Abstract
Effective family planning with modern contraception is an important intervention to prevent unintended pregnancies which also provides personal, familial, and societal benefits. Contraception is also the most cost-effective strategy to reduce the burden of mother-to-child HIV transmission for women living with HIV who wish to prevent pregnancy. There are concerns, however, that certain contraceptive methods, in particular the injectable contraceptive depot medroxyprogesterone acetate (DMPA), may increase a woman's risk of acquiring HIV or transmitting it to uninfected males. These concerns, if confirmed, could potentially have large public health implications. This paper briefly reviews the literature on use of contraception among women living with HIV or at high risk of HIV infection. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommendations place no restrictions on the use of hormonal contraceptive methods by women with or at high risk of HIV infection, although a clarification recommends that, given uncertainty in the current literature, women at high risk of HIV who choose progestogen-only injectable contraceptives should be informed that it may or may not increase their risk of HIV acquisition and should also be informed about and have access to HIV preventive measures, including male or female condoms.
Keywords: HIV, AIDS, Contraception, Hormonal contraception, Transmission
Globally, an estimated 41 % of pregnancies are unintended, with even higher rates in South America (64 %), Southern Africa (59%), and North America (48%) [1]. Increased use of contraceptive services to reduce unintended pregnancies is a cost-effective strategy with great personal, familial, and societal benefits, including reduced maternal and child mortality and better educational and economic gender equity [2, 3]. Contraception is also the most cost-effective strategy to reduce the burden of mother-to-child HIV transmission among women living with HIV who wish to prevent unintended pregnancy [4]. Unfortunately, some countries with the highest rates of HIV prevalence also have low rates of contraceptive use and high rates of unintended pregnancy. The World Health Organization (WHO), United Nations, and other global public health organizations recognize that preventions of new HIV infections and unintended pregnancies are both critical to improving health, preventing mother-to-child transmission, and reducing infant and maternal morbidity and mortality [5–7, 8•]. It is therefore a public health priority to improve contraceptive access and use for women living with and at high risk of HIV infection.
All contraceptive methods are not equally effective for pregnancy prevention, and no contraceptive method other than male and female condoms can prevent sexual transmission of HIV. Furthermore, the safety of contraceptive methods may depend on the presence of medical comorbidities, including HIV infection, or interactions with medications such as antiretrovirals (ARVs). Recent systematic reviews have synthesized emerging evidence on whether hormonal contraception affects the risk of HIV disease acquisition, disease progression, or female-to-male transmission [9, 10, 11•, 12•]. This article reviews the literature and potential biologic mechanisms by which hormonal contraception might affect HIV acquisition or female-to-male transmission risk.
Contraceptive Efficacy of Various Methods
While condoms are the only contraceptive method that can reduce the risk of sexually transmitted infections (STIs) including HIV, they have a high failure rate for pregnancy prevention due to poor adherence, with a typical-use rate of 18–21 % within the first year of use [13]. Furthermore, despite three decades of promotion of condoms for HIV prevention, consistent and correct use is reported to occur infrequently among high-risk individuals in settings where the virus is endemic [14].
Hormonal contraceptive methods have higher efficacy for pregnancy prevention than condoms, with a typical-use contraceptive failure rate of approximately 9 % for combined hormonal contraceptives (oral contraceptive pills, patch, or ring), 6% for injectable methods [depot medroxyprogesterone acetate (DMPA) or norethisterone enanthate (NET-EN)], and less than 1 % for contraceptive implant and intrauterine devices (IUDs) [13]. Nonhormonal contraceptives such as the diaphragm, cervical cap, and spermicides have lower contraceptive efficacy (12–28 % failure rate with typical use) [13]. Diaphragms have no proven benefit of reduced HIV infection or STI transmission risk [15], and some forms of spermicide, such as nonoxynol-9, have been associated with an increased risk of sexual HIV acquisition when applied frequently [16, 17]. Fertility awareness methods, such as the symptothermal method, can be highly efficacious when used perfectly [18], but typical-use failure rates for some forms of fertility awareness methods are as high as 24 % [19]. Surgical sterilization, with bilateral tubal ligation for women or vasectomy for men, is an extremely effective contraceptive method with a typical-use failure rate less than 1 %; however, access to these procedures may be limited in low-resource settings and lack of reversibility makes sterilization not ideal for pregnancy spacing [13].
Hormonal contraceptives have high acceptance rates in many communities with infrequent condom use or limited nonhormonal contraceptive options, such as the copper IUD [20]. Injectable contraception alone accounts for nearly half (47 %) of modern contraceptive use in sub-Saharan Africa [20].
Hormonal Contraceptive Methods and HIV Acquisition Risk
Despite excellent contraceptive efficacy and high global uptake, there are growing concerns that hormonal contraception, specifically progestogen-only injectables (such as DMPA or NET-EN), may increase the risk of HIV acquisition in uninfected women or, when used by women living with HIV, the risk of female-to-male HIV transmission [10, 11•, 12•, 21•, 22]. If confirmed, these risks would have important implications for health at the individual and population level.
