated measures ANOVA with the factors of Group (OC, aMCI, AD) and Lag (4, 12, 40). The initial analysis focused on uncorrected pattern separation rates (rate at which subjects correctly classified a lure item as “similar”) between the three groups. The ANOVA revealed effects of Group [F(2, 37) = 4.10, P = 0.025] and Lag [F(2, 74) = 4.84, P = 0.011], and an interaction of Group and Lag [F(4, 72) = 5.81, P < 0.001]. Follow-up independent-samples t-tests revealed that healthy older adults demonstrated higher uncorrected pattern separation rates compared to patients with aMCI [t(26) = 2.58, P = 0.016] and patients with AD [t(24) = 2.43, P = 0.023]. Post-hoc t-tests also revealed that when collapsed across Group, uncorrected pattern separation rates were higher at Lag 4 than at Lag 40 [t(39) = 2.80, P = 0.008].
When the bias-corrected BPS score (pattern separation rate – similar bias rate) was entered into the ANOVA, we again found effects of Group [F(2, 37) = 22.10, P < 0.001) and Lag [F(2, 74) = 5.17, P = 0.008], and an interaction of Group and Lag [F(4, 74) = 6.14, P < 0.001]. As in the uncorrected pattern separation analysis, the effect of Group was present because healthy older adults demonstrated higher BPS scores compared to patients with aMCI [t(26) = 3.43, P = 0.002] and patients with AD [t(24) = 6.02, P < 0.001]. Patients with aMCI also demonstrated overall higher BPS scores than patients with AD [t(24) = 2.91, P = 0.008]. Also similar to the uncorrected pattern separation analysis, when collapsed across Group, BPS score was higher at Lag 4 compared to Lag 40 [t(39) = 3.02, P = 0.004]. When compared to the healthy older adult group, the patients with aMCI showed no difference in BPS score at Lag of 4 [t(26) = 0.29, P = 0.767] but showed a lower BPS score at Lag 12 [t(26) = 2.92, P = 0.007] and at Lag 40 [t(26) = 4.99, P < 0.001]. In contrast, compared to the healthy older adults, patients with AD demonstrated lower BPS scores at all 3 Lags: 4 [t(24) = 4.60, P < 0.001], 12 [t(24) = 6.56, P < 0.001], and 40 [t(24) = 5.48, P < 0.001]. When the two patient groups were compared, an interesting pattern resulted. Patients with aMCI showed better BPS scores at Lag 4 [t(24) = 5.00, P < 0.001] and Lag 12 [t(24) = 2.37, P = 0.023], but not at Lag 40 [t(24) < 1, P = 0.712]. In other words, at Lag 4 patients with aMCI showed similar bias corrected pattern separation rates (BPS scores) as healthy older adults, but by Lag 40 patients with aMCI demonstrated similar bias corrected pattern separation rates as patients with AD. To further explore BPS score differences across the three Lags, within-subjects paired-sample t-tests were performed. Here, healthy older adults showed no difference in BPS scores over the three lags. Similarly, patients with AD showed no difference in BPS scores over the three lags. In contrast, patients with aMCI showed a higher BPS score at Lag 4 compared to Lag 12 [t(13) = 2.41, P = 0.032] and Lag 40 [t(13) = 7.09, P < 0.001], and a higher BPS score at Lag 12 compared to Lag 40 [t(13) = 2.22, P < 0.045]. Average BPS scores for all three groups at each Lag can be seen in Figure 2 and in Table 3.
FIGURE 2.
Bias-corrected pattern separation rates for all three groups at each lag.
Finally, we examined whether pattern completion rates across lags differed between the three groups. We submitted the uncorrected pattern completion rates (rate at which subjects incorrectly classified a lure item as “old”) into a repeated measures ANOVA to examine the factors of Group (OC, aMCI, AD) and Lag (4, 12, 40). The ANOVA revealed only an interaction of Group and Lag [F(4, 74) = 2.70, P = 0.037], indicating there were no differences in uncorrected pattern completion rates across Group or across Lag. Between-group independent samples t-tests revealed only a trend toward a higher uncorrected pattern completion rate for patients with aMCI compared to patients with AD at Lag 40 [t(24) = 1.89, P = 0.071]. Within-group paired samples t-tests revealed a marginally significant lower uncorrected pattern completion rate at Lag 40 compared to Lag 4 for the AD group [t(11) = 2.15, P = 0.056]. There were no other Group or Lag differences found during follow-up analyses.
