Abstract
Objective
The N-methyl-D-aspartate receptor antagonist ketamine has rapid antidepressant effects in treatment-resistant major depressive disorder (MDD) and bipolar depression. Clinical predictors may identify those more likely to benefit from ketamine within clinically-heterogeneous populations.
Method
Treatment-resistant inpatients with DSM-IV-TR-diagnosed MDD or bipolar I/II depression currently experiencing a moderate-to-severe major depressive episode were enrolled between November 2004 and March 2013. All subjects received a single subanesthetic (0.5mg/kg) ketamine infusion over 40 minutes. Patients were analyzed at the 230-minute post-infusion time point (N=108), at Day 1 (N=82), and at Day 7 (N=71). Univariate Pearson correlations were performed for each variable with change-from-baseline in the 17-item Hamilton Depression Rating Scale (HDRS). Multivariate linear regression was then conducted for statistically significant predictors (p<0.05, two-tailed).
Results
Higher body mass index (BMI) correlated with greater HDRS improvement at 230 minutes and at Day 1 (standardized β=−0.30, p=0.004), but not at Day 7 (standardized β=−0.18, p=0.10). Family history of an alcohol use disorder in a first-degree relative was associated with greater HDRS improvement at Day 1 (standardized β=−0.37, p=0.001) and Day 7 (standardized β=−0.41, p<0.001). No prior history of suicide attempt(s) was associated with greater improvement only at Day 7 (standardized β=0.28, p=0.01). The overall statistical model explained 13, 23, and 36 percent of HDRS percent change variance at 230 minutes, Day 1, and Day 7, respectively.
Conclusion
Despite its post-hoc nature, the study identified several clinical correlates of ketamine's rapid and durable antidepressant effects. Further investigation of these relationships is critical for individualized treatment of depression.
Keywords: clinical predictors, moderators, mediators, major depression, ketamine, NMDA receptor antagonist
Introduction
Developing clinical tools to predict treatment response is essential both for developing improved treatments and for personalizing medicine1. In mood disorders, a number of demographic and treatment characteristics that predict response to standard antidepressant treatments have been identified. For example, the large, real-world Sequenced Treatment Alternatives to Relieve Depression (STAR*D) effectiveness trial found that race, gender, employment status, level of education, and income were associated with higher remission rates after treatment with citalopram2. Comorbid psychiatric and substance use disorders, general medical conditions, longer index episodes, and lower psychosocial function were associated with lower remission rates2. The clinical and sociodemographic variables identified in the STAR*D trial were subsequently used to create an online calculator to assess risk for future treatment resistance3.
Although a large number of antidepressants are available, no reliable selection criteria currently exist. As a result, many patients are exposed to numerous and lengthy antidepressant trials before obtaining significant improvement. In recent years, however, there has been a shift in the field to develop more rapid-acting antidepressants, which may streamline this iterative process. For example, both the muscarinic cholinergic receptor antagonist scopolamine, and the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine have rapid-acting antidepressant effects4, 5, and clinical indicators and biomarkers of rapid treatment response to both medications have been investigated6. Baseline scores on the Profile of Mood States (POMS) depression subscale, as well as the restlessness, sad, and irritated items on the Visual Analog Scales (VAS), discriminated scopolamine responders from nonresponders in both major depressive disorder (MDD) and bipolar depression7. Increased functional magnetic resonance imaging (MRI) baseline blood-oxygen level dependent (BOLD) signal in the middle occipital cortex during the stimulus-processing component of an emotional memory task also predicted increased antidepressant response to scopolamine8.
For ketamine, a positive family history of alcohol dependence was associated with better antidepressant response in both MDD9 and bipolar depression10. In addition, although change in peripheral brain-derived neurotrophic factor (BDNF) levels did not correlate with ketamine's antidepressant response11, changes in peripheral BDNF (a major inducer of synaptic plasticity) were directly proportional to slow wave sleep (a surrogate marker of synaptic plasticity) change in ketamine responders12. Also, the BDNF val66met rs6265 single nucleotide polymorphism (SNP) predicted antidepressant response to ketamine; specifically, val/val homozygotes responded more often than met carriers13. Finally, baseline peripheral B12 (but not folic acid or homocysteine) levels predicted antidepressant response to ketamine in a small sample of patients with bipolar depression14.