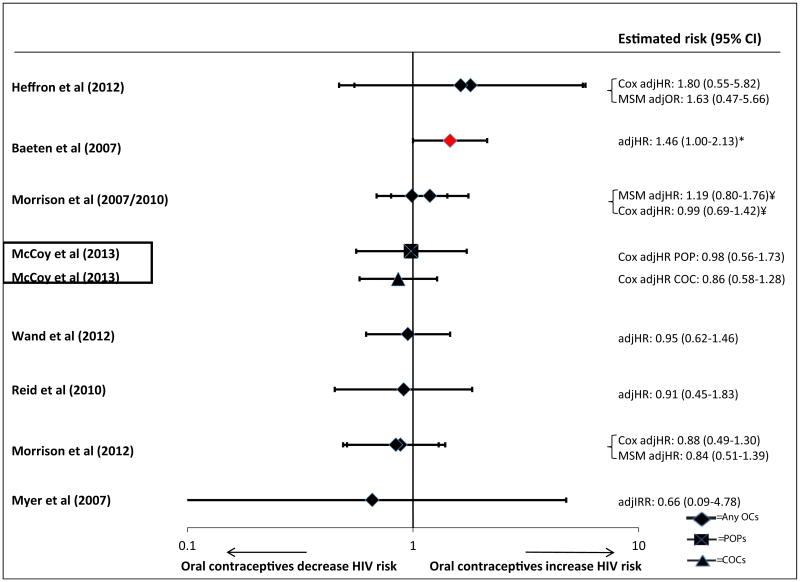
Research in nonhuman primates demonstrated several-fold increases in the risk of simian immunodeficiency virus (SIV) acquisition in rhesus macaques following exposure to large doses of DMPA [23]. The potential HIV acquisition risk associated with injectable contraception in humans has not been consistently observed across all studies, with some studies reporting no association with DMPA [24–27] and others reporting an increased risk of HIV acquisition [28–31]. Discrepancies in study design, population, sample size, contraceptive methods studied, dose, contraceptive adherence, analytic methods used, and rate of method discontinuation add to the complexity of interpreting results across these observational studies and limit the conclusions that can be drawn [32]. A recent systematic review [12•] of prospective studies noted that four of nine studies considered informative but with important limitations reported a 1.5 to 2.2 times increased risk of HIV acquisition in women using injectable contraception (DMPA or nonspecified injectable contraceptive) [33–36]. Notably, one of these studies [35] that suggested a statistically significant increase in risk using a marginal structural model found no statistically significant association using a Cox proportional hazards model [28]. The other five studies of injectable contraception that were informative but with important limitations reported no statistically significant association [25, 29, 37–39]. Of eight studies [21•, 25, 28, 29, 33–35, 37, 39] considered informative but with important limitations [12•] which evaluated oral contraceptive pills (OCPs), one found an increased risk of HIV acquisition [34]. Figures 1 and 2 present results of epidemiologic studies presented in the systematic review to evaluate the impact of OCPs and injectables on a risk of HIV acquisition in women [12•]. A recent, unpublished meta-analysis examining individual participant data from 18 studies including 37,124 women noted a significantly increased risk of acquisition [adjusted hazard ratio (aHR) 1.5; 95 % confidence interval (CI) 1.24–1.83] [40] for DMPA, with no increased risk from NET-EN or combined hormonal contraceptives. However, when the analysis was restricted to studies with lower risk of bias, the risk associated with DMPA was reduced and no longer statistically significant (adjusted HR 1.22; 95 % CI 0.99–1.50) [40]. A similar conclusion was reported from a recent analysis among 1,393 discordant couples in Zambia, which found that the use of injectable (aHR=1.2; 95 % CI 0.8–1.8), OCP (aHR=1.3; 95 % CI 0.9–1.9), or implant (aHR=0.9; 95 % CI 0.4–2.0) was not associated with HIV acquisition relative to nonhormonal contraceptive users after controlling for woman's age, literacy, sperm on a vaginal swab wet prep, genital ulceration/ inflammation, and time interval post-enrollment [41].
Fig. 1.
Use of injectable contraceptives and HIV acquisition (nine studies considered informative but with important limitations), reproduced with permission from Polis et al. [12•]. Error bars show 95 % CIs. Studies are arranged in order of decreasing magnitude of risk estimate, except if a single study disaggregated DMPA and NET-EN, in which case both estimates are adjacent (as indicated by a box around the study identifiers). For studies in which both Cox proportional hazards (Cox) and marginal structural model (MSM) analyses were reported, both are displayed on a single line (also identified by brackets), except for one study in which both Cox and MSM estimates for both DMPA and NET-EN separately were unavailable [61, 82]. OR odds ratio, IRR incidence risk ratio, HR hazard ratio. Asterisk analysis showing significant findings at p=0.05 (marker also displayed in red). Dagger estimate for Cox model taken from slightly updated analysis which controlled for a total number of unprotected sex acts. Section sign unpublished estimates disaggregated by injectable type; only disaggregated Cox estimates provided, in McCoy et al. [39], disaggregated MSM estimates not possible due to violation of the positivity assumption. Yen sign different statistical models adjusted for slightly different confounders
Fig. 2.
