1. Summary
Regulation of the pause and elongation by RNA polymerase (Pol) II is used widely by metazoans to attain the pattern of gene expression that is essential for optimal cell growth/renewal, differentiation and stress response. Currently, much of what we know about Pol II elongation control comes from pioneering studies of the HIV-1-encoded Tat protein and its host cellular co-factors. The interaction between the two fuels a powerful feedback circuit that activates HIV transcription and prevents the virus from entering latency. One of the key Tat cofactors is the human positive transcription elongation factor b (P-TEFb), which exists in a family of complexes with distinct functions during Tat transactivation. This article reviews recent progresses in HIV transcription research with an emphasis on the intricate control of the various P-TEFb complexes, structural and functional insights into their interactions with Tat, the multifaceted roles of post-translational modifications of Tat and epigenetic control of HIV chromatin in modulating Tat activity and HIV latency. The knowledge from these studies will not only help design better strategies to fight HIV infection and transcriptional latency, but also advance the overall understanding of the mechanism controlling transcriptional elongation in general.
2. Introduction
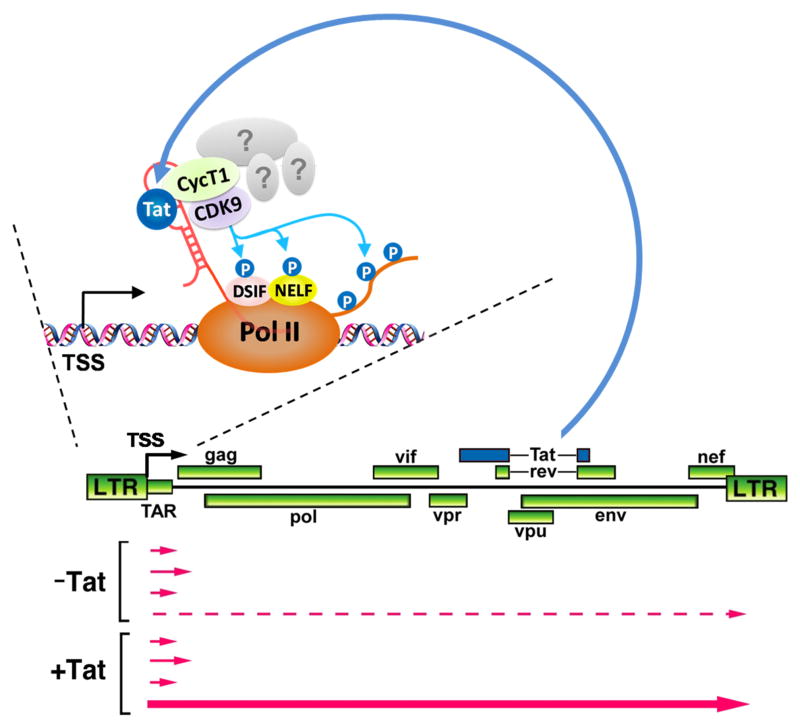
Transcription of the HIV-1 proviral DNA into mRNA is a critical step in the viral life cycle, as the mRNA serves not only as the template for the synthesis of all viral structural and accessory proteins but also as the genome for the next generation of viral particles. Upon reverse transcription of the viral RNA in CD4+ T cells or macrophages, the resulting HIV proviral DNA must be integrated into the human chromatin before it can be transcribed by host RNA polymerase II (Pol II). For all simple retroviruses, transcription is mediated exclusively by the host Pol II transcriptional apparatus. However, for a complicated retrovirus like HIV, this process is additionally controlled by the virus-encoded transcriptional transactivator Tat protein (Fig. 1). Without Tat, Pol II initiates transcription from the HIV promoter efficiently but only travels a short distance on the viral template, producing short abortive transcripts that do not support viral replication 1. To overcome this restriction, Tat is employed by HIV to dramatically increase the processivity of Pol II to produce the full-length viral transcripts (Fig. 1).
Fig. 1. HIV-encoded Tat protein stimulates production of full-length viral transcripts through binding to HIV TAR RNA stem-loop structure.
The genomic structure of the HIV-1 virus is shown with the coding region for the two-exon form of Tat highlighted in blue. Tat, in conjunction with other cellular cofactors (indicated by question marks), binds to the TAR RNA structure that is formed at the 5′ end of the nascent HIV transcript to stimulate the production of the full-length HIV mRNA (i.e. transcriptional elongation) by Pol II. TSS: transcription start site.
Different from most other transcriptional activators that target specific DNA sequences located in promoters or enhancer regions, HIV Tat stimulates Pol II elongation through interaction with the transactivation response (TAR) RNA element, a stem-loop structure located at the 5′ end of all nascent viral transcripts and synthesized by Pol II right before pausing (Fig. 1). Tat binds mainly to the 3-nucleotide bulge and its immediate surrounding sequences right below the 6-nucleotide TAR apical loop (Fig. 1). Although the loop sequence contributes minimally to the binding of Tat to TAR in vitro, it is absolutely required for Tat to transactivate HIV in vivo 2, suggesting that the loop sequence may have other important functions (see below).
Because Tat is produced from the same HIV mRNA whose production it stimulates, it fuels a powerful feedback circuit that can lead to rapid and robust HIV gene expression under optimal conditions 3. However, when HIV transcription initiation is restricted due to epigenetic silencing or low activity/levels of key transcriptional activators (e.g. NF-κB or Sp1) on the viral LTR, Tat concentrations can fall below threshold levels, leading to the establishment of HIV latency 4. Conversely, even a small increase in Tat expression can sometimes be enough to drive a provirus from its latent state into productive replication 5. It is worth noting that the origin of Tat could even be extracellular due to the fact that Tat is able to traverse lipid membranes via its arginine-rich transduction motif 6. As such, Tat secreted from productively infected cells can act as a viral growth factor to target neighboring cells to stimulate HIV replication and latency reactivation. All in all, the highly sensitive and signal amplification nature of the Tat-based feedback circuit establishes this small HIV protein as a molecular switch that determines the “on” and “off” states of HIV replication.
It is worth noting that HIV Tat has long been used as a model system for studying the factors and molecular mechanism that govern Pol II transcriptional elongation in general. Exactly twenty years after elongation was first recognized as a major check point that controls HIV gene expression 1, analyses conducted in both Drosophila and human stem cells revealed that a great number of cellular genes also employ the same mechanism to control their transcription 7. These genes, which are mostly involved in stress-response, cell growth/renewal and differentiation, contain paused Pol II at their promoter-proximal regions. It is generally believed that the de novo recruitment of Pol II and assembly of a functional pre-initiation complex (PIC) are very time-consuming. Thus, the paused Pol II at these gene promoters under resting, un-stimulated conditions enables highly sensitive, rapid, and synchronous induction of transcription that is essential for stress-response, cell-growth/renewal and development 8.
In this article, we review recent progresses in our understanding of HIV transcriptional control with an emphasis on the effects exerted by macromolecular assemblies containing HIV Tat/TAR and the host cellular co-factors. These progresses are critical to not only ongoing efforts to eradicate HIV/AIDS through reactivating and then eliminating latent HIV reservoirs that are in a transcriptionally silent state, but also the elucidation of the general mechanism that controls transcriptional elongation of numerous cellular genes.
3. P-TEFb is a key host cellular cofactor for Tat activation of HIV transcription
Although elongation is a major rate-limiting step during HIV transcription, and HIV employs its own Tat and TAR to overcome this restriction, mounting evidence had suggested that the mere binding of Tat to TAR is not sufficient to induce HIV transactivation and specific host cellular co-factors are required in this process 9. This notion is supported by many observations, and an especially important one shows that mutations in the apical loop of TAR strongly blocked Tat activation of HIV transcription but had no obvious effect on Tat binding in vitro 10.
The ensuing years saw many unsuccessful attempts to isolate and identify the specific Tat co-factors. After numerous trials and errors, a major breakthrough finally arrived in the late 1990’s with the identification of the human positive transcription elongation factor b (P-TEFb) as such a co-factor 11. These studies demonstrate unequivocally that the Tat-TAR-P-TEFb interaction at the HIV promoter is absolutely essential for efficient activation of viral transcription.
P-TEFb is composed of the cyclin-dependent kinase 9 (CDK9) and its regulatory partner cyclin T (CycT). While the 42-kDa isoform of CDK9, termed CDK9(42), is the predominant form of CDK9 in many cell types, there is also the 55-kDa isoform termed CDK9(55), which has a 117-residue amino terminal extension missing in CDK9(42) 12. Similarly, besides CycT1, minor CDK9-associated CycT2a and T2b molecules also exist in many cell types 13. However, Tat does not recognize CycT2a and T2b, and can only bind to CycT1 through a Tat:TAR recognition motif (TRM) located at the carboxy-terminal edge of the cyclin domain in T1 14.
Although P-TEFb is ubiquitously present in all cell types, in CD4+ T lymphocytes and monocytes, which are highly relevant for HIV infection and replication, the expression of CycT1 is normally low and repressed at the level of protein synthesis 15. In fact, the limited supply of P-TEFb could be an important contributing factor to viral latency in primary T cells 16. However, the induced activation of T cells or differentiation of monocyte into macrophages, which markedly enhances permissiveness of these cells to HIV infection, has been shown to increase the protein but not mRNA level of CycT1 15. Although the posttranscriptional mechanism restricting CycT1 production in resting CD4+ T cells is still unknown, a microRNA-dependent mechanism involving miR-198 has been reported to repress CycT1 mRNA translation and HIV replication in monocytes 17.
P-TEFb functions by phosphorylating serine residues located at the second position (Ser2) within the heptapeptide repeats that constitute the C-terminal domain (CTD) of the largest subunit of Pol II. The phosphorylated CTD functions as a scaffold upon which various transcription and RNA processing factors meet and operate. These factors collectively control the elongation and termination phases of Pol II transcription and also facilitate co-transcriptional processing of pre-mRNAs 18. In addition to the Pol II CTD, two negative transcription elongation factors, DSIF and NELF, are also phosphorylated by P-TEFb. The phosphorylation antagonizes their inhibitory actions, leading to the release of Pol II from promoter-proximal pausing and transition into productive elongation 18.
The identification of P-TEFb as a key host cofactor for Tat-transactivation has provided satisfactory explanations for a number of long-standing observations in the HIV transcription field. For example, comparing to many other cellular and viral gene promoters, transcription from the HIV LTR had long been indicated as particularly sensitive to kinase inhibitors such as DRB (5,6-dichloro-1-β-D-ribofuranosylbenzimidazole) and flavopiridol 19. The strong correlation detected between the ability of these two drugs to inhibit CDK9’s kinase activity and their suppression of Tat transactivation immediately reveals the reason behind this high sensitivity.
Moreover, it had long been known that Tat acts in a species-specific manner to efficiently transactivate the viral LTR in many human and primate cell types but not in cells from other species (e.g., yeast, Drosophila, and murine cells) 20. After the identification of a direct interaction between Tat and human CycT1, it was revealed that the interaction requires zinc as well as essential cysteine residues in both proteins 21. However, murine CycT1 lacks a critical cysteine (C261 in human CycT1) that is required for the stable interaction. The substitution of a cysteine for tyrosine at this position in murine CycT1 restores its zinc-dependent binding to Tat as well as its ability to support Tat-transactivation 14,22.
Finally, earlier studies had shown that the defect in Tat transactivation in murine cells is also at the level of TAR RNA recognition 23, and that a hypothetical cellular Tat cofactor encoded by a gene on human chromosome 12 confers on Tat the ability to activate HIV transcription in a TAR loop-dependent manner 24. This is in spite of the observation that no specific loop sequence is required for the Tat-TAR interaction in vitro. After the identification of human CycT1 as a direct binding partner of Tat, the discovery that the CycT1 gene indeed maps to human chromosome 12 and that CycT1 touches the TAR loop and forms a stable ternary complex with Tat and TAR with a stringent requirement for the wild-type TAR loop sequence 11b,20,25 provides the long-sought-after validation of the earlier observations.
4. Sequestration of P-TEFb in catalytically inactive 7SK snRNP
As a general transcription factor, P-TEFb is required for not only Tat activation of HIV transcription, but also efficient expression of a vast array of cellular genes. As such, the availability and activity of P-TEFb must be carefully controlled to respond to changes in the global transcriptional demand 26. For most CDKs, which belong to an extensive family of protein kinases that are discovered mainly for their roles in regulating the cell cycle, it is well known that specific inhibitors are used to regulate their kinase activities 27. Since CDK9 is a member of this superfamily, it is not surprising that the CDK9-CycT1 heterodimer, which constitutes the core P-TEFb, can also exist in a form that lacks catalytic activity. However, the way P-TEFb is inhibited involves a mechanism that is quite different from those used for other CDKs.