Non-invasive neuroimaging techniques have also revealed potential biomarkers of ketamine's antidepressant efficacy. For instance, increased pretreatment rostral anterior cingulate cortex (ACC) reactivity to fearful faces predicted augmented antidepressant response to ketamine15. Magnetoencephalography (MEG) response during a spatial working memory task also correlated with antidepressant response to ketamine16. Baseline slow wave sleep activity—particularly the delta sleep ratio [as defined by SWA1(NREM1)/SWA2(NREM2)]—positively correlated with ketamine's antidepressant effects17. Finally, increased tactile-stimulus evoked somatosensory cortical response from baseline correlated with better antidepressant response18. Potential biomarkers of antidepressant response to ketamine have also been studied via proton magnetic resonance spectroscopy (1H-MRS) and positron emission tomography (PET). Although change in 1H-MRS-detectable amino acids did not correlate with antidepressant response to ketamine in the occipital cortex19, in the dorsomedial/dorsoanterolateral and ventromedial prefrontal cortex, the differential ratio of amino acid neurotransmitters at baseline correlated with antidepressant response to ketamine20. Regional cerebral glucose metabolism at baseline and following ketamine infusion also found that regional metabolism decreased significantly in the right habenula, insula, and ventrolateral and dorsolateral prefrontal cortices, although whole-brain metabolism did not change significantly21. Conversely, metabolism increased in the bilateral occipital, right sensorimotor, left parahippocampal, and left inferior parietal cortices. Improvement in depression ratings correlated directly with change in right superior and middle temporal gyri glucose metabolism and inversely correlated with metabolic changes in the right parahippocampal gyrus and temporoparietal cortex.
Despite these intriguing findings, a major weakness associated with these exploratory studies is their limited sample size and their inability to assess multiple variables in aggregate. To weigh the predictive power of multiple variables—particularly clinical and demographic characteristics—larger samples sizes are typically needed. Towards this end, we pooled subject-level data from our ketamine studies, in order to better identify the clinical and treatment characteristics associated with acute and sustained antidepressant response to ketamine in individuals with MDD or bipolar depression. Several demographic variables analyzed in previous studies were included so that their associations in a multivariate context could be explored in this larger sample.
Method
We combined subject-level data from several independent studies investigating the use of ketamine in treatment-resistant MDD and bipolar I/II depression without psychotic features4, 22-24. Complete details regarding these patients have been previously published4, 22-24. Briefly, patients between the ages of 18 and 65 were admitted to the NIMH Mood and Anxiety Disorders research unit in Bethesda, MD, USA between November 2004 and March 2013. Participants gave written informed consent as approved by the NIH Combined Central Nervous System (CNS) Institutional Review Board. Patients were required to be experiencing a major depressive episode of at least moderate severity [≥184 on the 21-item Hamilton Depression Rating Scale (HDRS), or ≥2022, 24 or ≥2223 on the Montgomery-Asberg Depression Rating Scale (MADRS)] at screening and at the start of each infusion. All subjects met DSM-IV criteria for either MDD or bipolar depression as assessed by the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV Disorders (SCID), patient version, had no active substance use diagnosis (except caffeine or nicotine) for at least three months, and had no unstable medical illness as detected by clinical history, physical examination, and/or laboratory assessments.