Use of oral contraceptives and HIV acquisition (eight studies considered informative but with important limitations) reproduced with permission from Polis et al. [12•]. Error bars show 95% CIs. Studies are arranged in order of decreasing magnitude of risk estimate, except if a single study disaggregated progestin-only pills (POPs) and combined oral contraceptives (COCs), in which case both estimates are adjacent (as indicated by a box around the study identifiers). For studies which reported both Cox proportional hazards (Cox) and marginal structural model (MSM) estimates, both estimates are displayed on a single line (also identified by brackets). OR odds ratio, IRR incidence risk ratio, HR hazard ratio. Asterisk analysis showed significant findings at p=0.05 (marker also displayed in red). Yen sign different statistical models adjusted for slightly different confounders
Very limited data exist for the effects of contraceptive implant use on risk of HIV acquisition, with only one study identified in a systematic review [12•] as informative but with important limitations, finding no significant increase in HIV acquisition [22]. Also, no data exist on the effects of contraceptive patch, ring, combined injectable, or levonorgestrel IUD on risk of HIV acquisition [12•].
Hormonal Contraceptive Methods and Female-to-Male HIV Transmission Risk
Data on risk of female-to-male HIV transmission among women using hormonal contraceptive methods are sparse [11•]. A systematic review examining hormonal contraceptive methods and female-to-male HIV transmission risk identified only one study [21•] that evaluated the direct impact of hormonal contraception on HIV transmission, and that study suggested increased female-to-male HIV transmission risk with the use of injectable contraception [21•]. Additionally, the review identified 16 indirect studies that evaluated the influence of hormonal contraceptive methods on proxy markers for infectivity (i.e., plasma and/or genital HIV RNA levels) [11•]. Of 11 studies evaluating hormonal contraceptive methods and genital tract virus levels, detectability, or set point, results were mixed, while seven of eight studies assessing the impact of hormonal contraception on the amount of HIV in the blood reported no increase in plasma viral load or set point. More recent direct evidence from a retrospective cohort in Uganda suggested no increased HIV transmission risk with DMPA or oral contraceptives [42], but this study had some methodological limitations.
Potential Biologic Mechanisms for Increased HIV Acquisition and Transmission Risk Associated with Certain Hormonal Contraceptive Methods
Acquisition of HIV Infection Is a Multifaceted Process of Virus-Host Interactions
In addition to plasma viremia of the transmitter, factors that increase susceptibility of the partner to HIV infection include the presence of STIs, mucosal micro-abrasions, and secreted immune factors—all of which influence local mucosal immunity, the protective integrity of the epithelial barrier in the genital tract, and the activation status of HIV target cells involved in the early stages of infection [43•]. Evidence suggests that the robust nature and functional capacity of the innate and adaptive immune responses are influenced by female sex hormones [43•, 44, 45]. The immunoregulatory influence of estradiol and progesterone ensures a favorable environment for fertilization and pregnancy by altering the local female genital tract (FGT) immunologic milieu and the composition of immune cells.
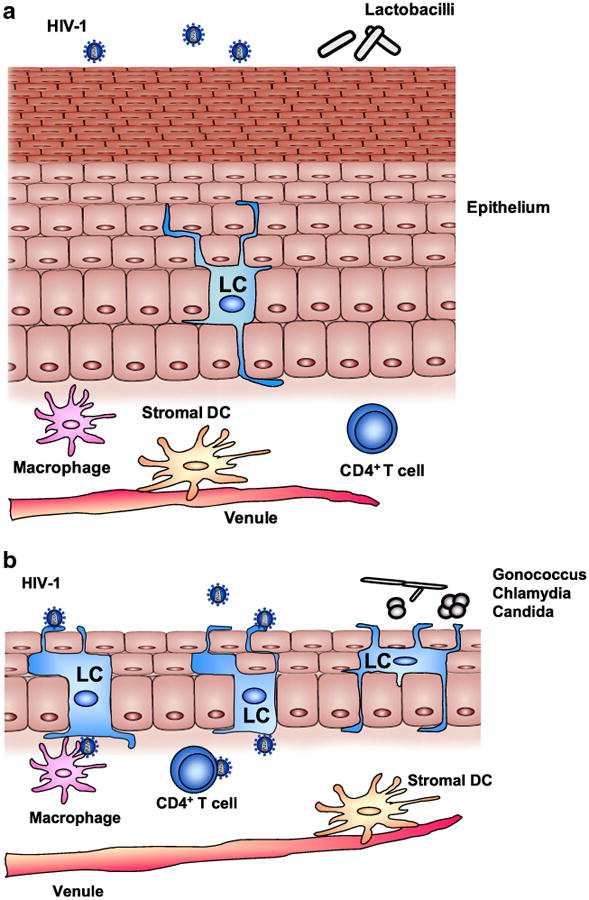
Animal studies among rhesus macaques have documented that systemic and topical administration of estrogen thickens the vaginal epithelial layer (Fig. 3a) [43•, 46, 47], reduces the population of Langerhans cells within the vaginal epithelium, alters vaginal epithelial tight junctions and mucosal permeability [48, 49], and supports healthy genital tract flora. Through these changes, estrogen makes the mucosa less susceptible to SIV by blocking its access to target cells within the epithelial and subepithelial layers. Contrary to the effect of estrogen, progesterone leads to thinning of the epithelial layer and increased concentrations of susceptible cells in female macaques, allowing SIVeasier access and availability to target cells (Fig. 3b) [50]. However, while animal studies demonstrate potential mechanisms to explain increased risk, these changes have not been robustly demonstrated to occur among humans [51, 52].
Fig. 3. Potential effects of female sex hormones on the vaginal mucosal tissue. Reproduced with permission from Hel et al. [43•].