What sets P-TEFb apart from other CDKs is the involvement of a small non-coding RNA molecule, termed the 7SK snRNA, in inhibiting CDK9’s kinase activity. Affinity-purified as an associated factor of CDK9 28, 7SK is an abundant small nuclear RNA of 331 nucleotides that is transcribed by RNA Pol III. When P-TEFb is present in a 7SK snRNA-containing large complex (Fig. 2), it displays little kinase activity toward the CTD heptapeptide repeats of RNA Pol II 28a. Although the 7SK-P-TEFb interaction was found to be necessary for CDK9’s inhibition, it was soon discovered that the 7SK snRNA alone was insufficient for this task and that additional factor(s) existing in the large 7SK/P-TEFb-containing complex, termed the 7SK snRNP (Fig. 2), must work together with the snRNA to suppress CDK9’s catalytic activity 28.
Fig. 2. P-TEFb is sequestered in 7SK snRNP and released in response to various signaling events.
Under normal conditions, the majority of nuclear P-TEFb is sequestered in the 7SK snRNP, where P-TEFb’s kinase activity is inhibited by HEXIM1 in a 7SK snRNA-dependent fashion. The stability of 7SK RNA is maintained by MePCE and LARP7, which bind to the 5′ and 3′ ends of 7SK, respectively. When cells are subjected to the indicated treatments (highlighted in red), various signal transduction pathways are turned on, leading to changes in posttranslational modifications that include phosphorylation and acetylation on the indicated 7SK snRNP subunits and the release of P-TEFb from 7SK snRNP.
Indeed, nuclear protein HEXIM1 was subsequently identified through affinity-purification of P-TEFb-associated factors and found to reside in the 7SK snRNP 29. It efficiently inhibits P-TEFb’s phosphorylation of the Pol II CTD on Ser2 only in the presence of 7SK snRNA. This is due to the fact that the 7SK snRNA is required to bridge the interaction of P-TEFb with the central region in HEXIM1 that contains positively charged residues reminiscent of the arginine-rich TAR RNA-binding motif in HIV Tat 30. In addition to HEXIM1, a close homologue named HEXIM2 was also found in the inactive P-TEFb-containing 7SK snRNP 31. Although these two HEXIM proteins exhibit distinct expression patterns in many human tissues and established cell lines, they have the potential to form stable homo- and hetero-dimers in a small set of 7SK snRNPs that display similar or highly related functions 31a,b. Consistent with this notion, in HEXIM1-knocked down cells, HEXIM2 was shown to functionally and quantitatively compensate for the loss of HEXIM1 to maintain a constant level of the 7SK snRNPs in vivo 29a. Despite the functional similarity between HEXIM1 and 2, HEXIM1 has a unique N-terminal region that possesses self-regulatory activity 29a, suggesting that these two homologous proteins may be regulated differently through their regions of unique sequences.
The 7SK snRNA is known to serve as a scaffold in maintaining the overall stability of the 7SK snRNP 32. Because of the stringent demand on its integrity, the stability of this snRNA is ensured by two additional 7SK snRNP components that bind to the 5′ and 3′ terminal regions of 7SK RNA, respectively (Fig. 2). At the 3′ end, the poly(U) sequence of the nascent 7SK snRNA is initially protected by the Lupus antigen (La) protein during transcription, and then replaced by the more specific La-related protein 7 (LARP7) after synthesis 33. LARP7 is a highly specific and abundant partner of 7SK snRNA, as approximately 90% of the snRNA in cells are bound to and rely on LARP7 for stability 33. Notably, it is frequently mutated in human gastric, breast, and cervical cancers, largely due to microsatellite instability-associated frame-shift mutations that result in C-terminally truncated proteins with no ability to bind to 7SK snRNA and sequester P-TEFb into the 7SK snRNP 32a,34. Consistent with the tumor suppressor role of a Drosophila homolog of LARP7, loss of LARP7 function in human breast epithelial cells has been shown to disrupt epithelial differentiation and cause P-TEFb-dependent malignant transformation 32a.
On the opposite end of the 7SK snRNA, MePCE, an S-adenosyl methionine-dependent methylphosphate capping enzyme, is responsible for mono-methylation of the 5′ triphosphate of 7SK snRNA, which helps protect against cleavage by exonucleases (Fig. 2) 32b,35. However, it appears that 7SK is capped by only the LARP7-free MePCE and in probably a co-transcriptional manner prior to its sequestration into the 7SK snRNP 32b. Once inside the snRNP, MePCE exerts a capping-independent function to promote the LARP7-7SK interaction. Thus, MePCE and LARP7 act cooperatively to stabilize 7SK RNA and maintain the integrity of 7SK snRNP, which sequesters P-TEFb in an inactive form 32b.
Recently, in vivo RNA–protein interaction assays have provided more details about the sequence and structural elements of human 7SK snRNA that direct the assembly of the 7SK-LARP7-MePCE core snRNP 36. While MePCE is shown to interact with the basal part of the 7SK 5′-hairpin (the so-called 5′-terminal G1-U4/U106-G111 helix-tail motif), LARP7 is found to bind to the 3′-terminal hairpin and the following poly(U) tail of 7SK. Furthermore, the direct 7SK-LARP7 binding is demonstrated as a prerequisite for recruiting P-TEFb into the 7SK snRNP, indicating that besides providing stability for 7SK snRNA, LARP7 possesses a more complex role in 7SK-mediated P-TEFb regulation than previously anticipated 36.
5. Signal-induced disruption of 7SK snRNP to release P-TEFb for stimulation of HIV and cellular gene transcription
Depending on the cell types, from 50 to 95% of cellular P-TEFb have been reported to reside in the 7SK snRNP 28a,37. Because of the sequestration of such high levels of P-TEFb, this complex is believed to serve as the primary cellular reservoir of unused P-TEFb 26c. Although the kinase activity of CDK9 is suppressed by HEXIM1/2 within the 7SK snRNP, CDK9 becomes fully active once released as it already has the phosphorylated Thr186 at the tip of the so-called T-loop (Fig. 2) and is thus suspended in a pre-activation state even before the release 38.
A variety of conditions that globally affect cell growth and differentiation have been shown to cause P-TEFb to reversibly associate with the 7SK snRNP (Fig. 2), and thus the level of the complex is dynamically controlled in cells 39. First of all, exposure of cells to various stress-inducing agents such as transcriptional inhibitors and DNA-damaging agents (e.g. flavopiridol, DRB, actinomycin D, staurosporine, camptothecin and UV irradiation, etc.) leads to the rapid dissociation of 7SK snRNP and an increase in the pool of active P-TEFb 38b,40. This process is presumably responsible for promoting the expression of many stress-responsive genes as part of the natural cellular response to stress. In fact, several of these agents (e.g. DRB and UV) are known to activate HIV transcription in a P-TEFb-dependent manner 41.
Besides playing a key role in stress response, the signal-induced release of active P-TEFb from the 7SK snRNP also contributes to activation of HIV and cellular gene transcription under certain growth-promoting conditions (Fig. 2). For example, the engagement of the T cell antigen receptor (TCR) or activation of T cells by phorbol esters has been shown to cause the disruption of 7SK snRNP and liberation of P-TEFb to increase HIV transcription and terminate viral latency 42. Moreover, conditions that induce cardiac hypertrophy also dissociate the 7SK snRNP to release P-TEFb, leading to a global increase in cellular RNA and protein contents and the enlargement of heart cells 43. Finally, the differentiation inducer hexamethylene bisacetamide (HMBA) has been found to induce a biphasic response in the cellular 7SK snRNP level in murine erythroleukemia cells, with an initial and transient disruption of 7SK snRNP that is followed by a permanent increase in the levels of HEXIM1 and 7SK snRNP after a prolonged treatment to induce terminal differentiation 39. Taken together, these observations reveal a strong correlation between signal-induced disruption of 7SK snRNP and the induction of HIV and cellular gene transcription during stress responses and cell proliferation.
As more and more agents and conditions that cause the release of P-TEFb from 7SK snRNP have been identified, the mechanisms and signaling pathways responsible for the release have also been gradually revealed (Fig. 2). For example, in HeLa cells, since the phosphorylation of Thr186 in the CDK9 T-loop is important for P-TEFb’s sequestration into 7SK snRNP 38b, one model proposes that protein phosphatases such as PP1α and PP2B are activated by HMBA or UV irradiation through a calcium-dependent signaling pathway, and then act cooperatively to dephosphorylate Thr186 to release P-TEFb, which is subsequently re-phosphorylated through a still unknown mechanism to regain activity 38a. The CDK9 T-loop can also be dephosphorylated by phosphatase PPM1A and, to lesser extent, PPM1B, leading to repression of HIV transcription 44. However, it is unclear whether the PPM1A-mediated dephosphorylation can occur in the context of 7SK snRNP to cause the disruption.
In experiments conducted in Jurkat T cells, HMBA is shown to activate the PI3K/Akt pathway, which in turn induces phosphorylation of HEXIM1 on the conserved Thr270 and Ser278 in the CycT1-binding domain and concomitant disruption of 7SK snRNP 45. In a separate study performed also in Jurkat T cells, the ERK kinase but not the PI3K/Akt pathway was indicated as important in the disruption of 7SK snRNP by activated TCR 37. Reaching a different conclusion, a recent report shows that protein kinase C (PKC) can phosphorylate Ser158 in HEXIM1 in response to the engagement of TCR or activation by phorbol esters 42a. Once Ser158 is phosphorylated, HEXIM1 neither binds to 7SK snRNA nor inhibits P-TEFb. Finally, adding yet another twist to the story, acetylation of CycT1 by the acetyltransferease p300 was reported to liberate P-TEFb from the 7SK snRNP, although this modification is apparently not required for the release of P-TEFb as induced by DRB, actinomycin D, or HMBA 46. Taken together, these observations suggest that multiple signaling pathways and mechanisms exist to facilitate the dissociation of P-TEFb from 7SK snRNP and that different agents, conditions or cell types may employ different pathways to achieve this goal. It will be interesting to test whether any of these pathways can crosstalk and coordinate their actions to increase the overall efficiency of 7SK snRNP disruption.
6. Tat extracts P-TEFb from 7SK snRNP
Extensive structural and functional analyses have so far revealed several interesting similarities between the Tat-TAR-P-TEFb complex and the 7SK snRNP. First of all, the arginine-rich TAR-binding motif in Tat is highly homologous to and functionally interchangeable with a portion of the 7SK-binding motif in HEXIM1 30a. Secondly, the Tat-binding site in the TAR RNA is structurally and functionally similar to a region in 7SK snRNA that is recognized by HEXIM1 30b. Thirdly, a small region near the cyclin box of CycT1 can be bound by either HEXIM1 or Tat 29b,47. These observations raise the possibility that Tat may take advantage of these similarities to directly extract P-TEFb from the 7SK snRNP.
Indeed, a number of in vitro and in vivo studies have shown that Tat does possess such a capability (Fig. 3) 48. Likely owing to this capability, a significant reduction in the level of 7SK snRNP and increase in the Tat-P-TEFb interaction has been observed in both human primary blood lymphocytes and cultured cell lines that are infected with HIV 48b,49. From the perspective of HIV, it makes perfect sense for Tat to directly target the 7SK snRNP to obtain P-TEFb for HIV transactivation. This is because the snRNP not only sequesters up to 95% of all the P-TEFb in the cell and is thus the principal source of unobligated P-TEFb, but also keeps P-TEFb in a pre-activated state that is marked by the phosphorylated CDK9 T-loop 38a.
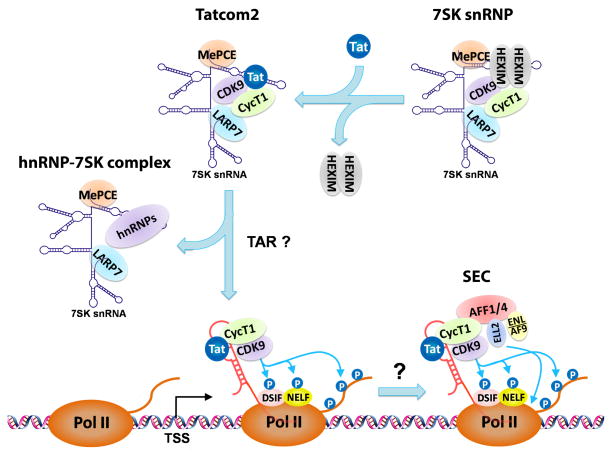
Fig. 3. Tat induces transfer of P-TEFb from 7SK snRNP via possibly Tatcom2 to SEC, where P-TEFb cooperates with ELL2 to synergistically activate HIV LTR transcription.