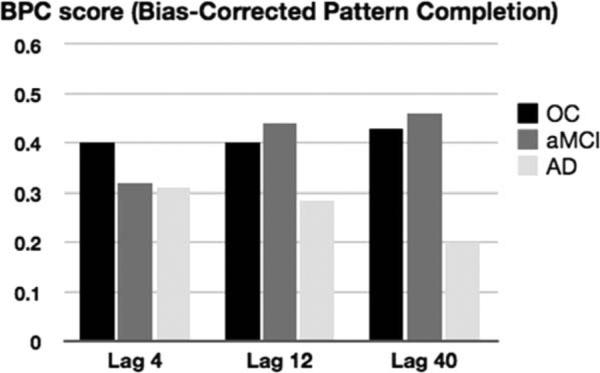
However, when the bias-corrected BPC score (pattern completion rate—baseline false alarm rate) was submitted into the ANOVA, the analysis revealed an effect of Group [F(2, 37) = 4.91, P = 0.013] and an Interaction of Group and Lag [F(4, 74) = 2.70, P = 0.037]. Follow-up independent-samples t-tests revealed that the effect of Group was present because healthy older adults [t(24) = 2.66, P = 0.014] and patients with aMCI [t(24) = 2.53, P = 0.018] demonstrated higher BPC scores than patients with AD when collapsed across Lag. There were no differences in BPC score when healthy older adults were compared with patients with aMCI [t(26) < 1]. Post-hoc t-tests following up on the interaction of Group and Lag revealed no differences between healthy older adults and patients with aMCI at any of the Lags. In contrast, healthy older adults [t(24) = 3.83, P < 0.001] and patients with aMCI [t(24) = 3.12, P = 0.005] demonstrated higher BPC scores than patients with AD at Lag 40. In addition to between group differences, post-hoc t-tests revealed within group differences. Healthy older adults and patients with aMCI showed no differences in BPC score across the three lags. In contrast, patients with AD demonstrated a higher BPC score at Lag 4 compared to Lag 40 [t(11) 2.15, P = 0.055] and at Lag 12 compared to Lag 40 [t(11) = 2.30, P = 0.043]. Average BPC scores for all three groups at each Lag can be seen in Figure 3 and in Table 3.
FIGURE 3.
Pattern completion rates for all three groups at each lag.
Old/New Recognition Accuracy
To examine differences in recognition accuracy, we calculated Pr (hit rate minus false alarm rate; Snodgrass & Corwin, 1988) for repeated and novel items only. Pr values were entered into a repeated measures ANOVA with the factors of Group (OC, aMCI, AD) and Lag (4, 12, 40). The ANOVA revealed effects of Group [F(2, 37) = 17.18, P < 0.001] and Lag [F(2, 74) = 3.21, P = 0.046], but no interaction of Group and Lag [F(4, 76] = 1.28, P = 0.287]. Follow-up independent samples t-tests reveal that when collapsed across Lag, the effect of Group was due to the fact that healthy older adults performed better than patients with aMCI [t(26) = 3.28, P = 0.003] and patients with AD [t(24) = 10.13, P < 0.001]. Additionally, patients with aMCI were more accurate than patients with AD [t(24) = 4.01, P = 0.001]. Follow-up t-tests also revealed that the effect of Lag was driven by the significantly better performance across all Groups on Lag 12 compared to Lag 40 [t(39) = 2.80, P 0.008]. Means and standard deviations of old/new accuracy appear in Table 3.
Post-hoc Analyses
Pearson's bivariate correlation analysis was completed to examine if there was a relationship between pattern completion rate and baseline false alarm rates. Results revealed no relationship between these two variables when all subjects were included in the analysis [r = 0.224, n = 40, P = 0.170] or when only the AD group was included [r = 0.497, n = 12, P = 0.101].