Other potential effects of progesterone on immune function that may increase HIV acquisition risk include suppression of antibody production and transepithelial transport within the FGT [53–55], inhibition of cytotoxic immune cell responses [56, 57], and immune cell infiltration within the genital tissue, reducing the functional capacity of antibody-dependent cell cytotoxicity [58–60]. Levels of expression/secretion of chemokines, cytokines, and endogenous antimicrobials in FGT secretions alter over the course of the menstrual cycle, during menopause, and with contraceptive use, suggesting a role for hormones in regulating their release [61–65]. Increased risk of HIV acquisition has been suggested to occur during pregnancy, a high-progesterone state [66]. Progesterone may compromise the integrity of the protective epithelial layers against HIV infection, promote enhanced homing of HIV-susceptible cells to the FGT, and reduce the robust nature of local innate antiviral and adaptive secretory immune responses.
Human data also suggest that progesterone treatment reduces the concentration of lactobacilli in the vaginal microbiota [52, 67]. This divergence from healthy vaginal flora is associated with increased HIV acquisition risk [68, 69]. Although coinfections with ulcerative STIs (e.g., syphilis, herpes, chancroid) or inflammatory STIs (e.g., gonorrhea, chlamydia) can increase the risk of HIV acquisition [70], evidence is conflicting on the influence of hormonal contraceptives on these infections [43•, 71].
For HIV transmission to male partners, several proposed biologic mechanisms could increase risk of female-to-male transmission with hormonal contraceptive methods, although it is unclear which mechanisms, if any, are relevant. Hormonal contraceptive methods could lead to higher genital tract viral loads either directly or indirectly, with increased shedding associated with increased HIV transmission [72]. For example, with increased hormonal levels associated with pregnancy, some studies have documented higher levels of HIV shedding [73–78] and suggested a higher transmission risk to male partners [66, 79]. Further, the immunomodulatory effects noted above that may influence acquisition risk may also impact local immune environment and genital compartmental viral replication, leading to increased shedding. Increases in local inflammation, through hormonal contraceptive influences on STIs or the vaginal microbiome, may additionally increase HIV shedding and, subsequently, transmission risk [80–82]. However, physiologic doses of DMPA in pigtail macaques did not increase plasma viremia or mucosal virus shedding during acute infections [83].
Different contraceptives have variable degrees of estrogenic, androgenic, anti-androgenic, glucocorticoid, and anti-mineralocorticoid activity [84, 85]. Medroxyprogesterone (MPA), for example, has androgenic activity, no anti-mineralocorticoid activity and potent glucocorticoid activity, higher than any other progestogen or endogenous progesterone. Activation of glucocorticoid receptors, seen with MPA, may influence glucocorticoid-like immunosuppressive effects on genes involved with immune function [86, 87]. These differences between contraceptives warrant further investigation and may influence relative contraceptive safety.
Nonhormonal Contraceptives and Risk of HIV Acquisition or Transmission
The copper IUD is one of the most effective contraceptive options available and is generally considered safe in women at high risk of HIV or who are living with HIV, but clinically well [8•]. For women with active stage 3 or 4 HIV disease, IUD insertion is not currently recommended; however, there are no restrictions on its continued use in women who have an IUD in place [8•]. To date, no studies suggest an increased risk of HIV acquisition or viral shedding among women living with HIV with the copper IUD [88, 89], but IUD use results in changes in genital tract immune cell populations which could theoretically alter risk in either direction [90].
Nonoxynol-9, the most common active ingredient in spermicides in the USA, may, with frequent use, cause irritation and vaginal epithelial erosion, which could increase risk of HIV acquisition and transmission. In a study of high-risk women, use of nonoxynol-9 more than three times a day increased the rate of HIV transmission to sexual partners [91]. According to Centers for Disease Control and Prevention (CDC)'s Medical Eligibility Criteria for Contraceptive Use, spermicides generally should not to be used by women with HIV and women at high risk of acquiring HIV [92].
Alternative nonhormonal contraceptives, such as lactational amenorrhea, diaphragms, fertility awareness methods, withdrawal, and cervical caps, are considered lower tier [13] in terms of contraceptive efficacy and do not reduce HIV transmission risk. Male condoms, as discussed above, are an important strategy for STI and HIV prevention but are less effective for pregnancy prevention during typical use than many alternative contraceptive options.
Dual Method Use
Dual method refers to the use of a highly effective form of contraception combined with consistent condom use, and it offers the greatest promise to prevent unintended pregnancy and STIs, including HIV. Reproductive health counseling guidelines recommend the use of a dual method strategy [93, 94]. However, rates of reported dual method use are typically low, ranging from 5.5 to 14.6 % in seronegative adolescents [93, 95–97]. Among South African seropositive young women, only 7 % reported dual method use [98]. In a longitudinal cohort study of seropositiveUS adult women, use of a dual method strategy was infrequent [99]. Further, even among dual method users, condom use is often inconsistent or incorrect [100].
Factors that Influence Contraceptive Choice May Impact HIV Risk
Several studies have examined factors associated with seronegative women's selection and use of contraceptive methods [96, 97, 101–103]. Among studies of adult HIV-seropositive women, the majority have been conducted in international settings, primarily in Africa [104–110], with few studies from the USA [111]. These reports highlight the importance of access to health care [104, 105], reporting women's concerns about contraceptive side effects [108] or potential interactions with ARVs [108], convenience of barrier contraceptive methods [104, 105, 111], and desire to have children [107–109] as determinants of contraceptive method use in women living with HIV [104]. Additionally, contraceptive use may differ by partner type; for instance, among a cohort of French women living with HIV, contraceptive use was more prevalent among women reporting recent sexual encounters with casual partners [112].