All patients received a single subanesthetic (0.5mg/kg) intravenous infusion of ketamine hydrocholoride over 40 minutes. Patients enrolled in the MDD ketamine cross-over4 and ketamineriluzole23 studies were off all psychotropic medications for at least two weeks (four weeks for fluoxetine) prior to the (first) infusion. Zarate et al. 20064 had a double-blind, placebo-controlled, cross-over design. Ibrahim et al. 201223 consisted of open-label ketamine followed six hours post-infusion by randomization to either riluzole (100-200 mg/day) or placebo for 28 days. During this period, no concomitant psychotropic medications were permitted. The two bipolar depression studies22, 24 were identically designed: randomized, double-blind, placebo-controlled, cross-over studies; all subjects were maintained on therapeutic levels of either lithium or valproate for at least two weeks prior to the first infusion22, 24. The remaining patients included in this report participated in a variety of neurophysiological studies. Like the MDD studies, these patients were free of psychotropic medications for at least two weeks prior to ketamine infusion.
As in the Zarate et al. 20064 study, the primary measure in this combined post-hoc analysis was the 17-item HDRS. Baseline and post-infusion scores were used to calculate percent HDRS change. Baseline ratings occurred 60 minutes before infusion. Data were examined at 230 minutes, Day 1, and Day 7 post-ketamine infusion. The Beck Depression Inventory (BDI), Young Mania Rating Scale (YMRS), and Brief Psychiatric Rating Scale (BPRS) were obtained at baseline and throughout the study period, including the aforementioned time points.
A variety of baseline sociodemographic and clinical variables were studied because of either prior evidence of an association with antidepressant response or their relationship with outcome measures in longitudinal studies of MDD or bipolar depression. Some clinical variables previously studied include gender2, 25, 26, age25, 26, and neuropsychiatric comorbidities2. Additional patient and family history data were analyzed alongside illness trajectory variables. Family history of alcohol dependence was defined as having at lease one first degree relative who met criteria for an alcohol use disorder.
Statistics
Pearson correlations were used to examine the associations between baseline variables and percent change in the 17-item HDRS. Scatterplots with predictors and HDRS percent change were used to visualize data and inspect for outliers. Separate correlations were run for each variable with changes in depression scores at 230 minutes, Day 1, and Day7 post-ketamine infusion. Statistically significant predictors in the univariate analysis advanced to a multivariate linear regression for each time point. Variables significant at any individual time point were analyzed at all time points for model consistency. Significance was evaluated at the p≤0.05 level, two-tailed, with no multiplicity correction.
For linear regression analyses, variables that were not significant at all time points were excluded from the model. Tolerance was examined to assess potential multi-collinearity problems. Standardized beta coefficients are reported for all remaining variables at each time point in order to facilitate the contribution of each factor to the overall model. Overall R2 values illustrate the amount of variance explained by this set of predictors.
Results
All 108 patients received a single subanesthetic (0.5mg/kg) ketamine infusion over 40 minutes, and data were available for all patients at the 230-minute time point. Patients who received a ketamine infusion and were later randomized to receive riluzole were excluded from analyses at Days 1 and 7. Due to withdrawal, not all 108 patients had available data at subsequent time points; 82 patients were analyzed at Day 1, and 71 patients were analyzed at Day 7.
Demographic characteristics are summarized in Table 1. The sample had moderate-to-severe depression [average HDRS = 21.2 (SD=4.4)] in the current episode lasting an average of 55.7 months (SD=97.2). The average age of onset was 20.2 (SD=11.3), and the average length of illness was 27.0 years (SD=12.9). The patient sample was predominantly Caucasian (85%, n=92) with most completing college (57%, n=59). Half of the sample was male (50%, n=54) and the majority had MDD (68%, n=74).
Table 1.