Tat is known to target the 7SK snRNP to capture P-TEFb and release HEXIM1. The Tatcom2 complex, whose composition is similar to that of 7SK snRNP except for the substitution of HEXIM1 with Tat, could be a reaction intermediate before the emergence of HIV TAR RNA. Once TAR is produced, P-TEFb and Tat are transferred onto the TAR structure, and through a still unknown mechanism, nucleate the formation of the multi-subunit SEC complex. Besides P-TEFb, which phosphorylates the Pol II CTD and negative elongation factors NELF and DSIF to antagonize their inhibitory effects, SEC also contains another well-characterized elongation stimulatory factor ELL2, which directly enhances the catalytic activity of Pol II. By acting on the same Pol II enzyme, P-TEFb and ELL2 synergistically activate HIV transcription.
Although the ability of Tat to extract P-TEFb from the 7SK snRNP has been clearly demonstrated, the underlying mechanism(s) for this extraction is less clear. It is possible that Tat may combine several different methods to maximize its chance for success. Based on the observations that the cysteine-rich domain of Tat binds to CycT1 with an affinity higher than that displayed by HEXIM1 48a,50, Tat has been proposed to use this domain to outcompete HEXIM1 for binding to CycT1. After P-TEFb is captured by Tat, a concomitant conformational change in the 7SK snRNA may permit the ejection of HEXIM1 from the snRNP 51. Another model proposes that the RNA-binding motif within the arginine-rich domain of Tat is responsible for P-TEFb’s extraction 30b. The idea is that Tat can use this motif to interact with the region of 7SK snRNA that is normally contacted by HEXIM1, thus displacing HEXIM1 and forming a transitional HEXIM1-free 7SK snRNP (Fig. 3) 30b. After the synthesis of HIV TAR RNA, Tat and P-TEFb could be transferred out of this complex to form the Tat/TAR/P-TEFb-containing complex on the viral LTR. Recently, Tatcom2, a novel Tat/P-TEFb-containing complex that lacks HEXIM1 but contains all the other 7SK snRNP subunits, has been identified 52. Detected in cells lacking the TAR RNA, this complex appears to fit the descriptions of a transitional complex that exists between the 7SK snRNP and the eventual Tat/TAR/P-TEFb-containing complex (Fig. 3).
The exact sub-nuclear location where Tat extracts P-TEFb from the 7SK snRNP has yet to be determined. Although the vast majority of the snRNP appears to exist off the chromatin and can be easily extracted from the nucleus 53, a small amount has recently been detected at the HIV promoter 54. This latter observation has led to the proposition that the Tat-P-TEFb elongation complex is assembled de novo on the HIV template via the Tat-TAR interaction with the concomitant release of P-TEFb from the 7SK snRNP 55. Finally, the process through which Tat extracts P-TEFb from 7SK snRNP may also be assisted by other cellular co-factors. For example, the interaction of Tat with protein phosphatase PP1 56, which contributes to stress-induced 7SK snRNP disruption through dephosphorylating Thr186 at the CDK9 T-loop 56, could conceivably lend a helping hand to Tat in the extraction process. In agreement with this notion, PP1 has been shown to play a positive role in Tat transactivation and HIV replication 57.
7. Tat assembles Super Elongation Complex (SEC) to activate HIV transcription
As discussed above, Tat can cause the release of P-TEFb from 7SK snRNP. However, it was unclear whether Tat delivers P-TEFb alone or in combination with additional factors to the HIV LTR to stimulate viral transcription. To answer this question, sequential affinity-purifications that target HA-tagged Tat, Flag-tagged CDK9 and any protein(s) associated with these two factors in a single complex were performed 58. This procedure has led to the identification of a set of chromosomal translocation partners of the mixed lineage leukemia (MLL) protein, including ELL2, AFF4, ENL and AF9, all of which are demonstrated transcription factors/co-factors, as novel components of the Tat-P-TEFb complex (Fig. 3). Notably, the same set of factors plus several others (e.g. the AFF4 homolog AFF1, the ELL2 homolog ELL1, and components of the polymerase-associated factor complex PAFc) were also independently isolated through a similar affinity-purification scheme that targets HIV Tat alone 52.
Because the form of CDK9 present in the 7SK snRNP has the phosphorylated Thr186 at the tip of the T-loop 38, it is expected to be catalytically active once captured by Tat from the 7SK snRNP. As such, it had long been thought that the Tat-P-TEFb interaction alone is sufficient to activate HIV transcription. The big surprise coming from the identification of several novel partners of the Tat-P-TEFb complex is that Tat not only recruits P-TEFb but also another well-characterized elongation factor ELL2 or 1 to the viral LTR (Fig. 3; our unpublished data suggest that Tat strongly prefers ELL2 over ELL1; Nanhai He and Qiang Zhou, unpublished observations).
Unlike P-TEFb, which stimulates transcriptional elongation through phosphorylating key target proteins (the Pol II CTD, DSIF and NELF), ELL2/1 directly increases the catalytic rate of Pol II by keeping the 3′ end of the nascent mRNA properly aligned with the catalytic center within the polymerase enzyme to prevent Pol II backtracking 59. Thus, Tat is able to deliver P-TEFb and ELL2/1, which belong to different classes of elongation factors, within a single complex to the viral LTR, where these two factors can act on the same paused Pol II to synergistically stimulate elongation (Fig. 3). This observation has significantly expanded the conventional view of the mechanism of Tat transactivation and also explains why Tat is such a powerful transcriptional activator. Because of the existence of at least two well-established elongation stimulatory factors in a single complex, this novel multi-subunit complex is now called the Super Elongation Complex (SEC; Fig. 3).
Recent structural and functional analyses of the SEC reveal that AFF1 and AFF4, which are likely present in separate but closely related SECs 60, function as a central scaffold to mediate the formation of the complexes 26b,58,61. Detailed mapping studies indicate that AFF4 (likely also the homologous AFF1) employs short hydrophobic regions along its structurally disordered axis to directly bind to and recruit other factors into a SEC 61. Direct binding partners CycT1, ELL1/2, and ENL or AF9 act as bridging components that link this complex to two major elongation factors, P-TEFb and PAFc. The unique scaffolding properties of AFF4 thus allow dynamic and flexible assembly of multiple elongation factors while connecting the components not only to each other, but also to a larger network of transcriptional regulators.
For a powerful elongation factor complex such as the SEC, it would be hard to imagine that it is used only by Tat to stimulate HIV transcription. The fact that all SEC subunits except P-TEFb have previously been reported as MLL fusion partners implies an intimate relationship between the SEC and leukemic pathogenesis. Indeed, at about the same time when the SEC was identified as a co-factor and binding partner of HIV Tat, independent biochemical purifications of several frequently occurring MLL chimaeras such as MLL-AFF1 and MLL-ENL and their interacting molecules have led to the isolation of the same SEC complex that turns out to be essential for MLL-mediated leukemogenesis 62. It is believed that this high order P-TEFb-containing complex, which is biochemically distinct from the MLL histone methyltransferase complex, promotes uncontrolled transcriptional elongation of MLL target genes to induce leukemic transformation 62.
8. ELL2 stabilization promotes SEC formation and HIV transcription
The Tat-SEC interaction, which provides the basis for SEC’s original identification, not only allows Tat to recruit the complex to the HIV LTR to activate viral transcription, but also promotes the SEC formation through stabilizing ELL2, which is otherwise a highly labile protein 58. Unlike all the other SEC subunits including its close homolog ELL1, ELL2 is stoichiometrically limiting and uniquely regulated at the level of protein stability. Recently, the RING domain protein Siah1 has been identified as the specific E3 ubiquitin ligase for ELL2 polyubiquitination and proteasomal degradation 63. Depletion of Siah1 was shown to promote the formation of SECs and enhance the SEC-dependent HIV transcription, whereas overexpression of Siah1 resulted in the degradation of ELL2 and to a lesser degree AFF1 and AFF4 63.
Consistent with an earlier observation that the interaction of ELL2 with the scaffolding protein AFF4 (likely also AFF1) dramatically increases the former’s half-life 58, Siah1 cannot access and ubiquitinate ELL2 that is bound to AFF4, although at high concentrations, it also degrades AFF1/4 to destroy existing SECs 63. Unlike AFF4, Tat does not appear to act by inhibiting ELL2 polyubiquitination, and the exact mechanism by which it stabilizes ELL2 remains elusive at this moment. There exists a possibility that Tat affects a step downstream of the Siah1-mediated ELL2 polyubiquitination to directly suppress proteasomal degradation of ELL2. In agreement with this idea, Tat has been shown to directly bind to the β subunits of the constitutive 20S proteasome thereby inhibiting the proteolytic activity of the proteasome in cells 64.
9. The Brd4-P-TEFb interaction and its control of HIV transcription and latency
Many cellular genes, especially those that function as primary response genes in stimulus-responsive pathways, often contain paused Pol II and negative elongation factors at their promoter-proximal regions before full induction 65. It has been shown that these genes employ the nuclear protein Brd4 as an adaptor to recruit P-TEFb to their chromatin loci 65b,66. To these genes, Brd4 serves as the cellular equivalent of HIV Tat to antagonize the inhibitory actions of negative elongation factors and promote Pol II transcriptional elongation in a P-TEFb-dependent manner.
As a member of the bromodomain and extra-terminal domain (BET) protein family, Brd4 has two N-terminal bromodomains that bind to acetylated histone H3 and H4 67, a P-TEFb-interaction domain (PID) at its C-terminus that interacts with P-TEFb 68, and a central extra-terminal (ET) domain that has been implicated in the interaction with a number of other proteins 69. Notably, Brd4 remains bound to the chromatin throughout the mitosis 67a,70. As an epigenetic reader, it is thus able to transmit epigenetic memory across cell divisions by recruiting and depositing P-TEFb onto promoters of key growth-promoting genes prior to the onset of G1 70–71. Besides exerting its gene-activating effect through interacting with acetylated chromatin, Brd4 is also known to bind to the mediator complex within the pre-initiation complex (PIC), which may further enhance its overall transcriptional activity 66a,72.
Just as Brd4 plays an important role in recruiting P-TEFb to many cellular gene promoters to release Pol II from pausing, it also produces a positive effect on basal, Tat-independent HIV transcription, although the effect obtained with the stably integrated proviral DNA is generally smaller than that with the transiently transfected HIV LTR reporter construct 66a,72–73, which cannot assemble into a chromatin template. Thus, the interaction of Brd4 with the mediator complex but not acetyl-histones may play an even more prominent role in mediating basal HIV transcription.
In contrast to the stimulatory effect of Brd4 on basal, Tat-independent HIV transcription, Brd4 has been demonstrated as a potent inhibitor of Tat transactivation 66a,68. This is because Tat and Brd4 can directly compete with each other for binding to P-TEFb and the Brd4-P-TEFb interaction precludes the more efficient and highly specific recruitment of P-TEFb/SEC to the viral LTR by Tat and TAR (Fig. 4A) 66a. As a result of this competition, overexpression of just the PID domain of Brd4 has been shown to antagonize Tat-dependent HIV transcription and latency reactivation 68.
Fig. 4. Brd4 is a potent suppressor of Tat-transactivation and BET bromodomain inhibitor JQ1 efficiently antagonizes this suppressive effect.
A. In the absence of JQ1, the promoter-bound Brd4 (through interacting with acetylated histones or Ac) competitively blocks the interaction between P-TEFb and Tat. Likewise, methylation of Tat by SETDB1 and PRMT6 also prevents this interaction. Meanwhile, most of cellular P-TEFb are sequestered in the inactive 7SK snRNP. All of these inhibit the ability of Tat to form on the HIV TAR RNA a functional SEC that is essential for activated viral transcription. B. JQ1 dissociates Brd4 from the HIV promoter and increases the local concentration of active P-TEFb for Tat to assemble into the SEC for efficient phosphorylation of the Pol II CTD, DSIF and NELF and activation of productive elongation. Additionally, JQ1 inhibits the expressions of SETDB1 and PRMT6 while promoting the production of SIRT1, which deacetylates Tat to enhance the Tat-P-TEFb interaction. Finally, JQ1 also disrupts the 7SK snRNP to release P-TEFb, providing another source of P-TEFb for SEC assembly at the HIV promoter.