To determine whether the current lag-based pattern separation task BPS score was able to discriminate between subject groups, we performed Receiver Operating Characteristic (ROC) analysis. The ROC curve is a graph of the relationship between the true-positive rate (sensitivity) and the false-positive rate (1-specificity). Figure 4a shows the ROC curve analysis between patients with aMCI and patients with AD at Lag 4, and Figure 4b shows the ROC curve analysis between patients with aMCI and healthy older adults at Lag 40. These two Lags were chosen because patients with aMCI performed highly similar to healthy older adults at Lag 4 (performing significantly better than patients with AD) and highly similar to patients with AD at Lag 40 (performing significantly worse than healthy older adults). These two Lags appear to discriminate patients with aMCI from healthy older adults and patients with AD with promising ability. Using a confidence interval of 0.95, performance at Lag 4 was able to provide excellent discrimination between patients with aMCI and patients with mild AD (area under the curve = 0.923), and performance at Lag 40 was able to provide excellent discrimination between patients with aMCI and healthy older adults (area under the curve = 0.969). See Supporting Information B for ROC curves for each lag, comparing each group.
FIGURE 4.
Receiver operating characteristic (ROC) curves. Area under the curve (AUC) discriminability of pattern separation task at Lag 4 between patients with AD and patients with aMCI. Area under the curve (AUC) discriminability of pattern separation task at Lag 40 between patients with aMCI and healthy older adults. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The goal of the current study was to examine performance on a task that relies on behavioral pattern separation in patients with aMCI and mild AD to help characterize the pattern of memory loss throughout the early course of the disease. We specifically used a lag-based continuous recognition task to determine whether patients with aMCI and mild AD properly encode representations that are rapidly forgotten, or whether the patients fail to properly encode the representations. In accordance with the rapid forgetting hypothesis of AD, we predicted that patients with aMCI and mild AD would show decreasing behavioral pattern separation rates and increasing pattern completion rates as the lag increased between studied and cue/test pictures. Consistent with this hypothesis, we found that patients with aMCI demonstrated decreasing behavioral pattern separation rates (BPS scores) as the lag of interfering objects increased. In fact, at the shortest lag of 4, patients with aMCI performed similarly to healthy older adults while at the longest lag of 40, patients with aMCI performed similarly to patients with AD. In contrast, patients with AD demonstrated consistently poor BPS scores across the three lags compared to healthy older adults. An ROC analysis showed that the current lag-based pattern separation task was able to provide excellent discrimination (AUC = 0.92) between patients with aMCI and patients with AD at Lag 4, as well as excellent discrimination (AUC = 0.97) between patients with aMCI and healthy older adults at Lag 40. Conflicting with our hypotheses, uncorrected pattern completion rates did not differ across group or across lag. Interestingly, when we corrected for response bias (BPC score), patients with AD showed lower pattern completion rates compared to healthy older adults and patients with aMCI, suggesting that these patients may have impaired behavioral pattern completion as well.
To our knowledge, there has not been a previous demonstration of rapidly degrading memory representations in patients with aMCI or mild AD. Behavioral pattern separation rates in patients with aMCI clearly decreased as the lag between intervening test items increased. However, this result stands in stark contrast to the performance of patients with AD who, like healthy older adults, demonstrated relatively stable pattern separation rates across the three lags. Healthy older adults showed stable pattern separation rates across the three lags, hovering near 50%. Similarly, patients with AD showed stable BPS scores across the three lags, but hovering much lower, near 15%. So the obvious question becomes, why do patients with aMCI show decreasing BPS scores over lag, but patients with AD do not? The most parsimonious explanation is that performance on our pattern separation task is directly affected by the normal progression of Alzheimer's pathology in the disease process. Neuropathologically, patients with clinical AD (Braak and Braak stages 3 and 4) have moderately dense hippocampal pathology in most hippocampal subfields (Braak & Braak, 1991). In contrast, patients with aMCI (Braak and Braak stages 1 and 2) tend to have pathologic involvement in transentorhinal and entorhinal regions, with relative sparing of hippocampus subfields CA3 and dentate gyrus (Braak & Braak, 1991; West et al., 1994; Braak et al., 2006). In fact, more recent work has indicated potential pathologic involvement of subfield CA1 in aMCI, but complete sparing of CA3 and dentate gyrus until the disease progresses (Apostolova et al., 2010). Further, microstructural changes within hippocampus subfields are not as evident in aMCI as they are in mild AD (Hong et al., 2013). Potentially, the relatively unaffected dentate gyrus/CA3 connections work to properly orthogonalize and encode distinct separate visual representations, but these representations degrade rapidly or are overwritten to the point that they cannot be used to successfully discriminate previously seen items from perceptually similar lures after a particular amount of interference (Newsome et al., 2012).