Univariate Analysis of Demographic and Clinical Correlates of Antidepressant Response to Ketamine In Treatment-Resistant Depression
| Time Following Ketamine Infusion |
||||||||
|---|---|---|---|---|---|---|---|---|
| 230 Min (n = 108) |
Day 1 (n = 82) |
Day 7 (n = 71) |
||||||
| Variable | Mean | SD | r | P | r | P | r | P |
| Age, y | 47.2 | 12.0 | –0.10 | .319 | 0.08 | .454 | 0.01 | .926 |
| Body mass index | 30.5 | 6.9 | –0.32 | .001* | –0.38 | <.00l* | –0.34 | .004* |
| Age at onset, y | 20.2 | 11.3 | –0.11 | .262 | 0.01 | .953 | <0.01 | .986 |
| Length of current episode, mo | 55.7 | 97.2 | 0.03 | .797 | 0.06 | .641 | –0.12 | .360 |
| Length of illness, y | 27.0 | 12.9 | 0.02 | .807 | 0.09 | .425 | 0.03 | .841 |
| No. of previous episodes | 26.2 | 37.6 | –0.10 | .334 | –0.01 | .927 | 0.02 | .880 |
| Clinical ratings | ||||||||
| HDRS (17 item) | 21.2 | 4.4 | <0.0) | .982 | –0.04 | .693 | –0.13 | .287 |
| BDI | 27.1 | 8.3 | 0.05 | .627 | 0.16 | .149 | –0.02 | .856 |
| YMRS | 4.9 | 2.6 | –0.03 | .721 | –0.04 | .744 | 0.06 | .607 |
| BPRS positive symptoms | 9.8 | 1.5 | –0.05 | .640 | –0.26 | .020* | –0.11 | .346 |
| Percent change in HDRS score | ||||||||
| 230 min | –39.6 | 25.3 | ||||||
| Day 1 | –35.9 | 30.7 | ||||||
| Day 7 | –23.4 | 29.7 | ||||||
| n |
% |
|||||||
| Diagnosis (bipolar disorder) | 31 | 31.5 | –0.03 | .729 | 0.07 | .537 | 0.24 | .042* |
| Male | 54 | 50.0 | –0.07 | .499 | –0.04 | .746 | 0.06 | .617 |
| Race/ethnicity (white) | 92 | 85.2 | 0.09 | .372 | 0.16 | .152 | 0.25 | .035* |
| College graduate | 59 | 56.7 | 0.02 | .854 | 0.06 | .576 | 0.03 | .806 |
| Psychiatric hospitalization | 69 | 71.1 | –0.07 | .482 | –0.24 | .045* | –0.02 | .851 |
| ECT | 37 | 41.0 | 0.18 | .096 | –0.09 | .502 | 0.11 | .457 |
| Suicide attempt(s) | 44 | 43.6 | 0.08 | .409 | –0.01 | .921 | 0.37 | .003* |
| Smoking (current) | 21 | 20.6 | –0.05 | .602 | 0.12 | .291 | 0.03 | .810 |
| Abuse | ||||||||
| Physical | 22 | 22.0 | –0.04 | .705 | –0.11 | .356 | –0.22 | .083 |
| Sexual | 22 | 22.0 | –0.13 | .199 | –0.14 | .218 | 0.16 | .216 |
| Family history | ||||||||
| Alcohol use disorder (first degree) | 40 | 39.2 | –0.21 | .031* | –0.33 | .004* | –0.47 | <.001* |
| Mood disorder (first degree) | 88 | 83.8 | –0.10 | .294 | –0.13 | .236 | –0.07 | .555 |
| Suicide | 29 | 34.9 | –0.02 | .882 | –0.18 | .168 | –0.04 | .794 |
| Alcohol use disorder | 12 | 28.6 | 0.02 | .922 | 0.10 | .565 | –0.14 | .437 |
| Comorbid diagnosis | ||||||||
| Agoraphobia (current) | 10 | 9.3 | 0.04 | .705 | 0.06 | .610 | 0.07 | .578 |
| Anxiety NOS (current) | 10 | 9.6 | –0.04 | .692 | –0.14 | .225 | –0.08 | .544 |
| GAD (current) | 15 | 14.4 | <0.01 | .977 | <0.01 | .981 | 0.09 | .467 |
| OCD (current) | 9 | 8.7 | –0.05 | .623 | 0.05 | .652 | <0.01 | .985 |
| Panic disorder (current) | 8 | 7.4 | 0.12 | .229 | 0.15 | .200 | 0.07 | .591 |
| PTSD (current) | 3 | 2.9 | 0.0) | .911 | 0.06 | .591 | 0.02 | .843 |
| Social phobia (current) | 29 | 26.9 | 0.05 | .642 | 0.11 | .348 | 0.15 | .235 |
| Specific phobia (current) | 12 | 11.5 | –0.09 | .381 | –0.12 | .276 | 0.11 | .393 |
| MDD subtype | ||||||||
| Atypical | 27 | 27.0 | –0.02 | .858 | 0.10 | .375 | –0.10 | .418 |
| Melancholic | 31 | 31.0 | –0.04 | .692 | 0.01 | .909 | 0.04 | .748 |
| Neither | 42 | 42.0 | 0.05 | .594 | –0.11 | .366 | 0.05 | .681 |
P≤.05, 2-tailed.