Some of the behavioral differences between women who choose hormonal contraception and other women may also be related to a woman's underlying risk of acquiring HIV infection. Observational studies can attempt to control for these factors, but unless they are accurately measured, residual (in addition to unmeasured) confounding can occur, complicating our understanding of the impact of hormonal contraceptive methods on HIV risk. For example, unprotected sexual encounters may change with contraceptive use and subsequently alter the risks to uninfected partners through exposure to HIV and other STIs [113–116]. Further, unprotected coitus associated with STI and semen exposure can alter the genital tract inflammatory environment, increasing the risk of HIV transmission. In vitro studies have shown that whole semen can double the rate of HIV infectivity [117], upregulate the expression of proinflammatory cytokines in the female genital tract [118–120], and increase recruitment of Langerhans cells to the vaginal mucosa [121]. Coinfections with STIs and other genital infections have also been associated with increased HIV shedding and transmission [80, 81, 119, 122]. This increased risk may be due to micro-ulcerations or local recruitment of activated immune cells to the genital tract [123]. Thus, inconsistent condom use, which is difficult to account for in observational studies, can alter HIV susceptibility and infectivity, thereby potentially affecting the association between contraceptive use and HIV acquisition and transmission.
The Influence of Combination Antiretroviral Therapy
Adherence to combination antiretroviral therapy (ART) reduces HIV transmission and clinical disease progression. Plasma viral load (VL) has been shown to be a very strong predictor of heterosexual transmission [124, 125], and ART decreases viral load in plasma and the genital tract. Results from the HIV Prevention Trials Network (HPTN) 052 study demonstrated that individuals living with HIV who initiated ART when their CD4 counts were >350 cells/μl were 96 % less likely to transmit HIV to uninfected partners than those who deferred initiation of ART [126]. Suboptimal adherence to ART remains the primary cause of persistent plasma viremia. Certain at-risk groups (e.g., adolescents and young adults) are more likely to have poor adherence [127–129].
However, several reports have documented detectable HIV in cervicovaginal fluid of women receiving ART, even when the woman has undetectable plasma viral load [125, 130–137]. Thus, it is important to address potential modulating factors that affect viral load in mucosal compartments [138, 139]. The impact of ART on genital HIV-1 RNA shedding in the setting of hormonal contraceptive use is also important, as ART may mitigate any potential increased HIV transmission risk associated with hormonal contraceptives. For example, while evidence is mixed regarding an association between DMPA use and genital HIV-1 RNA shedding among women living with HIV, a recent study in Kenya among women receiving ART demonstrated no association between DMPA use and plasma or cervical HIV-1 RNA [140]. This study suggests that DMPA is unlikely to increase HIV transmission risk among women who are adherent to ART.
Drug interactions have been documented between ART regimens containing certain antiretroviral drugs and certain hormonal contraceptives (primarily combined oral contraceptive pills and possibly implants) due to shared metabolic pathways utilizing the hepatic cytochrome P450 system [92, 141, 142]. Individual concerns about potential interactions between certain ART medications and certain forms of hormonal contraception resulting in decreased contraceptive effectiveness may reduce uptake of certain contraceptive methods. In a qualitative study of South African women, concerns that ART would diminish the efficacy of hormonal contraception were reported [108]. Further investigations are warranted to understand the potential interactions between uses of specific ART medications and various hormonal contraceptive methods.
Summary of the Evidence/Current Recommendations
WHO and CDC have determined that insufficient evidence exists to support a restriction on the use of hormonal contraceptives by women at risk of, or living with, HIV infection [8•, 143]. However, the following clarification applies for women using progestogen-only injectable contraception who are at high risk of HIV:
Available studies on the association between progestogen-only injectable contraception and HIV acquisition have important methodological limitations hindering their interpretation. Some studies suggest that women using progestogen-only injectable contraception may be at increased risk of HIV acquisition; other studies have not found this association. The public health impact of any such association would depend upon the local context, including rates of injectable contraceptive use, maternal mortality, and HIV prevalence. This must be considered when adapting guidelines to local contexts. WHO expert groups continue to actively monitor any emerging evidence. At the meeting in 2014, as at the 2012 technical consultation, it was agreed that the epidemiological data did not warrant a change to the Medical Eligibility Criteria for Contraceptive Use (MEC). Given the importance of this issue, women at high risk of HIV infection should be informed that progestogen-only injectables may or may not increase their risk of HIV acquisition. Women and couples at high risk of HIV acquisition considering progestogen-only injectables should also be informed about and have access to HIV preventive measures, including male and female condoms.
Furthermore, WHO states that women taking ART are eligible for all hormonal contraceptive methods, but special consideration may be necessary for women on certain antiretrovirals, specifically efavirenz, nevirapine, or some protease inhibitors [8•]. CDC guide lines for contraceptive use among women with HIV infection or other medical conditions can be found at http://www.cdc.gov/reproductivehealth/unintendedpregnancy/usmec.htm [92]. Global recommendations for contraceptive use among women with HIV infection or other medical conditions can be found at http://www.who.int/reproductivehealth/topics/family_planning/en/ [8•]. These guidelines are regularly evaluated and updated.