In contrast, patients with AD in the current study likely have extensive hippocampal and parahippocampal damage that does not allow them to properly form or encode information in distinct, orthogonal representations. Our results showed significantly diminished BPS scores across all three lags. The possibility exists that patients with AD do have the ability to pattern separate at very short lags, but our Lag of 4 in the current study was unable to capture this as patients might have been at functional floor. However, recent work investigating visual working memory in patients with AD shows that patients have difficulty maintaining multifeature visual objects at delays as short as 900 ms (Parra et al., 2009, 2010). While we understand that encoding and maintaining visual representations in working memory may require different processing than encoding and maintaining visual representations in long-term memory, we feel the findings from Parra and colleagues support the assertion that patients with AD have significant difficulty at the level of encoding even at very short delays. The possibility also exists that more posterior brain regions in the visual-ventral stream have pathology burden that does not allow patients with AD to form even basic perceptual or visual representations to be encoded (Done & Hajilou, 2005; Hussey et al., 2012). This hypothesis might help to explain some of the past discrepant findings in the conceptualization of memory loss in the course of AD and lend itself to somewhat of a continuum whereby aMCI is a disorder of intact encoding and rapid forgetting, while clinical AD is a disorder of encoding (and potentially pre-encoding perceptual processes).
These pattern separation results generally fit with previous work using the dual process model of recognition memory in these patient populations. The dual process model posits that recognition of single visual objects can occur on the basis of familiarity and/or recollection. Familiarity is described as an acontextual, vague sense that an item has been previously encountered, whereas recollection is described as a vivid re-experiencing of the event bound to some context or source (Yonelinas, 2002). Presently, it is unclear exactly how behavioral pattern separation and pattern completion relate to the dual process model of recognition memory, but an initial investigation has shown that it is certainly not a one-to-one relationship (Kim & Yassa, 2013). Since pattern separation requires participants to discriminate studied items from novel items and perceptually similar lure items, one might assume that pattern separation requires some form of recollection, where the participant must be able to re-experience the study item to discriminate between that item and a perceptually similar lure (Kim & Yassa, 2013). Patients with AD have severely impaired estimates of recollection (Ally et al., 2009a; Embree et al., 2012) and have difficulty updating perceptual representations at very short delays (Ally et al., 2006; Parra et al., 2009), which lends support to our assertion that patients with AD are unable to create distinct, orthogonalized visual representations of perceptually similar items at the time of encoding.
In contrast, studies in patients with aMCI have been mixed with respect to recollection and familiarity. These patients appear to be able to rely on recollection and familiarity to varying degrees depending on the task and stimulus type (see Ally, 2012 for review). Indeed, visual object recognition work using stimuli similar to those in the present study have found intact behavioral and neurophysiologic estimates of familiarity, but impaired estimates of recollection, compared to healthy older adults (Ally et al., 2009b; Embree et al., 2012). It is possible that patients with aMCI accurately reported lures as “similar” at early delays, not due to pattern separation, but due to enhanced familiarity. However, given that patients with aMCI demonstrate intact familiarity even at long delays with interference (Deason et al., 2012; Embree et al., 2012), it is less likely that pattern separation rates would decrease over lag in the current study if they were able to rely on familiarity to discriminate between a studied item and a perceptually similar lure. The current results suggest that patients with aMCI can likely rely on recollection in order to successfully perform the pattern separation task, but this ability is short-lived (unim-paired at Lag 4 only).