10. Reactivation of HIV transcription and latency by BET bromodomain inhibitors
Latent reservoirs of HIV are the principal impediment to eradication of infection as they harbor transcriptionally silent proviruses that can evade immune surveillance and resume replication once therapy is disrupted 74. Strategies, generally referred to as “Shock and Kill”, are currently being developed to reactivate latent HIV, which can then be cleared by HAART and the immune system 75. In devising such strategies, focus has been placed on finding specific and effective ways to reactivate latent HIV without causing generalized T cell activation. To this end, several laboratories have recently reported that the BET bromodomain inhibitors JQ1 and iBet151, which can competitively bind to the Brd4 bromodomains and displace it from chromatin, efficiently reactivate latent HIV in a variety of cell line-based latency models 76.
In depth analyses reveal that the mechanism of JQ1-inducted HIV latency reactivation is mainly through antagonizing Brd4’s inhibition of Tat-transactivation (Fig. 4B) 73a,77. Through displacing Brd4 from the LTR region of HIV chromatin thereby decreasing Brd4’s local concentration at the promoter, JQ1 significantly increases the association of P-TEFb/SEC with Tat 73a,77. As a result of this enhanced association, more SECs are recruited by Tat/TAR onto the viral LTR to promote HIV transcriptional elongation (Fig. 4B) 73a. Further confirming the functional significance of the SEC in HIV latency reactivation, the siRNA-mediated knockdown of the key SEC component ELL2 significantly reduces JQ1’s activation of the LTR 73a.
Similar to JQ1, stable ectopic expression of a CDK9 mutant carrying the Ser175 to Ala mutation (S175A) in latently infected cells has also been shown to induce a robust Tat-dependent reactivation of the provirus 78. Although S175A destroys CDK9 phosphorylation on Ser175 that is induced by TCR or phorbol ester (PMA) signaling, it only slightly reduced the Tat-P-TEFb interaction. In contrast, the binding of Brd4 to P-TEFb is completely blocked by this mutation 66a,78. Since Brd4 is unable to compete with Tat for binding to CDK9 carrying S175A, just like JQ1, the mutation effectively removes the inhibition by Brd4, thus allowing the Tat-mediated HIV transactivation and latency activation to occur.
Besides JQ1’s direct and prominent enhancement of Tat transactivation, additional activities displayed by this compound may also contribute to its stimulation of HIV transcription. For example, JQ1 has been shown to partially and transiently release P-TEFb from the 7SK snRNP (Fig. 4B) 73a,79, which could be responsible for its weak activation of basal HIV transcription in the absence of Tat 73a,77. Although this effect of JQ1 is weaker than that on Tat-transactivation, it may help trigger the first few rounds of productive elongation to help build up the cellular Tat level and establish a positive feedback circuit. Another potentially beneficial effect caused by JQ1 is the induction of post-translational modifications of Tat (Fig. 4B) 76. This may be achieved through JQ1’s induction of the expression of SIRT1, a deacetylase and positive regulator of Tat, and down-regulation of the expression of methyltransferases SETDB1 and PRMT6, which are two negative modifiers/regulators of Tat (see below). However, it remains to be determined whether Tat indeed undergoes the implicated changes in the modifications in JQ1-treated cells, and if yes, whether these changes directly contribute to the JQ1-induced HIV latency activation.
It should be pointed out that in primary CD4+ T cells derived from patients on long-term HAART, the JQ1-induced latency reactivation is relatively inefficient and highly heterogeneous 76–77. This is likely due to the fact that JQ1 targets primarily the Tat-transactivation step, which depends exquisitely on the availability of the Tat cofactor P-TEFb/SEC, and that the level of the key SEC component P-TEFb is very low in resting CD4+ T cells 17,80. However, since full latency activation must rely on Tat and its interaction with the SEC, which is strongly promoted by JQ1, future studies will be very informative to reveal whether a cocktail of drugs that contains JQ1 as a key component plus other classes of latency activators can be used to efficiently reactivate HIV latency through overcoming multiple restrictions in resting CD4+ T cells. As a proof-of-concept of this idea, JQ1 has been shown to synergize with prostratin, another well-known latency activator that works by enhancing loading of RNA Pol II onto the HIV promoter thereby promoting transcriptional initiation, to reactivate latent HIV 73a. This synergism is detected in both Jurkat T cell-based HIV latency models as well as in pools of resting CD4+ T cells isolated from HIV-infected, HAART-treated patients 73a,77.
JQ1 has been proposed as an anti-cancer drug that works by reducing the Brd4-dependent c-myc expression, leading to the induction of differentiation and growth arrest of cancer cells that are addicted to the c-myc oncogene 81. Consistent with its general anti-growth property, JQ1 has been shown to potently suppress T cell proliferation with minimal cytotoxic effect 76. This inability to cause generalized T cell activation, together with its use of a largely Tat-specific mechanism in reactivating latent HIV, make JQ1 and its derivatives attractive candidates for implementing the “shock” phase of the “Shock and Kill” strategy to reactivate latent HIV reservoirs for subsequent eradication 75b.
11. Structural and functional characterization of Tat interactions with its partners
The Tat gene has two exons and the first one encoding amino acid residues 1–72 is both necessary and sufficient for HIV transactivation. Among the five conserved regions within the first exon, the first three (Acidic/proline-rich, Cystein-rich/Zn-finger, and Core) constitute the minimal transactivation domain that is vital for the Tat-P-TEFb interaction. According to the crystal structure of the Tat-P-TEFb complex solved by Tahirov et al. 55, this 49-aa minimal transactivation domain, which is the only well-resolved portion of the 86-aa recombinant Tat protein in the crystal structure, interacts extensively with residues located mainly in the cleft between the two cyclin box repeats of CycT1 and also extends to partially touch the CDK9 T-loop. While an intramolecular zinc finger coordinated by Cys22, Cys34, Cys37, and His33 is clearly visible within the Tat structure, a proposed intermolecular zinc finger involving Cys261 of CycT1 and Cys25, Cys27, and Cys30 of Tat, which are not conserved in Tat proteins from other species, has been questioned for its physiological relevance 73b. Nevertheless, as the interaction surface between CDK9 and CycT1 is ~40% smaller than in other CDK-cyclin pairs 82, the extensive Tat-P-TEFb interaction with the surface area twice as large as the average value for stable protein-protein interactions 55,83 explains how Tat stabilizes the P-TEFb complex 84.
It is interesting to note that when existing alone in solution without any binding partners, Tat is highly flexible and has no defined secondary structure along most of its sequence 85. It is only upon the binding to P-TEFb does Tat obtain its defined structure 55. In fact, Tat adopts a conformation that is highly complementary to the surface of P-TEFb. In return, Tat also induces significant conformational changes in P-TEFb 55,82, leading to the establishment of a more active kinase complex 84.
As a key regulatory protein encoded by a small virus, the flexibility of Tat and its dependence on host partners to adopt defined structures can be advantageous in several aspects. First of all, they may allow Tat to interact with more than one host factor complex and thus facilitate the transfer of Tat from one complex (e.g. Tatcom2; 52) to another (e.g. the SEC; 58) at different stages of HIV transactivation. Secondly, they are economical to the relatively small HIV genome, which does not need to encode an exceptionally long polypeptide that may be necessary to stabilize the Tat structure 86. Finally, they afford Tat a relatively high tolerance to sequence variations (up to 40%) without losing transactivation activity 85.
As the Tat-P-TEFb complex turns out to be only a non-physiological subcomplex of the larger functional Tat-SEC assembly recruited to the HIV promoter for transactivation, it is important to determine how the interactions between P-TEFb and other SEC subunits may impact the Tat-P-TEFb interaction. As an important step toward this goal, the crystal structure of a tripartite complex containing the recognition regions of P-TEFb and AFF4, which functions as a scaffold in SEC, has recently been solved87. AFF4 is shown to use the Leu34-Ile66 segment to meander over the surface of CycT1 on the opposite side of the CDK9-binding site, thus making no direct contact with CDK9 (Fig. 5). Interface mutations are found to reduce CycT1 binding and AFF4-dependent HIV transcription. Although Tat is not present in the structure, a unique inter-subunit pocket created cooperatively by AFF4 and CycT1 would provide an excellent fit for Tat (Fig. 5). Consistent with this prediction, Tat is shown to significantly increase the affinity of AFF4 for P-TEFb by more than 10-fold, which could be the reason for Tat to recruit the whole SEC but not P-TEFb alone to the viral LTR. Interestingly, one particular residue in Tat that is predicted to directly contact AFF4 is Lys28 (Fig. 5), which has been reported previously as important for the formation of the Tat-TAR-P-TEFb complex 88. As Lys28 is strictly conserved and exquisitely regulated by reversible acetylation 73b,89, it will be interesting to investigate whether this residue plays a key role in Tat-mediated recruitment of SEC to TAR as well as termination of the TAR-dependent phase of Tat transactivation (reviewed below).
Fig. 5. HIV Tat and SEC component AFF4 are predicted to make direct contacts on CycT1.

Superposition of the crystal structures of the AFF4-P-TEFb complex and the Tat-P-TEFb complex using the CycT1 subunit (yellow) shows the close proximity of AFF4 (blue) and Tat (red). The resulting Tat-AFF4-P-TEFb complex model is shown in two different orientations that are rotated by about 90 degrees (CDK9 is in gray). The side chains of Tat are from only those residues that are known to have an effect on transcription when mutated and that don’t have any identified binding partner or structural function. Lys28, which is well known for its influence on complex stability upon acetylation, is engaged in the AFF4 interaction in this model but exposed to solvent in the Tat-P-TEFb complex. Arg49, which is the last visible Tat residue in the structure, indicates where the RNA binding domain will be located [figure courtesy of Ursula Schulze-Gahmen and Tom Alber of UC Berkeley].
At this point, none of the published structures of human P-TEFb and P-TEFb-containing complexes contains the HIV TAR RNA 55,82,87. Although the ARM region of Tat was not visible in the solved Tat-P-TEFb structure 55, it is safe to predict that this region will likely adopt a well-defined conformation once bound to the recognition region in TAR. Indeed, a recent crystal structure of the tripartite ribonucleoprotein complex formed by EIAV Tat and TAR and the corresponding equine CycT1 strongly supports this notion 90. In this structure, both the C-terminal part of EIAV Tat (aa 41–69) that encompasses the ARM as well as the associated TAR RNA were resolved. While the core and the C-terminal hydrophobic regions of EIAV Tat wrap around the surface of equine CycT1 at the first cyclin box repeat, the ARM, which lies in between the two Tat regions, interacts with the major groove of TAR RNA. The interactions are largely electrostatic in nature and involve the basic residues in the Tat ARM and the negatively charged phosphate backbone of TAR. These electrostatic interactions, although not expected to afford high selectivity among the various TAR species of different base pair compositions, can still be modulated through reversible acetylation of specific lysine residues in the ARM to alter its charge property (reviewed below). Finally, in the tripartite structure formed by EIAV Tat/TAR and equine CycT1, the TAR displays a stable six-nucleotide hairpin-loop, with the fifth nucleotide directly contacting CycT1, which contributes to the specificity of the overall interaction 90.
Although much progress has been made toward the structural and functional characterization of the interactions of Tat with a subset of its binding partners, the more challenging and also urgent task facing us is to solve the structures of several complete macromolecular assemblies, such as the 7SK snRNP, Tatcom2, the Brd4-P-TEFb and Tat-TAR-SEC complexes, that are essential for the proper function of Tat. Obtaining such structures will provide unprecedented insights into the molecular mechanism by which Tat and its cofactors activate HIV transcription. It may also reveal novel druggable targets and assist the design of small molecule inhibitors that can specifically target the Tat-associated transactivation machinery with minimal cytotoxic side effects.
12. Post-translational modifications of Tat and their effects on HIV transcription
Given its central role in HIV transactivation, it is not surprising to see that the activity of Tat is elaborately controlled by post-translational modifications that include methylation, acetylation, phosphorylation, and polyubiquitination (Fig. 6). Most of these modifications are located in the arginine-rich motif (ARM) of Tat and can influence the formation or disruption of the Tat-TAR-SEC complex. Notably, many of these sites are targeted by both the “writing” and “erasing” enzymes, which permit the fine-tuning of Tat function during different phases of HIV transactivation. Interestingly, many of the Tat modification enzymes are also involved in histone modifications, thus linking the control of Tat activity to the chromatin status of an integrated HIV provirus.