Conclusion
The impact of hormonal contraception, specifically progestogen-only injectables, on risk of HIV acquisition or transmission remains inconclusive. If an increased risk is determined with certain contraceptives, this would need to be balanced against consequences of reducing availability of hormonal contraception, especially in regions with high rates of injectable use and maternal morbidity and mortality. However, in certain regions with high HIV prevalence, particularly South Africa, should increased risk of HIV acquisition or transmission via specific contraceptive methods be proven, the overall public health impact of increased HIV incidence might warrant policy changes [144, 145]. Any policies must carefully balance regional HIV risk, contraceptive method safety, regional maternalmortality, and availability and acceptance of alternative effective methods. Both HIV and pregnancy risk reduction strategies, such as the development of female-controlled prevention technology, should be the focus of research efforts. Dual protection with consistent and correct condom use in addition to more effective contraception is a critical strategy that needs to be further promoted. Lastly high-quality counseling is imperative to optimize patient selection and maintenance of effective contraception.
Acknowledgments
Jennifer Brown has received a grant from the NIH.
Rana Chakraborty has received a grant from the CDC and Gilead.
Igho Ofotokun has received a grant from Bristol-Myers Squibb.
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Lisa B. Haddad, Chelsea B. Polis, Anandi N. Sheth, Athena P. Kourtis, and Caroline King declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the United States Agency for International Development.
Contributor Information
Lisa B. Haddad, Email: lbhadda@emory.edu, Department of Gynecology and Obstetrics, Emory University School of Medicine, 49 Jesse Hill Jr. Drive, Atlanta, GA 30303, USA.
Chelsea B. Polis, Office of Population and Reproductive Health, United States Agency for International Development (USAID), Washington, DC, USA
Anandi N. Sheth, Department of Medicine, Division of Infectious Diseases and Grady Healthcare System, Emory University School of Medicine, Atlanta, GA, USA
Jennifer Brown, Department of Psychological Sciences, Texas Tech University, Lubbock, TX, USA.
Athena P. Kourtis, Division of Reproductive Health, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, GA, USA
Caroline King, Division of Reproductive Health, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Atlanta, GA, USA.
Rana Chakraborty, Department of Pediatrics, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, USA.
Igho Ofotokun, Department of Medicine, Division of Infectious Diseases and Grady Healthcare System, Emory University School of Medicine, Atlanta, GA, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of major importance
- 1.Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Stud Fam Plan. 2010;41(4):241–50. doi: 10.1111/j.1728-4465.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 2.Mavranezouli I. Health economics of contraception. Best Pract Res Clin Obstet Gynaecol. 2009;23(2):187–98. doi: 10.1016/j.bpobgyn.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Darroch JE. Adding it up: costs and benefits of contraceptive services: estimates for 2012. 2012 [Google Scholar]
- 4.Wilcher R, et al. From effectiveness to impact: contraception as an HIV prevention intervention. Sex Transm Infect. 2008;84(Suppl 2):ii54–60. doi: 10.1136/sti.2008.030098. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Hormonal contraception and HIV technical statement. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 6.The Inter-agency Task Team for Prevention and Treatment of HIV Infection in Pregnant Women, M, and their Children. Preventing HIV and unintended pregnancies: strategic framework 2011–2015. 2012 http://www.unfpa.org/webdav/site/global/shared/documents/publications/2012/PreventingHIV_UnintendedPregnancies_SF2011_2015.pdf.
- 7.Johnston B, et al. Meeting the family planning needs of women living with HIV in US government global health programs. AIDS. 2013;27(Suppl 1):S121–5. doi: 10.1097/QAD.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.World Health Organization. Dissemination and evaluation of the 2014 guidance statement on hormonal contraceptive methods for women at high risk and living with HIV. Geneva: WHO; 2014. This report reviews the most recent evidence and summarizes the current guidelines on the topic from the World Health Organization. [PubMed] [Google Scholar]
- 9.Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a systematic review. AIDS. 2013;27(5):787–94. doi: 10.1097/QAD.0b013e32835bb672. [DOI] [PubMed] [Google Scholar]
- 1.0 Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 11•.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27(4):493–505. doi: 10.1097/QAD.0b013e32835ad539. This is a systematic review of the literature through 2012 evaluating the epidemiological evidence on use of various hormonal contraceptive methods and female-to-male HIV transmission. [DOI] [PubMed] [Google Scholar]
- 12•.Polis CB, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–90. doi: 10.1016/j.contraception.2014.07.009. This is a systematic review of the epidemiological evidence on use of evaluating use of various hormonal contraceptive methods and HIV acquisition in women. [DOI] [PubMed] [Google Scholar]
- 13.Hatcher R, et al. Contraceptive technology. 20th. New York: Ardent Media; 2011. [Google Scholar]
- 14.Adair T. DHS working papers—men's condom use in higher-risk sex: trends and determinants in five Sub-Saharan countries. Maryland: USAID; 2008. [Google Scholar]
- 15.Padian NS, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370(9583):251–61. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson D, et al. Nonoxynol-9 for preventing vaginal acquisition of sexually transmitted infections by women from men. Cochrane Database Syst Rev. 2002;4:CD003939. doi: 10.1002/14651858.CD003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson D, et al. Nonoxynol-9 for preventing vaginal acquisition of HIV infection by women from men. Cochrane Database Syst Rev. 2002;4:CD003936. doi: 10.1002/14651858.CD003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank-Herrmann P, et al. The effectiveness of a fertility awareness based method to avoid pregnancy in relation to a couple's sexual behaviour during the fertile time: a prospective longitudinal study. Hum Reprod. 2007;22(5):1310–9. doi: 10.1093/humrep/dem003. [DOI] [PubMed] [Google Scholar]
- 19.Trussell J. Contraceptive efficacy. Arch Dermatol. 1995;131(9):1064–8. [PubMed] [Google Scholar]
- 20.United Nations. World contraceptive pattern 2013. 2013 [June 21, 2014]. http://www.un.org/en/development/desa/population/publications/pdf/family/worldContraceptivePatternsWallChart2013.pdf.