Previous work has suggested that when pattern separation fails, pattern completion compensates to help integrate incoming stimuli into stored representations (Yassa & Stark, 2011). Therefore, we hypothesized that as BPS scores decreased and lag increased, pattern completion rates would increase as well. Studies of patients with aMCI and mild AD have highlighted increased false alarm rates in these populations when there is high perceptual overlap between study and test cues (Budson et al., 2003; Newsome et al., 2012). Further, it has been speculated that elevated perceptually based false alarm rates result from severely diminished recollection of item-specific details, allowing either incoming perceptual information to overwrite or re-write existing representations (Newsome et al., 2012) or perceptual representations to degrade over time with only conceptual information left to make recognition judgments at test (Budson et al., 2003; O’Connor & Ally, 2010). Analysis of pattern completion rates in patients with aMCI and mild AD revealed findings in opposition to our hypotheses. Our first set of analyses focused on uncorrected pattern completion rates. Not only did pattern completion rates remain stable over the three lags, but also there were no differences in uncorrected pattern completion rate across the three groups. However, when the bias-corrected BPC score was entered into the analysis, we found that patients with AD actually demonstrated lower BPC scores than patients with aMCI and healthy older adults. Post-hoc analyses revealed that this effect of group was mainly driven by differences at the longest lag, with AD patients showing the lowest BPC scores at Lag 40. Interestingly, correlational analyses failed to show a relationship between baseline false alarm rate and pattern completion rate for the AD patient group.
These findings have two potential implications. First, the finding that pattern completion rates remained relatively stable (or decreased) over the three lags for patients with aMCI and AD in the face of significant pattern separation rate deficits suggests that pattern completion and pattern separation are likely two individual processes rather than two ends of a unitary process (Hunsaker & Kesner, 2013). Some have speculated that enhanced pattern completion is a result of impaired pattern separation and vice-versa (see Aimone et al., 2011; Sahay et al., 2011 for discussion of this interpretation). In contrast, our data show diminished BPC scores with severely impaired and stable BPS scores in patients with AD. When examining Figure 5, if behavioral pattern separation and pattern completion were at the ends of a unitary process, one would expect the total for the separation/completion ratio to be at or near one for all groups, with different proportions of black and gray within the ratio bar. For participants with relatively healthy memory, indeed the ratio bar is near one with near equal proportion of separation (black) and completion (gray) within the bar. However, for both patient groups with impaired memory, the ratio bar is far below one and completion (gray) has not compensated for impaired separation (black), providing evidence that pattern completion does not always compensate for impaired pattern separation. Second, it appears that the process of behavioral pattern completion is not directly responsible for elevated rates of false recognition in patients with AD. Not only did BPC scores remain stable across lag for patients with aMCI and decrease across lag for patients with AD, but post-hoc analysis showed that there was no relationship with BPC score and baseline false alarm rates. Although further investigation is needed, we believe these results provides suggestive evidence that pattern separation is not directly related to increased false alarm rates in patients with AD.
FIGURE 5.
Pattern separation/pattern completion ratios collapsed across the three lags.
Indeed, we would be remiss not to discuss the limitations of the current behavioral methodology or how the results could be interpreted using other theoretical models or frameworks. A significant criticism of behavioral pattern separation and pattern completion in humans is that these two constructs do not integrate well with previously established work in recognition memory (Hunsaker & Kesner, 2013). For example, participant behavioral response types (hits, false alarms, etc.) do not easily map on to the theoretical or computational constructs of pattern separation and pattern completion. In their review, the behavioral framework provided by Yassa and Stark (2011) defines pattern completion as the endorsement of a similar lure as “old,” allowing for accurate generalization in the face of noisy or partial input. Under this assumption, pattern completion results in failure of recollection, subsequently leading to false recognition (Kim & Yassa, 2013). However, theoretical and computational models define pattern completion as the recollection of a previously studied item based only a partial or degraded cue. Further, these models view pattern completion as a retrieval process that does not generalize across perceptually similar stimuli but works to help retrieval when only partial information is available at test (see Hunsaker & Kesner, 2013 for review). The difference in these two conceptual definitions of pattern completion potentially stems from the types of stimuli used in computational and behavioral designs. Behavioral pattern completion studies utilize perceptually similar lures, which are not actually partial cues, but “noisy” cues that share greater pattern overlap with the studied item compared to partial cues. In the example used by Hunsaker and Kesner (2013), partial cues are simply degraded versions of the original studied item (i.e., partial cues with 50% cue degradation = 50% overlap with original item). In contrast, noisy cues tend to be a perceptual or exemplar variation of the studied item, which engages the pattern separation properties of the hippo-campal feedforward circuit rather than the pattern completion circuit (i.e., noisy cues with 10% degradation replaced with 10% noise = 90% overlap with the original item). Pattern completion processes can act on noisy cues, but increased pattern completion rates in these studies have been viewed as pattern separation failure rather than evidence of “enhanced” pattern completion (see Hunsaker & Kesner, 2013 for review).