Abbreviations: BDI = Beck Depression inventory, BPRS = Brief Psychiatric Rating Scale, ECT = electroconvulsive therapy, GAD = generalized anxiety disorder, HDRS= Hamilton Depression Rating Scale, MDD = major depressive disorder, NOS = not otherwise specified, OCD = obsessive-compulsive disorder, PTSD = posttraumatic stress disorder, YMRS = Young Mania Rating Scale.
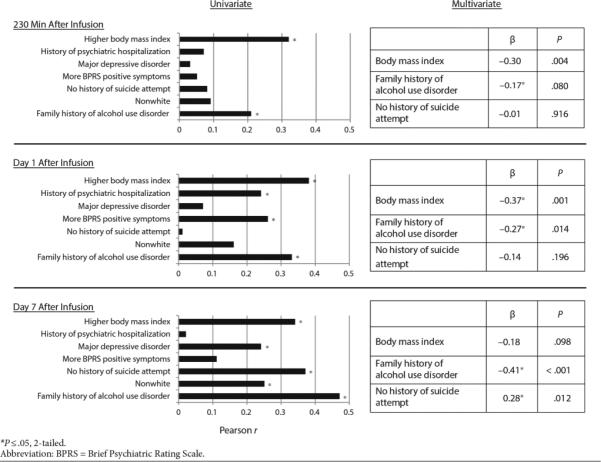
In the univariate analysis, a higher body mass index (BMI) and a positive family history of alcohol use disorder (FHP) correlated with greater HDRS improvement from baseline to 230 minutes. The same relationships were significant at Days 1 and 7 post-infusion. One day after infusion, patients with more positive symptoms on the BPRS at baseline and a history of psychiatric hospitalization displayed greater change from baseline. These factors were not related to change in depression severity at Day 7. One week after ketamine infusion, participants with no prior suicide attempt had more substantial change in depressive symptoms. Finally, in the univariate analysis, MDD patients had a greater reduction in HDRS score one week after receiving ketamine compared to patients with bipolar depression patients and patients who were not Caucasian.
Clinical predictors that were significant at the p≤0.05 level at one time point in the univariate analysis advanced to the multivariate linear regressions: BMI, FHP, history of suicide attempt(s), history of psychiatric hospitalization, diagnosis, and baseline BPRS positive symptoms. Given the small proportion of non-Caucasian patients in the sample, race/ethnicity was not included in the multivariate analysis due to reliability concerns. After running initial regression models, history of psychiatric hospitalization, diagnosis, and baseline BPRS positive symptoms were not significant independent predictors at any time point, so these were removed from the final model.
In the final model, higher BMI correlated with greater improvement in HDRS at 230 minutes and Day 1, but not at Day 7 post-infusion. FHP patients had greater improvement in HDRS on Days 1 and 7, but not at 230 minutes. Having no prior history of suicide attempt was associated with greater improvement at Day 7 only. Tolerances remained above 0.7 for all models, so multicollinearity was not an issue. The overall statistical models explained a total of 13, 23, and 36 percent of the variance in HDRS percent change at 230 minutes, Day 1, and Day 7, respectively.