Fig. 6. Post-translational modifications of Tat and their effects on HIV transcription.
The modifications (modified residues shown in boxes) can be classified into two types based on their impact on Tat’s transactivation activity: positive (green) and negative (red). The physiological consequences of the modifications during different phases of the HIV and Tat transactivation cycles are displayed in blue. The black arrows accompanied by a plus “+” sign mean that the modifications promote the formation of the indicated complexes. Me1, Me2 and Me3 denote mono-, di-, and tri-methylation, respectively. Ac: acetylation; Pi: phosphorylation; (Ub)n: polyubiquitination. The amino acid sequence of the 86-aa form of HIV-1 Tat is shown at the bottom with the modified residues indicated in boldface type and their positions shown above and below the sequence.
The most heavily modified residue in Tat is Lys51 (Fig. 6), which plays a central role in the interaction with HIV TAR RNA 90. Monomethylation of Lys51 by SET7/9 (KMT7) enhances Tat activity likely through strengthening the Tat-TAR interaction 90–91. Since SET7/9 itself has been shown to bind to TAR 91, its proximity to Tat may allow efficient methylation of Lys51 to facilitate the recruitment of the Tat-SEC complex to the TAR. Interestingly, while monomethylation of Lys51 is an activation marker for Tat, removing this methyl group by the lysine-specific demethylase LSD1 (KDM1) and its cofactor CoREST is apparently also required for the full transactivating function of Tat as well as the reactivation of latent HIV 92. To reconcile these seemingly contradictory observations, it has been proposed that Tat transactivation is actually composed of two distinctive phases, namely the TAR-dependent early phase and the subsequent TAR-independent phase 73b. In the TAR-dependent phase, Lys51 is monomethylated by SET7/9 to facilitate the recruitment of the Tat-SEC complex to the TAR RNA. Subsequently, LSD1 and CoREST demethylate the same lysine to facilitate the dissociation of the Tat-TAR-SEC complex and prepare Tat for acetylation at Lys51, which are important for the subsequent TAR-independent phase of Tat-transactivation (Fig. 6) 73b. During this second phase, Tat is proposed to directly interact with Pol II 93 and recruit the histone acetyltransferase (HAT) PCAF and chromatin remodeling complex PBAF to generate a further relaxed chromatin environment in a TAR-independent manner. Thus, Lys51 acts as a molecular switch, with its monomethylation promoting the Tat-TAR-SEC formation and acetylation enhancing the Tat-PBAF-PCAF assembly (Fig. 6).
In order to efficiently disrupt the Tat-TAR-SEC complex, upon the demethylation of Lys51, Lys51 and its neighboring Lys50 must be acetylated by acetyltransferases p300/CBP (KAT3B) or GCN5 (KAT2A) (Fig. 6) 89,94. The acetyl groups neutralize the positive charges in the highly basic ARM to dissociate Tat from TAR. This step is critical to terminate the TAR-dependent phase and enable the transition into the TAR-independent phase 95. Of note, Lys50 acetylation also facilitates the recruitment of chromatin-modification transcriptional co-activators such as PCAF (KAT2B) and the SWI/SNF complex PBAF onto Tat 96.
Just like the demethylation of Lys51, the deacetylation of Lys50 by sirtuin 1 (SIRT1) is also required for the full transactivating function of Tat 91. A possible reason for this requirement is that the deacetylation happens toward the end of productive transcription to recycle Tat back to the unacetylated state so that it can bind to TAR/SEC again and start over a new round of transactivation 73b. Paradoxically, the overexpression of SIRT1 was found to significantly hinder Tat transactivation in latently infected cells 97. This is probably due to the fact that the Tat level is very low at the beginning of latency reactivation and too much SIRT1 activity will interfere with the TAR-independent Tat transactivation step and disrupt the establishment of the positive Tat feedback circuit that is critical for latency reactivation 97.
The above observations concerning reversible modifications at Lys50 and Lys51 indicate that the activity of Tat is elaborately regulated by both “writers” and “erasers” of posttranslational modification marks. The functions of individual modification enzymes cannot be simply classified as positive or negative with regard to the overall Tat transactivation and latency reactivation process. Rather, they should be viewed as indispensible components of a complete, interrelated regulatory network (Fig. 6) that exerts proper control of HIV transcription in response to changing conditions and viral replication status within an infected cell.
Like Lys50 and Lys51, Lys28, which is located in the cysteine-rich region of Tat, can also be acetylated. Catalyzed by PCAF (KAT2B), the acetylation of Lys28 enhances the binding of Tat to P-TEFb/SEC and TAR 89,96a to promote Tat transactivation. Unlike the deacetylation on Lys50, deacetylation on Lys28 by HDAC6, a class II HDAC also responsible for deacetylating α-tubulin 98, inhibits Tat transactivation as it destabilizes the Tat-TAR-P-TEFb/SEC complex 99. The interaction between HDAC6 and Tat appears to depend upon microtubules 99, and the binding of Lys28-acetylated Tat to tubulin/microtubules perturbs microtubule dynamics, leading to apoptosis of T lymphocytes 100. Since HDAC6 can suppress both Tat transactivation and Tat-induced apoptosis, it may serve as a potent host cell antagonist of HIV/AIDS.
Besides the above-mentioned reversible modifications, Tat is also subject to posttranslational modifications of which no “erasers” have been identified. These modifications span across the entire length of Tat and include di-/tri-methylation, phosphorylation, and polyubiquitination. For example, Lys50 and Lys51 can be di- or tri-methylated by SETDB1 (KMT1E) 101. Likewise, the neighboring Arg52 and Arg53 can also be methylated by another methyltransferase PRMT6 (Fig. 6) 102. There is currently no enzyme that can erase these modifications. Located in the Tat ARM region, these methylation events interfere with the formation of the Tat-TAR-P-TEFb/SEC complex probably through steric hindrance 90. Of note, the methylations on Arg52 and Arg53 have an additional role in increasing the stability of Tat 103, suggesting that they may serve to tag a stable pool of inactive Tat in latently infected cells.
HIV Tat can also be phosphorylated by the PKR kinase and the CDK2/cyclin E kinase complex 104. PKR phosphorylates Ser62, Thr64, and Ser68, which results in faster and stronger binding of Tat to TAR and promotes Tat transactivation 104a. CDK2/cyclin E phosphorylates two highly conserved Tat residues Ser16 and Ser46, and this event is reported as important for the expression from a transiently transfected HIV LTR-LacZ reporter construct and viral replication in a HeLa-based proviral system 104b.
Polyubiquitination is frequently used as a tag that marks a protein for degradation by the proteasome. However, in the case of Tat, this particular modification has been shown to promote the transactivating function of Tat without inducing Tat degradation 105. The polyubiquitination appears to be catalyzed by the proto-oncoprotein Hdm2, which interacts with Tat and functions as a specific E3 ligase to induce ubiquitination on Lys71 (Fig. 6). At this moment, the mechanism by which the polyubiquitinated Tat enhances HIV transactivation is unclear. Through targeting and dissociating the 26S proteasome, Tat has been shown to activate HIV transcription in a manner that does not depend on the proteolytic activity of the proteasome 106. It is tempting to speculate that the polyubiquitinated Tat in association with key proteasomal components may play a key role in this process.
13. Epigenetic regulation of HIV transcription
Besides the central role that the Tat-TAR-SEC axis plays in overcoming the restriction on Pol II elongation, HIV transcription is also subjected to epigenetic regulation. The types of regulation directly affect the compactness of the HIV chromatin, thus altering the accessibility of a provirus to general transcription factors/co-factors and transcriptional regulators, which in turn determines whether the provirus enters the repressive or active transcription state. In the context of HIV transcription, the epigenetic regulation can be divided into two categories: (1) covalent modifications that include histone acetylation, histone methylation, and DNA methylation; and (2) chromatin remodeling that moves nucleosomes around relative to key binding sites in a ATP-dependent manner. One particular nucleosome called Nuc-1, which is located ~50-bp downstream of the HIV transcription start site (TSS), plays a central role in blocking Pol II transcription of the provirus 96c,107. Thus, most types of epigenetic regulation that affect the transcriptional state of the HIV provirus frequently target this nucleosome.
Histone deacetylation is known to induce the compact chromatin structure that represses the recruitment and assembly of active transcriptional machinery at the promoter. In the case of HIV, two transcriptional repressors, LSF and YY-1, have been reported to interact with the Nuc-1 region to recruit histone deacetylase 1 (HDAC-1) to the viral promoter and repress transcription 108. Besides LSF and YY-1, a number of other proteins can also recruit HDACs to different regions of the HIV LTR. For example, the homodimer formed by the NF-κB p50 subunit binds to the NF-κB binding sites located in the HIV enhancer region, leading to the recruitment of HDAC1 to the LTR and inhibition of viral transcription 109. Consistently, the RNAi-mediated p50 knockdown reduces HDAC1 binding to the LTR, which in turn results in enhanced recruitment of Pol II and reactivation of latent HIV in J-Lat 6.3 cells 109. Upon stimulation by the pro-inflammatory cytokine TNF-α, the NF-κB p50/p65 heterodimer displaces the p50 homodimer and activates HIV transcription 110.
As a transcriptional repressor in the Notch signaling pathway, CBF-1 and its cofactors CIR and mSIN3A can also bind to the NF-κB binding sites in the viral LTR and recruit HDACs 16,111. In a latency model based on primary CD4+ T cells, CBF-1, CIR, and mSin3A, together with HDAC1 and other markers of restrictive chromatin, were all detected on the proviral chromatin before induction 16. The induction with the α-CD3/CD28 antibodies substantially decreased the levels of CBF-1, CIR, mSIN3A, and HDAC-1, but increased the level of Pol II at the promoter. Further expanding the list of sequence-specific transcription factors that are capable of recruiting HDACs to the HIV LTR, both HDAC1 and HDAC2 can also be recruited by the corepressor COUP-TF interacting protein (CTIP2) through binding to the Sp1 elements in the LTR 108b,112. Finally, several other transcription factors such as AP-4, c-Myc and Sp1 can also recruit HDAC1 to the HIV-1 promoter 113 Taken together, these studies indicate the recruitment of HDACs to the viral promoter to repress transcription and establish latency as a common property shared by many transcription factors that interact with the enhancer or promoter-proximal region of the HIV LTR.
While histone deacetylation contributes to the repressive chromatin structure, acetylation of histones produces the opposite effect. As for HIV, histone acetylation at the viral promoter is associated with induced latency reactivation. Various stimuli can induce the accumulation of the NF-κB p65 subunit in the nucleus, which results in the formation of the p50/p65 heterodimer, recruitment of cellular HATs such as p300/CBP, PCAF, and GCN5 to the viral enhancer region, acetylation of chromatin, improved accessibility for Pol II, and eventually activation of HIV transcription initiation 114. The recruitment of HATs may in turn stabilize NF-κB on the LTR, as acetylation of p65 increases NF-κB’s DNA-binding affinity 115. In addition to NF-κB, several other transcription factors such as NFAT, GR, C/EBP, LEF-1, IRF, Ets-1, AP-1, c-Myb, and Sp1 can also recruit HATs onto the LTR to activate transcription initiation 114c,116. Finally, besides these cellular transcription factors, HIV Tat has also been reported to recruit p300 and PCAF to the LTR through binding to the TAR RNA, which may further contribute to its ability to activate the integrated provirus 114d.
The initiation of HIV transcription is also repressed by methylation of histone H3 such as di- or tri-methylation on Lys9 (H3K9me2 or H3K9me3) and tri-methylation on Lys27 (H3K27me3) 4c,112b,117. Histone lysine methyltransferase (HKMT) SUV39H1 is responsible for H3K9me3, which is frequently associated with heterochromatin formation and serves as a platform to recruit the chromodomain protein HP1γ 117α. Of note, SUV39H1 can also interact with CTIP2, which is able to recruit HDAC1 and HDAC2 to the HIV LTR as mentioned above. Thus, through enforcing two kinds of repressive histone modifications of the HIV chromatin, the SUV39H1-CTIP2 interaction cooperatively contributes to the establishment of a highly repressive chromatin structure 112b. While H3K9me3 is generated by SUV39H1, H3K9me2 is induced by another HKMT called G9A. Like H3K9me3, H3K9me2 also contributes to the maintenance of HIV latency as demonstrated by the treatment with BIX01924, a specific inhibitor targeting G9A, which caused the virus to exit latency 117b. Finally, the HKMT responsible for H3K27me3 is a nuclear protein called EZH2, which is a component of the Polycomb Repressive Complex 2 (PRC2) 118. Knockdown and specific inhibition of EZH2 show that it appears to play an even more important role in maintaining HIV latency than do SUV39H1 and G9A, and thus may serve as a central regulator of HIV epigenetic silencing 118.