It should also be mentioned that years of previous work and hard-won theoretical distinctions in the memory literature have shown that an individual could falsely endorse a similar item as “old” (behavioral index of pattern completion) simply due to increased familiarity or faulty recollection (Schacter et al., 1998), suggesting that healthy older adults and patients with aMCI in the current study could have demonstrated similar pattern completion rates for completely different reasons. For example, pattern completion may remain relatively intact in healthy older adults, whereas pattern completion may be impaired in patients with aMCI, but the increased false recognition of similar items due to enhanced familiarity could have contributed to what appears to be similar pattern completion performance as healthy older adults. There is significant evidence to suggest patients with aMCI and mild AD have disordered recollection (Wolk et al., 2008; Ally et al., 2009a) and potentially misinterpret the strength or accuracy of the familiarity signal (Gold & Budson, 2008). Indeed, a significant amount of previous work with these patients has highlighted an overdependence on aberrant familiarity as the basis of elevated false recognition rates (Pierce et al., 2005; Budson et al., 2006; Gallo et al., 2006; Beth et al., 2009). In the current study, we believe this is evident when examining the similar bias rates shown in Table 3. Patients with aMCI and mild AD endorsed completely novel items as “similar” significantly more than healthy older adults. This elevated similar bias rate likely reflects aberrant familiarity or potentially poor memory confidence. However, given that all groups demonstrated higher false alarm rates to similar lures than to novel items, we cannot rule out the possibility that pattern completion rates in the current study were due to modulation of familiarity, particularly for the patient groups.
In conclusion, the current study used a lag-based continuous recognition task to examine behavioral pattern separation in patients with aMCI and mild AD. Our results provide robust evidence of rapidly degrading visual memory representations in patients with aMCI. These results stood in contrast to patients with AD, who demonstrated very poor pattern separation rates, even at the shortest lag. We propose that aMCI can be conceptualized as a disorder of rapid forgetting, while clinical AD can be conceptualized as a disorder of encoding. Additionally, our results showed that the process of pattern completion does not compensate for impaired pattern separation, suggesting that pattern separation and pattern completion are two independent processes. While more work in this area needs to be undertaken, there also appears to be no strong relationship between behavioral pattern completion and elevated baseline false alarms in patients with AD. The study of behavioral pattern separation and pattern completion is still in its infancy, particularly with patient populations. Future work should be aimed at the relationship between pattern separation and pattern completion and the dual process model of recognition, as well as whether perceptual and conceptual information differentially influence the processes of pattern separation and pattern completion.