Discussion
This secondary data analysis of 108 treatment-resistant patients with MDD or bipolar depression currently experiencing a major depressive episode who received a single subanesthetic infusion of ketamine assessed numerous demographic and clinical factors as potential predictors of antidepressant response to ketamine. In the univariate analysis, the following variables emerged as significant predictors of improvement, at least at a single post-infusion time point: increased BMI, FHP, no history of suicide attempt(s), prior psychiatric hospitalization, MDD diagnosis, and more BPRS positive symptoms at baseline. In the multivariate analysis, only increased BMI, FHP, and no history of suicide attempt(s) remained significant; BMI tended to be associated with acute improvement, while FHP and no history of suicide attempt were associated with durability of antidepressant response to ketamine.
The association between BMI and acute antidepressant response may be related to clinically effective dose, as patients with the highest dose (in mg) had greater improvements in HDRS scores. Our group and others have postulated that ketamine's mechanism of action is mediated by increased presynaptic release of glutamate (“glutamate surge”) and the subsequent activation of 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA) receptors27-29. In this model, a higher dose of ketamine may result in greater NMDA antagonism and parallel increases in both presynaptic glutamate release and postsynaptic AMPA throughput. The extracellular glutamate levels resulting from ketamine have been reported to have “inverted U”-shaped pharmacodynamics, i.e. a lower dose of ketamine increases extracellular glutamate levels until a peak, with subsequent decreases at higher doses30. The resultant biochemical and neurophysiological events involved in ketamine's antidepressant effects may reflect these “inverted U” pharmacodynamics31, 32. Although a structured dose-finding study with ketamine in depression is currently underway (ClinicalTrials.gov identifier: NCT01558063), a previous study in healthy volunteers reported that a 0.1 mg/kg ketamine infusion was associated with fewer psychotomimetic and dissociative effects than 0.5 mg/kg33; this indicates that ketamine's adverse effects result from differences in NMDA receptor antagonism. It may be important, therefore, in dose-finding studies to take BMI into account, as an imbalance in weight among the different dose level groups may lead to differences in antidepressant efficacy. It is also interesting to note that a prior study found that increased BMI was associated with higher rates of tachyphylaxis following treatment with selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibiors (SNRIs)34 Thus, while BMI may predict initial response to ketamine, our results are in agreement with prior studies suggesting that patients with higher BMIs may not sustain their initial antidepressant response.
Improvement in depressive symptoms at Day 7 post-ketamine infusion (indicating more sustained antidepressant effects) confirmed our previous findings of antidepressant durability in FHP patients with treatment-resistant depression; it should be noted, however, that the current dataset overlaps these prior reports9, 10. Previous studies found that glutamatergic dysfunction is involved in the pathophysiology of mood disorders, both alone35 and co-occurring with alcoholism36. One study found that patients with bipolar disorder in long-term remission from alcoholism showed reduced dorsolateral prefrontal cortex glutamate levels compared to bipolar disorder patients who never developed alcoholism36. At the genetic level, Schumann and colleagues37 found a significant association between risky drinking behavior in adolescents and the genes encoding mGluR5 and the NR2A subunit of the NMDA receptor. Because ketamine has antagonist effects at NR2A, and alcohol is also a weak NMDA receptor antagonist38, it is feasible that genetic polymorphisms in NMDA receptor subunits may render FHP patients more responsive to the antidepressant effects of ketamine. The antidepressant efficacy of ketamine may also be augmented in patients with comorbid depression and alcoholism, a hypothesis currently under investigation (ClinicalTrials.gov Identifier: NCT01551329). Future genetic studies may unveil polymorphisms in NMDA receptor complex subunits that will further elucidate glutamatergic dysfunction in mood disorders and alcoholism, and their relationship to ketamine's antidepressant effects.