Like the methylation of histone H3 mentioned above, DNA methylation is also a repressive mark on the HIV promoter and detected on cytosine residues in the two CpG islands flanking the HIV transcription start site (TSS) in latently infected Jurkat and primary CD4+ T cells 119. The methyl-CpG binding domain protein 2 (MBD2) binds to the second CpG island and recruits HDAC2 to help maintain the repressive chromatin structure during latency 119. Inhibition of cytosine methylation by 5-aza-2′-deoxycytidine (aza-CdR) has been shown to abrogate the recruitment of MBD2 and HDAC2. As such, aza-CdR can synergize with prostratin or TNF-α to reactivate latent HIV 119. Further supporting the role of CpG methylation in HIV latency, it has been found that in the latent reservoirs of HIV-infected individuals with non-detectable plasma viremia, the HIV promoter and enhancer are hypermethylated and resistant to reactivation, as opposed to the hypomethylated 5′ LTR in viremic patients 120. Finally, implicating a strong correlation between DNA methylation and histone methylation and deacetylation in maintaining HIV latency, a latently infected Jurkat cell line with densely methylated HIV promoter displays much higher level of H3K27me3 and is more efficiently reactivated by the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) than other cell lines containing latent proviruses with little or no 5′ LTR DNA methylation 120.
SWI/SNF is an ATP-dependent multi-subunit nucleosome-remodeling complex with two key subunits Brg-1 and Brm that possess ATP-dependent nucleosome remodeling activities. It can exist in two different forms: PBAF with the defining subunits Brd7, BAF180 and BAF200; and BAF with the defining subunit BAF250a (ARID1a). Both forms participate in HIV transcriptional control albeit with opposing functions: While BAF represses HIV transcription initiation by positioning Nuc-1 downstream of the TSS 121, PBAF enhances initiation by removing Nuc-1 96c,122. Suggesting that these two SWI/SNF complexes of opposing activities may function at different stages of the HIV life cycle, upon activation of HIV transcription, BAF dissociates from the LTR region, and PBAF is then recruited by the Lys50-acetylated Tat to the promoter to stimulate transcription (Fig. 6) 121,123. Besides inducing a more relaxed chromatin conformation, the acetylated histones at the HIV promoter, especially those induced by the Tat-recruited PCAF 114d, may also serve to stabilize PBAF complex on HIV promoter through binding to the bromodomain of PBAF’s component Brg-1 124.
14. Future perspectives
Over the past 15 years or so, remarkable advances have been made toward the elucidation of the mechanism and factors that regulate HIV transcriptional elongation. Among these, the identification of P-TEFb as a key host cellular cofactor for Tat transactivation, the isolation and in-depth analyses of several P-TEFb-containing complexes and their impact on HIV transcription, and the determination of crystal structures of Tat in complex with its key binding partners are some of the highlights that have provided unparalleled molecular insights into the intricate control of HIV transcription.
As expected, these advances have raised new questions even though some of the old ones still remain unanswered. For example, high-resolution structures of the various P-TEFb-containing complexes are yet to be determined to reveal how P-TEFb activity is regulated in these complexes and what triggers P-TEFb’s transfer from one complex to the other. It is also important to investigate whether the assembly, stability and function of these complexes are regulated by posttranslational modifications, of which only a small number have been identified and studied. Moreover, structural and functional analyses are yet to be performed to determine how HIV Tat and TAR interact with the complete SEC complex and whether these interactions present any useful therapeutic targets for developing novel antiviral drugs. Finally, critical epigenetic controls of the HIV chromatin and their precise roles in contributing to the establishment, maintenance and termination of viral latency remain to be further elucidated. Judging by the accelerated rate of discovery in this field, answers to some of these questions will undoubtedly be obtained within the next few years, which will provide exciting opportunities to design novel strategies to eradicate latent HIV reservoirs and achieve a real cure for HIV/AIDS.
Acknowledgments
We thank Drs. Ursula Schulze-Gahmen and Tom Alber of UC Berkeley for providing the structural model used in Fig. 5. This work is supported by grants (R01AI41757 and R01AI095057) from the National Institutes of Health to Q.Z. and grants from the National Natural Science Foundation of China (81201276) and Natural Science Foundation of Fujian (2012J05067) to Y.X.
Biographies

Huasong Lu received his B.S. degree in Bioinformatics in 2009 from Zhejiang University, China and M.S. degree in Marine Biology in 2012 from the Chinese Third Institute of Oceanography, where studied the invertebrate innate immune response to pathogen infection. After that, he worked as a Research Specialist in the Department of Molecular and Cell Biology at UC Berkeley for two years. Huasong is currently pursuing his Ph.D. degree in School of Pharmaceutical Sciences, Xiamen University, where his research focuses on the molecular mechanism utilized by the HIV-encoded Tat protein to stimulate viral transcriptional elongation.

Zichong Li obtained his B.S. degree from Laiyang Agricultural College in 2006 and M.S. degree from Xiamen University in 2010. Initially joining Dr. Qiang Zhou’s Lab as a visiting scholar in 2010, he is presently a graduate student in the Department of Molecular and Cell Biology at UC Berkeley. His research interest lies in the regulation of the SECs, and the transfer of elongation factors between different complexes. In 2012, he was among the first to identify the effect of a newly identified epigenetic drug, JQ1, on reactivation of HIV latency. His data show that the host transcription factor Brd4 is a previously overlooked inhibitory factor of the HIV-encoded Tat protein and that JQ1 antagonizes Brd4’s inhibitory action to reactivate HIV latency. This discovery may contribute to the design of “Shock-and-Kill” approaches to purge latent HIV reservoirs from infected patients.

Yuhua Xue obtained her BS degree from Langfang Normal University in 2004. She subsequently went to Xiamen University, China to pursue graduate study. Since 2007, she has first served as a visiting student and then a postdoctoral researcher at UC Berkeley, where she completed a project investigating how two P-TEFb-associated factors MePCE and LARP7 control the formation of the human 7SK snRNP, which plays a key role in regulating HIV gene expression. In 2011, she joined the School of Pharmaceutical Sciences at Xiamen University as an assistant professor, where she is studying the interaction between HIV and its human host cells at the molecular level and identifying small molecule drugs that can block this interaction.

Qiang Zhou obtained his BS degree from the University of Science and Technology of China in 1986. Supported by the prestigious CUSBEA fellowship, he then went to UCLA to pursue graduate study. He was among the first to purify and characterize the general transcription factor TFIID, a multi-subunit complex required for RNA polymerase II transcription initiation. Supported by a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research, he received postdoctoral training from 1993 to 1996 in Dr. Phillip Sharp’s laboratory at MIT, where he studied the mechanism by which the HIV-encoded Tat protein stimulates viral transcriptional elongation and identified and characterized a nuclear protein called Tat-SF1 as a key contributor to this process. In 1997, he joined the Department of Molecular and Cell Biology (MCB) at UC Berkeley, where he is presently a full professor. Dr. Zhou’s main research interest lies in the investigation of viral-host interactions at the molecular level, with a special emphasis on mechanisms and host factors that control HIV transcription and latency. His research has made important contributions to the elucidation of the mechanisms that control both cellular and HIV transcriptional elongation. His laboratory was the first to discover that most of P-TEFb, a key human transcription elongation factor and HIV Tat cofactor, is normally sequestered in a catalytically inactive state in the 7SK snRNP. He identified 7SK snRNA as well as HEXIM1/2 and LARP7 as key subunits of 7SK snRNP and determined their roles in inhibiting CDK9 kinase activity, maintaining 7SK snRNP integrity and suppressing cellular transformation. In addition, he was also the first to identify the bromodomain protein Brd4 as a key factor to recruit P-TEFb to many cellular promoters as well as the HIV LTR in the absence of Tat. Recently, his lab has uncovered a connection between HIV Tat and the Super Elongation Complexes (SECs) and shown that Tat recruits SECs to the viral LTR, where SEC subunits P-TEFb and ELL2 synergistically activate HIV transcription. This result has expanded the conventional view of the mechanism of Tat-transactivation.
References
- 1.Kao SY, Calman AF, Luciw PA, Peterlin BM. Nature. 1987;330:489. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 2.(a) Feng S, Holland EC. Nature. 1988;334:165. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]; (b) Selby MJ, Bain ES, Luciw PA, Peterlin BM. Genes Dev. 1989;3:547. doi: 10.1101/gad.3.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Debaisieux S, Rayne F, Yezid H, Beaumelle B. Traffic. 2012;13:355. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Donahue DA, Kuhl BD, Sloan RD, Wainberg MA. J Virol. 2012;86:3253. doi: 10.1128/JVI.06648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Burnett JC, Miller-Jensen K, Shah PS, Arkin AP, Schaffer DV. PLoS Pathog. 2009;5:e1000260. doi: 10.1371/journal.ppat.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pearson R, Kim YK, Hokello J, Lassen K, Friedman J, Tyagi M, Karn J. J Virol. 2008;82:12291. doi: 10.1128/JVI.01383-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Cell. 2005;122:169. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 6.(a) Frankel AD, Pabo CO. Cell. 1988;55:1189. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]; (b) Green M, Loewenstein PM. Cell. 1988;55:1179. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 7.(a) Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. Cell. 2007;130:77. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, Adelman K. Nat Genet. 2007;39:1507. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine M. Cell. 2011;145:502. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones KA, Peterlin BM. Annu Rev Biochem. 1994;63:717. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Delling U, Chen CH, Rosen CA, Sonenberg N. Genes Dev. 1990;4:1365. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- 11.(a) Mancebo HS, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D, Flores O. Genes Dev. 1997;11:2633. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. Cell. 1998;92:451. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]; (c) Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews MB, Price DH. Genes Dev. 1997;11:2622. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shore SM, Byers SA, Maury W, Price DH. Gene. 2003;307:175. doi: 10.1016/s0378-1119(03)00466-9. [DOI] [PubMed] [Google Scholar]
- 13.(a) Fu TJ, Peng J, Lee G, Price DH, Flores O. J Biol Chem. 1999;274:34527. doi: 10.1074/jbc.274.49.34527. [DOI] [PubMed] [Google Scholar]; (b) Peng J, Zhu Y, Milton JT, Price DH. Genes Dev. 1998;12:755. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov D, Kwak YT, Nee E, Guo J, Garcia-Martinez LF, Gaynor RB. J Mol Biol. 1999;288:41. doi: 10.1006/jmbi.1999.2663. [DOI] [PubMed] [Google Scholar]
- 15.Rice AP, Herrmann CH. Curr HIV Res. 2003;1:395. doi: 10.2174/1570162033485159. [DOI] [PubMed] [Google Scholar]