Supplementary Material
Acknowledgments
Grant sponsor: NIH; Grant number: AG031925, AG038471.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Adjutant General's Office . Army individual test battery: Manual of directions and scoring. War Department; Washington, DC: 1944. [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA. Using pictures and words to understand recognition memory deterioration in the course of Alzheimer's disease: A review. Curr Neurol Neurosci Rep. 2012;12:687–694. doi: 10.1007/s11910-012-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. The picture superiority effect in patients with Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2009a;47:595–598. doi: 10.1016/j.neuropsychologia.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Jones GE, Cole JA, Budson AE. The P300 component in patients with Alzheimer's disease and their biological children. Biol Psychol. 2006;72:180–187. doi: 10.1016/j.biopsycho.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Ally BA, McKeever JD, Waring JD, Budson AE. Preserved frontal memorial processes for pictures in patients with mild cognitive impairment. Neuropsychologia. 2009b;47:2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Thompson PM, Green AE, Hwang KS, Zoumalan C, Jack CR, Jr, Harvey DJ, Petersen RC, Thal LJ, Aisen PS, Toga AW, Cummings JL, Decarli CS. 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp. 2010;31:786–797. doi: 10.1002/hbm.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Boller F, Saxton J, McGonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer's disease. Cortex. 1987;23:59–72. doi: 10.1016/s0010-9452(87)80019-9. [DOI] [PubMed] [Google Scholar]
- Beth EH, Waring JD, Budson AE, Ally BA. Response bias for picture recognition in patients with Alzheimer's disease. Cogn Behav Neurol. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Budson AE, Michalska KJ, Sullivan AL, Rentz DM, Daffner KR, Schacter DL. False recognition in Alzheimer's disease: Evidence from categorized pictures. Cogn Behav Neurol. 2003;16:16–27. doi: 10.1097/00146965-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Waring JD, Sullivan AL, Hussion T, Schacter DL. Memory for the September 11, 2001, terrorist attacks one year later in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Cortex. 2007;43:875–888. doi: 10.1016/s0010-9452(08)70687-7. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: Separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Fadda L, Bonci A, Caltagirone C. Differential rates of forgetting from long-term memory in Alzheimer's and multi-infarct dementia. Int J Neurosci. 1993;73:1–11. doi: 10.3109/00207459308987206. [DOI] [PubMed] [Google Scholar]
- Christensen H, Kopelman MD, Stanhope N, Lorentz L, Owen P. Rates of forgetting in Alzheimer's dementia. Neuropsychologia. 1998;36:547–557. doi: 10.1016/s0028-3932(97)00116-4. [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Tinklenberg JR, Pfefferbaum A, Hollister LE, Kopell BS. Physostigmine: Improvement of long-term memory processes in normal humans. Science. 1978;201:272–274. doi: 10.1126/science.351807. [DOI] [PubMed] [Google Scholar]
- Deason RG, Hussey EP, Budson AE, Ally BA. Gist-based conceptual processing of pictures remains intact in patients with amnestic mild cognitive impairment. Neuropsychology. 2012;26:202–208. doi: 10.1037/a0026958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done DJ, Hajilou BB. Loss of high-level perceptual knowledge of object structure in DAT. Neuropsychologia. 2005;43:60–68. doi: 10.1016/j.neuropsychologia.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27:783–790. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- Embree LM, Budson AE, Ally BA. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50:2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: A review of recent evidence. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, Budson AE. Overdependence on degraded gist memory in Alzheimer's disease. Neuropsychology. 2006;20:625–632. doi: 10.1037/0894-4105.20.6.625. [DOI] [PubMed] [Google Scholar]
- Gold CA, Budson AE. Memory loss in Alzheimer's disease: Implications for development of therapeutics. Expert Rev Neurotherap. 2008;8:1879–1891. doi: 10.1586/14737175.8.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Butters N. Associative encoding and retrieval in Alzheimer's and Huntington's disease. Brain Cogn. 1988;7:335–347. doi: 10.1016/0278-2626(88)90007-3. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Harkins SW, Taylor JR. Rate of forgetting in mild Alzheimer's-type dementia. Brain Cogn. 1988;7:31–38. doi: 10.1016/0278-2626(88)90019-x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: Role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hong YJ, Yoon B, Lim SC, Shim YS, Kim JY, Ahn KJ, Han IW, Yang DW. Microstructural changes in the hippocampus and posterior cingulate in mild cognitive impairment and Alzheimer's disease: A diffusion tensor imaging study. Neurol Sci. 2013;34:1215–1221. doi: 10.1007/s10072-012-1225-4. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013;37:36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Hussey EP, Smolinsky JG, Piryatinski I, Budson AE, Ally BA. Using mental imagery to improve memory in patients with Alzheimer's disease: Trouble inspecting or remembering the mind's eye? Alzheimer Dis Assoc Dis. 2012;26:124–134. doi: 10.1097/WAD.0b013e31822e0f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. The neuropathological diagnosis of Alzheimer's disease: Clinical-pathological studies. Neurobiol Aging. 