- 21•.Heffron R, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. This frequently cited paper is a secondary analysis from a large cohort study. The analysis suggested an increased risk of HIV acquisition and transmission to men associated with use of injectable contraception, and increased global attention to this potential risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavreys L, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS. 2004;18(4):695–7. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 23.Marx PA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 24.Kapiga SH, et al. The incidence of HIV infection among women using family planningmethods in Dar es Salaam, Tanzania. AIDS. 1998;12(1):75–84. doi: 10.1097/00002030-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Myer L, et al. Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. Int J Epidemiol. 2007;36(1):166–74. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 26.Taneepanichskul S, Phuapradit W, Chaturachinda K. Association of contraceptives and HIV-1 infection in Thai female commercial sex workers. Aust N Z J Obstet Gynaecol. 1997;37(1):86–8. doi: 10.1111/j.1479-828x.1997.tb02224.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiddugavu M, et al. Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. AIDS. 2003;17(2):233–40. doi: 10.1097/00002030-200301240-00014. [DOI] [PubMed] [Google Scholar]
- 28.Morrison CS, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 29.Morrison CS, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS. 2012;26(4):497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 30.Bulterys M, et al. Hormonal contraception and incident HIV-1 infection: new insight and continuing challenges. AIDS. 2007;21(1):97–9. doi: 10.1097/QAD.0b013e3280117cb5. [DOI] [PubMed] [Google Scholar]
- 31.Morrison CS. Commentary: hormonal contraception and HIV acquisition—current evidence and ongoing research needs. Int J Epidemiol. 2007;36(1):175–7. doi: 10.1093/ije/dyl304. [DOI] [PubMed] [Google Scholar]
- 32.Polis CB, et al. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. AIDS. 2013;27(Suppl 1):S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012;26(3):375–80. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 34.Baeten JM, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21(13):1771–7. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 35.Morrison CS, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–81. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heffron R, et al. Use of hormonal contraceptives and risk of HIV-1 transmission—authors'reply. Lancet Infect Dis. 2012;12(8):510–1. doi: 10.1016/S1473-3099(12)70115-9. [DOI] [PubMed] [Google Scholar]
- 37.Reid SE, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53(5):606–13. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinschmidt I, et al. Injectable progestin contraceptive use and risk of HIV infection in a South African family planning cohort. Contraception. 2007;75(6):461–7. doi: 10.1016/j.contraception.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.McCoy SI, et al. Oral and injectable contraception use and risk of HIV acquisition among women in sub-Saharan Africa. AIDS. 2013;27(6):1001–9. doi: 10.1097/QAD.0b013e32835da401. [DOI] [PubMed] [Google Scholar]
- 40.Morrison C, et al. Presented at AIDS 2014. Melbourne, Australia: Jul 20–25, 2014. Hormonal contraception and HIV infection: results from a large individual participant data meta-analysis. [Google Scholar]
- 41.Wall K, et al. Presented at AIDS 2014. Melbourne, Australia: Jul 20–25, 2014. Weighing 17 years of evidence: does hormonal contraception increase HIV acquisition risk among Zambian women in discordant couples? [Google Scholar]
- 42.Lutalo T, et al. Effects of hormonal contraceptive use on HIV acquisition and transmission among HIV-discordant couples. AIDS. 2013;27(Suppl 1):S27–34. doi: 10.1097/QAD.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 43•.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31(1):79–97. doi: 10.1210/er.2009-0018. This paper reviews the potential biologic mechanisms from hormones that could affect HIV risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnex C. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74(1):11–9. doi: 10.1136/sti.74.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trunova N, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352(1):169–77. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182(3):708–15. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18(12):1637–43. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 48.Gorodeski GI. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause. 2007;14(6):1012–9. doi: 10.1097/gme.0b013e3180587eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148(1):218–31. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goode D, et al. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS ONE. 2014;9(5):e97767. doi: 10.1371/journal.pone.0097767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahamondes MV, et al. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception. 2014 doi: 10.1016/j.contraception.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell CM, et al. Long-term effect of depotmedroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014 doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kutteh WH, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retrovir. 1998;14(Suppl 1):S51–5. [PubMed] [Google Scholar]
- 54.White HD, et al. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37(1):30–8. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 55.Lu FX, et al. Effects of ovarian steroids on immunoglobulin-secreting cell function in healthy women. Clin Diagn Lab Immunol. 2003;10(5):944–9. doi: 10.1128/CDLI.10.5.944-949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cherpes TL, et al. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol. 2008;181(2):969–75. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laskarin G, et al. Progesterone directly and indirectly affects perforin expression in cytolytic cells. Am J Reprod Immunol. 1999;42(5):312–20. doi: 10.1111/j.1600-0897.1999.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 58.Scanlan JM, et al. Natural killer cell activity is reduced in association with oral contraceptive use. Psychoneuroendocrinology. 1995;20(3):281–7. doi: 10.1016/0306-4530(94)00059-j. [DOI] [PubMed] [Google Scholar]