- 16.Tyagi M, Pearson RJ, Karn J. J Virol. 2010;84:6425. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung TL, Rice AP. PLoS Pathog. 2009;5:e1000263. doi: 10.1371/journal.ppat.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Li T, Price DH. Annu Rev Biochem. 2012;81:119. doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Marciniak RA, Sharp PA. Embo J. 1991;10:4189. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, Peterlin BM, Price DH. J Biol Chem. 2000;275:28345. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 20.Garber ME, Wei P, KewalRamani VN, Mayall TP, Herrmann CH, Rice AP, Littman DR, Jones KA. Genes Dev. 1998;12:3512. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. Proc Natl Acad Sci U S A. 1999;96:7791. doi: 10.1073/pnas.96.14.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Das C, Edgcomb SP, Peteranderl R, Chen L, Frankel AD. Virology. 2004;318:306. doi: 10.1016/j.virol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Fujinaga K, Taube R, Wimmer J, Cujec TP, Peterlin BM. Proc Natl Acad Sci U S A. 1999;96:1285. doi: 10.1073/pnas.96.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciniak RA, Garcia-Blanco MA, Sharp PA. Proc Natl Acad Sci U S A. 1990;87:3624. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Herrmann CH, Rice AP. Virology. 1993;197:601. doi: 10.1006/viro.1993.1634. [DOI] [PubMed] [Google Scholar]; (b) Herrmann CH, Rice AP. J Virol. 1995;69:1612. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West MJ, Karn J. Embo J. 1999;18:1378. doi: 10.1093/emboj/18.5.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(a) Bres V, Yoh SM, Jones KA. Curr Opin Cell Biol. 2008;20:334. doi: 10.1016/j.ceb.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He N, Zhou Q. J Neuroimmune Pharmacol. 2011;6:260. doi: 10.1007/s11481-011-9267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhou Q, Yik JH. Microbiol Mol Biol Rev. 2006;70:646. doi: 10.1128/MMBR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr CJ, Roberts JM. Genes Dev. 1999;13:1501. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 28.(a) Yang Z, Zhu Q, Luo K, Zhou Q. Nature. 2001;414:317. doi: 10.1038/35104575. [DOI] [PubMed] [Google Scholar]; (b) Nguyen VT, Kiss T, Michels AA, Bensaude O. Nature. 2001;414:322. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 29.(a) Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. Mol Cell. 2003;12:971. doi: 10.1016/s1097-2765(03)00388-5. [DOI] [PubMed] [Google Scholar]; (b) Michels AA, Nguyen VT, Fraldi A, Labas V, Edwards M, Bonnet F, Lania L, Bensaude O. Mol Cell Biol. 2003;23:4859. doi: 10.1128/MCB.23.14.4859-4869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Yik JH, Chen R, Pezda AC, Samford CS, Zhou Q. Mol Cell Biol. 2004;24:5094. doi: 10.1128/MCB.24.12.5094-5105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Muniz L, Egloff S, Ughy B, Jady BE, Kiss T. PLoS Pathog. 2010;6:e1001152. doi: 10.1371/journal.ppat.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(a) Yik JH, Chen R, Pezda AC, Zhou Q. J Biol Chem. 2005;280:16368. doi: 10.1074/jbc.M500912200. [DOI] [PubMed] [Google Scholar]; (b) Byers SA, Price JP, Cooper JJ, Li Q, Price DH. J Biol Chem. 2005;280:16360. doi: 10.1074/jbc.M500424200. [DOI] [PubMed] [Google Scholar]; (c) Dulac C, Michels AA, Fraldi A, Bonnet F, Nguyen VT, Napolitano G, Lania L, Bensaude O. J Biol Chem. 2005;280:30619. doi: 10.1074/jbc.M502471200. [DOI] [PubMed] [Google Scholar]
- 32.(a) He N, Jahchan NS, Hong E, Li Q, Bayfield MA, Maraia RJ, Luo K, Zhou Q. Mol Cell. 2008;29:588. doi: 10.1016/j.molcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xue Y, Yang Z, Chen R, Zhou Q. Nucleic Acids Res. 2010;38:360. doi: 10.1093/nar/gkp977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayfield MA, Yang R, Maraia RJ. Biochim Biophys Acta. 2010;1799:365. doi: 10.1016/j.bbagrm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(a) Biewenga P, Buist MR, Moerland PD, Ver Loren van Themaat E, van Kampen AH, ten Kate FJ, Baas F. Gynecol Oncol. 2008;108:520. doi: 10.1016/j.ygyno.2007.11.024. [DOI] [PubMed] [Google Scholar]; (b) Krueger BJ, Jeronimo C, Roy BB, Bouchard A, Barrandon C, Byers SA, Searcey CE, Cooper JJ, Bensaude O, Cohen EA, Coulombe B, Price DH. Nucleic Acids Res. 2008;36:2219. doi: 10.1093/nar/gkn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, Therien C, Bergeron D, Bourassa S, Greenblatt J, Chabot B, Poirier GG, Hughes TR, Blanchette M, Price DH, Coulombe B. Mol Cell. 2007;27:262. doi: 10.1016/j.molcel.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muniz L, Egloff S, Kiss T. Nucleic Acids Res. 2013;41:4686. doi: 10.1093/nar/gkt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YK, Mbonye U, Hokello J, Karn J. J Mol Biol. 2011;410:896. doi: 10.1016/j.jmb.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(a) Chen R, Liu M, Li H, Xue Y, Ramey WN, He N, Ai N, Luo H, Zhu Y, Zhou N, Zhou Q. Genes Dev. 2008;22:1356. doi: 10.1101/gad.1636008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen R, Yang Z, Zhou Q. J Biol Chem. 2004;279:4153. doi: 10.1074/jbc.M310044200. [DOI] [PubMed] [Google Scholar]
- 39.He N, Pezda AC, Zhou Q. Mol Cell Biol. 2006;26:7068. doi: 10.1128/MCB.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.(a) Biglione S, Byers SA, Price JP, Nguyen VT, Bensaude O, Price DH, Maury W. Retrovirology. 2007;4:47. doi: 10.1186/1742-4690-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Amente S, Gargano B, Napolitano G, Lania L, Majello B. Cell Cycle. 2009;8:1249. doi: 10.4161/cc.8.8.8286. [DOI] [PubMed] [Google Scholar]
- 41.(a) Casse C, Giannoni F, Nguyen VT, Dubois MF, Bensaude O. J Biol Chem. 1999;274:16097. doi: 10.1074/jbc.274.23.16097. [DOI] [PubMed] [Google Scholar]; (b) Valerie K, Delers A, Bruck C, Thiriart C, Rosenberg H, Debouck C, Rosenberg M. Nature. 1988;333:78. doi: 10.1038/333078a0. [DOI] [PubMed] [Google Scholar]
- 42.(a) Fujinaga K, Barboric M, Li Q, Luo Z, Price DH, Peterlin BM. Nucleic Acids Res. 2012;40:9160. doi: 10.1093/nar/gks682. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Natarajan M, August A, Henderson AJ. J Biol Chem. 2010;285:17338. doi: 10.1074/jbc.M109.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. Nat Med. 2002;8:1310. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 44.(a) Wang Y, Dow EC, Liang YY, Ramakrishnan R, Liu H, Sung TL, Lin X, Rice AP. J Biol Chem. 2008;283:33578. doi: 10.1074/jbc.M807495200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Budhiraja S, Ramakrishnan R, Rice AP. Retrovirology. 2012;9:52. doi: 10.1186/1742-4690-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Contreras X, Barboric M, Lenasi T, Peterlin BM. PLoS Pathog. 2007;3:1459. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho S, Schroeder S, Kaehlcke K, Kwon HS, Pedal A, Herker E, Schnoelzer M, Ott M. Embo J. 2009;28:1407. doi: 10.1038/emboj.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karn J. J Mol Biol. 1999;293:235. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- 48.(a) Schulte A, Czudnochowski N, Barboric M, Schonichen A, Blazek D, Peterlin BM, Geyer M. J Biol Chem. 2005;280:24968. doi: 10.1074/jbc.M501431200. [DOI] [PubMed] [Google Scholar]; (b) Barboric M, Yik JH, Czudnochowski N, Yang Z, Chen R, Contreras X, Geyer M, Matija Peterlin B, Zhou Q. Nucleic Acids Res. 2007;35:2003. doi: 10.1093/nar/gkm063. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sedore SC, Byers SA, Biglione S, Price JP, Maury WJ, Price DH. Nucleic Acids Res. 2007;35:4347. doi: 10.1093/nar/gkm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu S, Urano E, Futahashi Y, Miyauchi K, Isogai M, Matsuda Z, Nohtomi K, Onogi T, Takebe Y, Yamamoto N, Komano J. Aids. 2007;21:575. doi: 10.1097/QAD.0b013e32801424a5. [DOI] [PubMed] [Google Scholar]
- 50.Dames SA, Schonichen A, Schulte A, Barboric M, Peterlin BM, Grzesiek S, Geyer M. Proc Natl Acad Sci U S A. 2007;104:14312. doi: 10.1073/pnas.0701848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krueger BJ, Varzavand K, Cooper JJ, Price DH. PLoS One. 2010;5:e12335. doi: 10.1371/journal.pone.0012335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobhian B, Laguette N, Yatim A, Nakamura M, Levy Y, Kiernan R, Benkirane M. Mol Cell. 2010;38:439. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qintong Li JPP, Byers Sarah A, Cheng Dongmei, Peng Junmin, Price David H. The Journal of Biological Chemistry. 2005;280:28819. doi: 10.1074/jbc.M502712200. [DOI] [PubMed] [Google Scholar]
- 54.(a) Barboric M, Lenasi T. Nat Struct Mol Biol. 2010;17:928. doi: 10.1038/nsmb0810-928. [DOI] [PubMed] [Google Scholar]; (b) D’Orso I, Frankel AD. Nat Struct Mol Biol. 2010;17:815. doi: 10.1038/nsmb.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tahirov TH, Babayeva ND, Varzavand K, Cooper JJ, Sedore SC, Price DH. Nature. 2010;465:747. doi: 10.1038/nature09131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ammosova T, Jerebtsova M, Beullens M, Lesage B, Jackson A, Kashanchi F, Southerland W, Gordeuk VR, Bollen M, Nekhai S. J Biol Chem. 2005;280:36364. doi: 10.1074/jbc.M503673200. [DOI] [PubMed] [Google Scholar]
- 57.Ammosova T, Yedavalli VR, Niu X, Jerebtsova M, Van Eynde A, Beullens M, Bollen M, Jeang KT, Nekhai S. J Biol Chem. 2011;286:3798. doi: 10.1074/jbc.M110.196493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. Mol Cell. 2010;38:428. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shilatifard A, Lane WS, Jackson KW, Conaway RC, Conaway JW. Science. 1996;271:1873. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 60.Debabrata Biswas TAM, Basrur Venkatesha, Kim Jaehoon, Elenitoba-Johnson Kojo SJ, David Allis C, Roeder Robert G. PNAS. 2011;108:15751. doi: 10.1073/pnas.1111498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chou S, Upton H, Bao K, Schulze-Gahmen U, Samelson AJ, He N, Nowak A, Lu H, Krogan NJ, Zhou Q, Alber T. Proc Natl Acad Sci U S A. 2013;110:E123. doi: 10.1073/pnas.1216971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.(a) Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. Mol Cell. 2010;37:429. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. Cancer Cell. 2010;17:198. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Slany RK. Haematologica. 2009;94:984. doi: 10.3324/haematol.2008.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu M, Hsu J, Chan C, Li Z, Zhou Q. Mol Cell. 2012;46:325. doi: 10.1016/j.molcel.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Kruger E. FEBS Lett. 2003;553:200. doi: 10.1016/s0014-5793(03)01025-1. [DOI] [PubMed] [Google Scholar]