1997;18:S27–S32. doi: 10.1016/s0197-4580(97)00066-3. [DOI] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: A functional MRI study. Neurology. 2001;57:812–816. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus. 2013 doi: 10.1002/hipo.22087. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Hartshorn A, Stark SM, Goodrich-Hunsaker NJ, Hopkins RO, Stark CE. Pattern separation deficit following damage to the hippocampus. Neuropsychologia. 2012;50:2408–2414. doi: 10.1016/j.neuropsychologia.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. Quantitative characterization of verbal learning deficits in patients with Alzheimer's disease. J Clin Exp Neuropsychol. 1994;16:749–753. doi: 10.1080/01688639408402688. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Remote and autobiographical memory temporal context memory and frontal atrophy in Korsakoff and Alzheimer's patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-type dementia. Brain. 1991;114:117–137. [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer's disease. J Gerontol. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc London B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Overmier JB. Present imperfect: A critical review of animal models of the mnemonic impairments in Alzheimer's disease. Neurosci Biobehav Rev. 1998;22:99–120. doi: 10.1016/s0149-7634(97)00024-9. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs RC, Davis KL. A signal detectability analysis of the effect of physostigmine on memory in patients with Alzheimer's disease. Neurobiol Aging. 1982;3:105–110. doi: 10.1016/0197-4580(82)90004-5. [DOI] [PubMed] [Google Scholar]
- Money EA, Kirk RC, McNaughton N. Alzheimer's dementia produces a loss of discrimination but no increase in rate of memory decay in delayed matching to sample. Neuropsychologia. 1992;30:133–143. doi: 10.1016/0028-3932(92)90023-f. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsycho-logical assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Morris R, Wheatley J, Britton P. Retrieval from long-term memory in senile dementia; cued recall revisited. Br J Clin Psychol. 1983;22:141–142. doi: 10.1111/j.2044-8260.1983.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule neurons mediate pattern separation, whereas old granule neurons facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Newsome RN, Duarte A, Barense MD. Reducing perceptual interference improves visual discrimination in mild cognitive impairment: Implications for a model of perirhinal cortex function. Hippocampus. 2012;22:1990–1999. doi: 10.1002/hipo.22071. [DOI] [PubMed] [Google Scholar]
- O'Connor MK, Ally BA. Using stimulus form change to understand memorial familiarity for pictures and words in patients with mild cognitive impairment and Alzheimer's disease. Neuropsychologia. 2010;48:2068–2074. doi: 10.1016/j.neuropsychologia.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie RH, Luzzi S, Della Sala S. Short-term memory binding deficits in Alzheimer's disease. Brain. 2009;132:1057–1066. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133:2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Pierce BH, Sullivan AL, Schacter DL, Budson AE. Comparing source-based and gist-based false recognition in aging and Alzheimer's disease. Neuropsychology. 2005;19:411–419. doi: 10.1037/0894-4105.19.4.411. [DOI] [PubMed] [Google Scholar]
- Pluta J, Yushkevich P, Das S, Wolk DA. In vivo analysis of hippocampal subfield atrophy in mild cognitive impairment via semi-automatic segmentation of T2-weighted MRI. J Alzheimer's Dis. 2012;31:85–99. doi: 10.3233/JAD-2012-111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, Haupt M, Romero B, Kurz A. Is impaired recall in dementia of the Alzheimer type a consequence of a contextual retrieval deficit? Dementia. 1993;4:102–108. doi: 10.1159/000107304. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O'Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Paller KA, Mungas D. Impaired acquisition and rapid forgetting of patterned visual stimuli in Alzheimer's disease. J Clin Exp Neuropsychol. 1998;20:738–749. doi: 10.1076/jcen.20.5.738.1123. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF. A critical evaluation of monkey models of amnesia and dementia. Brain Res Rev. 1991;16:15–37. doi: 10.1016/0165-0173(91)90018-4. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer's disease during memory encoding. Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ. The representational-hierarchical view of amnesia: Translation from animal to human. Neuropsychologia. 2010;48:2370–2384. doi: 10.1016/j.neuropsychologia.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Granholm E, McCullough D, Butters N, Grant I. Recognition memory span in mildly and moderately demented patients with Alzheimer's disease. J Clin Exp Neuropsychol. 1989;11:429–443. doi: 10.1080/01688638908400904. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuro-science of constructive memory. Ann Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatr. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2012.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST. Recollection and familiarity in amnestic mild cognitive impairment: A global decline in recognition memory. Neuropsychologia. 2008;46:1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trend Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.