In the STAR*D trial, race, gender, employment status, level of education, and income were associated with greater rates of remission with citalopram, while psychiatric comorbidities, general medical conditions, longer index episodes, and lower psychosocial function were associated with lower remission rates2. However, gender, education level, and neuropsychiatric and medical comorbidities were not associated with antidepressant response to ketamine in our sample. There are several potential explanations for these differences. First, the selected STAR*D population (2,876)2 was much larger than the 108 patients in this study. Second, STAR*D was an “all-comers” outpatient study while our ketamine protocols only included treatment-resistant inpatients. Third, there may have been putative differences in the antidepressant mechanism of action of conventional monoaminergic antidepressants and cognitive-behavioral therapy in STAR*D vs. ketamine's glutamatergic properties. Finally, differences in the outcome measures used may have played a role; STAR*D examined remission, while the present study used response rates.
One of the major strengths of this study is our well-characterized group of treatment-resistant depressed inpatients. All patients were hospitalized at the same site for an average of six weeks before receiving ketamine, thus providing ample resources and time to precisely describe their clinical and treatment characteristics. This inpatient milieu allowed us to pay attentive detail to the timing of response and side effect profile in the post-infusion period. Another significant strength was our relatively large sample studied under similar conditions, thus permitting the exploration of multiple variables and their variance alone and in aggregate. In addition, our group examined multiple time points and studied the potential variables longitudinally in a multivariate context. Potential limitations of the study include its post-hoc design, the pooled patient group that included patients with treatment-refractory MDD as well as bipolar depression, and the combination of open-label and randomized, placebo-controlled, cross-over designs.
Despite the fact that all predictors were studied post-hoc, this study is an important first step in the search for potential correlates of ketamine's rapid and relatively sustained antidepressant properties. While ketamine remains a promising key for future research into rapidly acting antidepressants, further investigation into its mechanism, safety, and feasibility is needed to determine its ultimate clinical utility. Nonetheless, uncovering clinically-relevant predictors of antidepressant response remains an important step towards improving the clinical care of depressed patients. Notably, obtaining BMI, identifying family history of alcohol use disorders in relatives, and asking about a past history of suicide attempt(s) are all part of the initial psychiatric interview. In the future, we seek to integrate such research findings to advance our predictive capacity for personalized antidepressant selection.
Clinical Points.
- Clinical and demographic predictors of treatment response are critical in the search for more personalized treatments for depression. These have only recently begun to be investigated for the rapid-acting antidepressant ketamine.
- Increased body mass index (BMI), a family history of an alcohol use disorder in a first-degree relative (FHP), and no personal history of suicide attempt(s) were significant clinical predictors antidepressant response to ketamine in the largest sample of treatment-resistant inpatients with depression to date.
Figure 1.
Significant Predictors of Ketamine's Antidepressant Effects in Treatment-Resistant Depression
Table 2.
Multivariate Linear Regression of Significant Predictors of Antidepressant Response to Ketamine in Treatment-Resistant Depression
| 230 Min |
Day 1 |
Day 7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Standardized β | P | r 2 | Standardized β | P | r 2 | Standardized β | P | r 2 |
| Body mass index | –0.30 | .004* | –0.37 | .001* | –0.18 | .098 | |||
| Family history of alcohol use disorder (first degree) | –0.17 | .080 | –0.27 | .014* | –0.41 | <.001* | |||
| Suicide attempt(s) | –0.01 | .916 | –0.14 | .196 | 0.28 | .012* | |||
| Total | 0.130 | 0.229 | 0.360 | ||||||
P ≤ .05, 2-tailed.
Acknowledgements
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), and thank the 7SE Inpatient Mood and Anxiety Disorders Research Unit for their clinical support during the writing of this manuscript. Ioline Henter, M.A., National Institute of Mental Health, provided excellent editorial assistance.