- 65.(a) Adelman K, Lis JT. Nat Rev Genet. 2012;13:720. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hargreaves DC, Horng T, Medzhitov R. Cell. 2009;138:129. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.(a) Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Mol Cell. 2005;19:535. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]; (b) Wu SY, Chiang CM. J Biol Chem. 2007;282:13141. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]; (c) Vollmuth F, Blankenfeldt W, Geyer M. J Biol Chem. 2009;284:36547. doi: 10.1074/jbc.M109.033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.(a) Dey A, Ellenberg J, Farina A, Coleman AE, Maruyama T, Sciortino S, Lippincott-Schwartz J, Ozato K. Mol Cell Biol. 2000;20:6537. doi: 10.1128/mcb.20.17.6537-6549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jeanmougin F, Wurtz JM, Le Douarin B, Chambon P, Losson R. Trends Biochem Sci. 1997;22:151. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- 68.Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Proc Natl Acad Sci U S A. 2007;104:13690. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.(a) Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, Howley PM. Molecular and cellular biology. 31:2641. doi: 10.1128/MCB.01341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin YJ, Umehara T, Inoue M, Saito K, Kigawa T, Jang MK, Ozato K, Yokoyama S, Padmanabhan B, Guntert P. Protein Sci. 2008;17:2174. doi: 10.1110/ps.037580.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.(a) Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. Proc Natl Acad Sci U S A. 2003;100:8758. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Z, He N, Zhou Q. Mol Cell Biol. 2008;28:967. doi: 10.1128/MCB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loyola A, Almouzni G. Trends Cell Biol. 2004;14:279. doi: 10.1016/j.tcb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. Mol Cell. 2005;19:523. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 73.(a) Li Z, Guo J, Wu Y, Zhou Q. Nucleic Acids Res. 2013;41:277. doi: 10.1093/nar/gks976. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ott M, Geyer M, Zhou Q. Cell Host Microbe. 2011;10:426. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.(a) Joos B, Fischer M, Kuster H, Pillai SK, Wong JK, Boni J, Hirschel B, Weber R, Trkola A, Gunthard HF. Proc Natl Acad Sci U S A. 2008;105:16725. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Nat Med. 1999;5:512. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 75.(a) Karn J. Curr Opin HIV AIDS. 2011;6:4. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. Science. 2009;323:1304. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 76.Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M. J Leukoc Biol. 2012;92:1147. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu J, Gaiha GD, John SP, Pertel T, Chin CR, Gao G, Qu H, Walker BD, Elledge SJ, Brass AL. Cell Rep. 2012;2:807. doi: 10.1016/j.celrep.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mbonye UR, Gokulrangan G, Datt M, Dobrowolski C, Cooper M, Chance MR, Karn J. PLoS Pathogens. 2013;9:e1003338. doi: 10.1371/journal.ppat.1003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. J Biol Chem. 2012;287:36609. doi: 10.1074/jbc.M112.410746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.(a) Chiang K, Rice AP. Viruses. 2012;4:1390. doi: 10.3390/v4091390. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ghose R, Liou LY, Herrmann CH, Rice AP. J Virol. 2001;75:11336. doi: 10.1128/JVI.75.23.11336-11343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.(a) Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS. Cell. 2011;146:904. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., 3rd Proc Natl Acad Sci U S A. 2011;108:16669. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. Nature. 2011;478:524. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baumli S, Lolli G, Lowe ED, Troiani S, Rusconi L, Bullock AN, Debreczeni JE, Knapp S, Johnson LN. Embo J. 2008;27:1907. doi: 10.1038/emboj.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janin J. Nat Struct Biol. 1997;4:973. doi: 10.1038/nsb1297-973. [DOI] [PubMed] [Google Scholar]
- 84.Zhou M, Halanski MA, Radonovich MF, Kashanchi F, Peng J, Price DH, Brady JN. Mol Cell Biol. 2000;20:5077. doi: 10.1128/mcb.20.14.5077-5086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell GR, Loret EP. Retrovirology. 2009;6:50. doi: 10.1186/1742-4690-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.(a) Dunker AK, Silman I, Uversky VN, Sussman JL. Curr Opin Struct Biol. 2008;18:756. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]; (b) Nobeli I, Favia AD, Thornton JM. Nat Biotechnol. 2009;27:157. doi: 10.1038/nbt1519. [DOI] [PubMed] [Google Scholar]
- 87.Ursula Schulze-Gahmen HU, Birnberg Andrew, Bao Katherine, Chou Seemay, Krogan Nevan, Zhou Qiang, Alber Tom. eLife. 2013:00327R1. doi: 10.7554/eLife.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Orso I, Frankel AD. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3101. doi: 10.1073/pnas.0900012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, Benkirane M, Van Lint C. Embo J. 1999;18:6106. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anand K, Schulte A, Vogel-Bachmayr K, Scheffzek K, Geyer M. Nat Struct Mol Biol. 2008;15:1287. doi: 10.1038/nsmb.1513. [DOI] [PubMed] [Google Scholar]
- 91.Pagans S, Kauder SE, Kaehlcke K, Sakane N, Schroeder S, Dormeyer W, Trievel RC, Verdin E, Schnolzer M, Ott M. Cell Host Microbe. 2010;7:234. doi: 10.1016/j.chom.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakane N, Kwon HS, Pagans S, Kaehlcke K, Mizusawa Y, Kamada M, Lassen KG, Chan J, Greene WC, Schnoelzer M, Ott M. PLoS Pathog. 2011;7:e1002184. doi: 10.1371/journal.ppat.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cujec TP, Cho H, Maldonado E, Meyer J, Reinberg D, Peterlin BM. Mol Cell Biol. 1997;17:1817. doi: 10.1128/mcb.17.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.(a) Col E, Caron C, Seigneurin-Berny D, Gracia J, Favier A, Khochbin S. J Biol Chem. 2001;276:28179. doi: 10.1074/jbc.M101385200. [DOI] [PubMed] [Google Scholar]; (b) Ott M, Schnolzer M, Garnica J, Fischle W, Emiliani S, Rackwitz HR, Verdin E. Curr Biol. 1999;9:1489. doi: 10.1016/s0960-9822(00)80120-7. [DOI] [PubMed] [Google Scholar]
- 95.Kaehlcke K, Dorr A, Hetzer-Egger C, Kiermer V, Henklein P, Schnoelzer M, Loret E, Cole PA, Verdin E, Ott M. Mol Cell. 2003;12:167. doi: 10.1016/s1097-2765(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 96.(a) Bres V, Tagami H, Peloponese JM, Loret E, Jeang KT, Nakatani Y, Emiliani S, Benkirane M, Kiernan RE. Embo J. 2002;21:6811. doi: 10.1093/emboj/cdf669. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dorr A, Kiermer V, Pedal A, Rackwitz HR, Henklein P, Schubert U, Zhou MM, Verdin E, Ott M. Embo J. 2002;21:2715. doi: 10.1093/emboj/21.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mahmoudi T, Parra M, Vries RG, Kauder SE, Verrijzer CP, Ott M, Verdin E. J Biol Chem. 2006;281:19960. doi: 10.1074/jbc.M603336200. [DOI] [PubMed] [Google Scholar]
- 97.Weinberger LS, Dar RD, Simpson ML. Nat Genet. 2008;40:466. doi: 10.1038/ng.116. [DOI] [PubMed] [Google Scholar]
- 98.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. Nature. 2002;417:455. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 99.Huo L, Li D, Sun X, Shi X, Karna P, Yang W, Liu M, Qiao W, Aneja R, Zhou J. J Biol Chem. 2011;286:9280. doi: 10.1074/jbc.M110.208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.(a) Chen D, Wang M, Zhou S, Zhou Q. Embo J. 2002;21:6801. doi: 10.1093/emboj/cdf683. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huo L, Li D, Sun L, Liu M, Shi X, Sun X, Li J, Dong B, Dong X, Zhou J. J Pathol. 2011;223:28. doi: 10.1002/path.2768. [DOI] [PubMed] [Google Scholar]
- 101.Van Duyne R, Easley R, Wu W, Berro R, Pedati C, Klase Z, Kehn-Hall K, Flynn EK, Symer DE, Kashanchi F. Retrovirology. 2008;5:40. doi: 10.1186/1742-4690-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie B, Invernizzi CF, Richard S, Wainberg MA. J Virol. 2007;81:4226. doi: 10.1128/JVI.01888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sivakumaran H, van der Horst A, Fulcher AJ, Apolloni A, Lin MH, Jans DA, Harrich D. J Virol. 2009;83:11694. doi: 10.1128/JVI.00499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.(a) Endo-Munoz L, Warby T, Harrich D, McMillan NA. Virol J. 2005;2:17. doi: 10.1186/1743-422X-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ammosova T, Berro R, Jerebtsova M, Jackson A, Charles S, Klase Z, Southerland W, Gordeuk VR, Kashanchi F, Nekhai S. Retrovirology. 2006;3:78. doi: 10.1186/1742-4690-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bres V, Kiernan RE, Linares LK, Chable-Bessia C, Plechakova O, Treand C, Emiliani S, Peloponese JM, Jeang KT, Coux O, Scheffner M, Benkirane M. Nat Cell Biol. 2003;5:754. doi: 10.1038/ncb1023. [DOI] [PubMed] [Google Scholar]
- 106.Lassot I, Latreille D, Rousset E, Sourisseau M, Linares LK, Chable-Bessia C, Coux O, Benkirane M, Kiernan RE. Mol Cell. 2007;25:369. doi: 10.1016/j.molcel.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 107.(a) Verdin E, Paras P, Jr, Van Lint C. Embo J. 1993;12:3249. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Van Lint C, Emiliani S, Ott M, Verdin E. Embo J. 1996;15:1112. [PMC free article] [PubMed] [Google Scholar]
- 108.(a) Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, Davie JR, Shi Y, Hansen U, Margolis DM. J Virol. 2000;74:6790. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keedy KS, Archin NM, Gates AT, Espeseth A, Hazuda DJ, Margolis DM. J Virol. 2009;83:4749. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He G, Margolis DM. Mol Cell Biol. 2002;22:2965. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Margolis DM, Somasundaran M, Green MR. J Virol. 1994;68:905. doi: 10.1128/jvi.68.2.905-910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. Embo J. 2006;25:139. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.(a) Oakes JW, Bagasra O, Duan L, Pomerantz RJ. AIDS Res Hum Retroviruses. 1994;10:1213. doi: 10.1089/aid.1994.10.1213. [DOI] [PubMed] [Google Scholar]; (b) Thieblemont N, Haeffner-Cavaillon N, Haeffner A, Cholley B, Weiss L, Kazatchkine MD. J Immunol. 1995;155:4861. [PubMed] [Google Scholar]
- 111.Tyagi M, Karn J. Embo J. 2007;26:4985. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.(a) Marban C, Redel L, Suzanne S, Van Lint C, Lecestre D, Chasserot-Golaz S, Leid M, Aunis D, Schaeffer E, Rohr O. Nucleic Acids Res. 2005;33:2318. doi: 10.1093/nar/gki529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; (b) Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, Aunis D, Rohr O. Embo J. 2007;26:412. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.(a) Jiang G, Espeseth A, Hazuda DJ, Margolis DM. J Virol. 2007;81:10914. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Imai K, Okamoto T. J Biol Chem. 2006;281:12495. doi: 10.1074/jbc.M511773200. [DOI] [PubMed] [Google Scholar]
- 114.(a) Williams SA, Kwon H, Chen LF, Greene WC. J Virol. 2007;81:6043. doi: 10.1128/JVI.02074-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hottiger MO, Felzien LK, Nabel GJ. Embo J. 1998;17:3124. doi: 10.1093/emboj/17.11.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lusic M, Marcello A, Cereseto A, Giacca M. Embo J. 2003;22:6550. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Benkirane M, Chun RF, Xiao H, Ogryzko VV, Howard BH, Nakatani Y, Jeang KT. J Biol Chem. 1998;273:24898. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]; (e) Marzio G, Tyagi M, Gutierrez MI, Giacca M. Proc Natl Acad Sci U S A. 1998;95:13519. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen L, Fischle W, Verdin E, Greene WC. Science. 2001;293:1653. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 116.(a) Garcia-Rodriguez C, Rao A. J Exp Med. 1998;187:2031. doi: 10.1084/jem.187.12.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Marsili G, Remoli AL, Sgarbanti M, Battistini A. Ann N Y Acad Sci. 2004;1030:636. doi: 10.1196/annals.1329.074. [DOI] [PubMed] [Google Scholar]; (c) Colin L, Van Lint C. Retrovirology. 2009;6:111. doi: 10.1186/1742-4690-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.(a) du Chene I, Basyuk E, Lin YL, Triboulet R, Knezevich A, Chable-Bessia C, Mettling C, Baillat V, Reynes J, Corbeau P, Bertrand E, Marcello A, Emiliani S, Kiernan R, Benkirane M. Embo J. 2007;26:424. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Imai K, Togami H, Okamoto T. J Biol Chem. 2010;285:16538. doi: 10.1074/jbc.M110.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kouzarides T. Curr Opin Genet Dev. 2002;12:198. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 118.Friedman J, Cho WK, Chu CK, Keedy KS, Archin NM, Margolis DM, Karn J. J Virol. 2011;85:9078. doi: 10.1128/JVI.00836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, Hirsch I. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rafati H, Parra M, Hakre S, Moshkin Y, Verdin E, Mahmoudi T. PLoS Biol. 2011;9:e1001206. doi: 10.1371/journal.pbio.1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.(a) Agbottah E, Deng L, Dannenberg LO, Pumfery A, Kashanchi F. Retrovirology. 2006;3:48. doi: 10.1186/1742-4690-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Treand C, du Chene I, Bres V, Kiernan R, Benarous R, Benkirane M, Emiliani S. Embo J. 2006;25:1690. doi: 10.1038/sj.emboj.7601074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Easley R, Carpio L, Dannenberg L, Choi S, Alani D, Van Duyne R, Guendel I, Klase Z, Agbottah E, Kehn-Hall K, Kashanchi F. Virology. 2010;405:322. doi: 10.1016/j.virol.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.(a) Hassan AH, Prochasson P, Neely KE, Galasinski SC, Chandy M, Carrozza MJ, Workman JL. Cell. 2002;111:369. doi: 10.1016/s0092-8674(02)01005-x. [DOI] [PubMed] [Google Scholar]; (b) Agalioti T, Chen G, Thanos D. Cell. 2002;111:381. doi: 10.1016/s0092-8674(02)01077-2. [DOI] [PubMed] [Google Scholar]