Funding and Disclosures: Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH), by a NARSAD Independent Investigator Award to CAZ, and by the Brain & Behavior Mood Disorders Research Award to CAZ. Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. The remaining authors have no conflict of interest to disclose, financial or otherwise.
Footnotes
Ms. Henter reports no potential conflicts of interest.
References
- 1.Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments? Am J Psychiatry. 2010;167(12):1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Perlis RH. A clinical risk stratification tool for predicting treatment resistance in major depressive disorder. Biol Psychiatry. 2013;74(1):7–14. doi: 10.1016/j.biopsych.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr., Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr., Mathews DC, Furey ML. Human Biomarkers of Rapid Antidepressant Effects. Biol Psychiatry. 2013;73(12):1142–55. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furey ML, Nugent AC, Speer AM, et al. Baseline mood-state measures as predictors of antidepressant response to scopolamine. Psychiatry Res. 2012;196(1):62–67. doi: 10.1016/j.psychres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70(3):280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65(2):181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luckenbaugh DA, Ibrahim L, Brutsche N, et al. Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord. 2012;14(8):880–887. doi: 10.1111/bdi.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70(12):1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan WC, Sarasso S, Ferrarelli F, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2012;7:1–11. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laje G, Lally N, Mathews D, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biol Psychiatry. 2012;72(11):e27–8. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Permoda-Osip A, Dorszewska J, Bartkowska-Sniatkowska A, Chlopocka-Wozniak M, Rybakowski JK. Vitamin B12 Level may be Related to the Efficacy of Single Ketamine Infusion in Bipolar Depression. Pharmacopsychiatry. 2013;46(6):227–8. doi: 10.1055/s-0033-1349861. [DOI] [PubMed] [Google Scholar]
- 15.Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvadore G, Cornwell BR, Sambataro F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan WC, Jr., Selter J, Brutsche N, Sarasso S, Zarate CA., Jr. Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord. 2013;145(1):115–9. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornwell BR, Salvadore G, Furey M, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72(7):555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentine GW, Mason GF, Gomez R, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191(2):122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvadore G, van der Veen JW, Zhang Y, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15(8):1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson PJ, Diazgranados N, Nugent AC, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary PET study. Biol Psychiatry. 2013;73(12):1213–21. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. Aug. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim L, Diazgranados N, Franco-Chaves J, et al. Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology. 2012;37(6):1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarate CA, Jr., Brutsche NE, Ibrahim L, et al. Replication of Ketamine's Antidepressant Efficacy in Bipolar Depression: A Randomized Controlled Add-On Trial. Biol Psychiatry. 2012;71(11):939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biol Psychiatry. 2002;51(3):253–260. doi: 10.1016/s0006-3223(01)01206-9. [DOI] [PubMed] [Google Scholar]
- 26.Zarate CA, Jr., Narendran R, Tohen M, et al. Clinical predictors of acute response with olanzapine in psychotic mood disorders. J Clin Psychiatry. 1998;59(1):24–28. doi: 10.4088/jcp.v59n0106. [DOI] [PubMed] [Google Scholar]
- 27.Maeng S, Zarate CA, Jr., Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niciu MJ, Ionescu DF, Mathews DC, Richards EM, Zarate CA. Second messenger/signal transduction pathways in major mood disorders: moving from membrane to mechanism of action, part I: major depressive disorder. CNS Spectr. 2013:1–10. doi: 10.1017/S1092852913000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 34.Rothschild AJ, Dunlop BW, Dunner DL, et al. Assessing rates and predictors of tachyphylaxis during the prevention of recurrent episodes of depression with venlafaxine ER for two years (PREVENT) study. Psychopharmacol Bull. 2009;42(3):5–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68(9):785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nery FG, Stanley JA, Chen HH, et al. Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J Psychiatr Res. 2010;44(5):278–285. doi: 10.1016/j.jpsychires.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumann G, Johann M, Frank J, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65(7):826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 38.Krystal JH, Petrakis IL, Webb E, et al. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55(4):354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]