- 59.Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81(2):254–62. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- 60.Chandra N, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retrovir. 2013;29(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ochiel DO, et al. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Womens Health Rev. 2008;4(2):102–17. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185(11):1606–13. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 64.Keller MJ, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21(4):467–76. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 65.Li A, et al. Effect of mifepristone on the expression of endometrial secretory leukocyte protease inhibitor in new medroxyprogesterone acetate users. Fertil Steril. 2008;90(3):872–5. doi: 10.1016/j.fertnstert.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 66.Mugo NR, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller L, et al. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96(3):431–9. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 68.Hayes R, et al. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? AIDS. 2010;24(Suppl 4):S15–26. doi: 10.1097/01.aids.0000390704.35642.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Low N, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8(2):e1000416. doi: 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClelland RS, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis. 2005;191(3):333–8. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 71.Morrison CS, et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis. 2004;31(9):561–7. doi: 10.1097/01.olq.0000137904.56037.70. [DOI] [PubMed] [Google Scholar]
- 72.Baeten JM, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clemetson DB, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269(22):2860–4. [PubMed] [Google Scholar]
- 74.Tanton C, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS ONE. 2011;6(3):e17480. doi: 10.1371/journal.pone.0017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreiss J, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170(6):1597–601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 76.Morrison CS, et al. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS. 2010;24(4):573–82. doi: 10.1097/QAD.0b013e32833433df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henin Y, et al. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syndr. 1993;6(1):72–5. [PubMed] [Google Scholar]
- 78.Gardella B, et al. HIV shedding in cervico-vaginal secretions in pregnant women. Curr HIV Res. 2011;9(5):313–20. doi: 10.2174/157016211797636017. [DOI] [PubMed] [Google Scholar]
- 79.Ghanem KG, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis. 2005;191(3):358–66. doi: 10.1086/427190. [DOI] [PubMed] [Google Scholar]
- 80.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35(11):946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 81.Rotchford K, Strum AW, Wilkinson D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis. 2000;27(5):243–8. doi: 10.1097/00007435-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radzio J, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS. 2014;28(10):1431–9. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 84.Africander D, et al. Differential regulation of endogenous proinflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception. 2011;84(4):423–35. doi: 10.1016/j.contraception.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76(7):636–52. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Hapgood JP, et al. Differential glucocorticoid receptor-mediated effects on immunomodulatory gene expression by progestin contraceptives: implications for HIV-1 pathogenesis. Am J Reprod Immunol. 2014;71(6):505–12. doi: 10.1111/aji.12214. [DOI] [PubMed] [Google Scholar]
- 87.Govender Y, et al. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS ONE. 2014;9(5):e96497. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richardson BA, et al. Effect of intrauterine device use on cervical shedding of HIV-1 DNA. AIDS. 1999;13(15):2091–7. doi: 10.1097/00002030-199910220-00012. [DOI] [PubMed] [Google Scholar]
- 89.European Study Group on Heterosexual Transmission of HIV. Comparison of female to male and male to female transmission of HIV in 563 stable couples. BMJ. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Achilles SL, et al. Changes in genital tract immune cell populations after initiation of intrauterine contraception. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Damme L, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention (CDC) U.S. medical eligibility criteria for contraceptive use. Morbidity and Mortality Weekly Report (MMWR) 2010 [Google Scholar]
- 93.Anderson JE, Santelli J, Gilbert BC. Adolescent dual use of condoms and hormonal contraception: trends and correlates 1991-2001. Sex Transm Dis. 2003;30(9):719–22. doi: 10.1097/01.OLQ.0000078628.84288.66. [DOI] [PubMed] [Google Scholar]
- 94.Greydanus DE, Patel DR, Rimsza ME. Contraception in the adolescent: an update. Pediatrics. 2001;107(3):562–73. doi: 10.1542/peds.107.3.562. [DOI] [PubMed] [Google Scholar]
- 95.Anderson JE, Santelli J, Mugalla C. Changes in HIV-related preventive behavior in the US population: data from national surveys, 1987-2002. J Acquir Immune Defic Syndr. 2003;34(2):195–202. doi: 10.1097/00126334-200310010-00010. 1999. [DOI] [PubMed] [Google Scholar]
- 96.Crosby RA, et al. Correlates of using dual methods for sexually transmitted diseases and pregnancy prevention among high-risk African-American female teens. J Adolesc Health. 2001;28(5):410–4. doi: 10.1016/s1054-139x(00)00210-x. [DOI] [PubMed] [Google Scholar]
- 97.Sales JM, et al. Differences between dual-method and non-dual-method protection use in a sample of young African American women residing in the Southeastern United States. Arch Pediatr Adolesc Med. 2010;164(12):1125–31. doi: 10.1001/archpediatrics.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacPhail C, et al. Predictors of dualmethod use for pregnancy and HIV prevention among adolescent South African women. Contraception. 2007;75(5):383–9. doi: 10.1016/j.contraception.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Massad LS, et al. Contraceptive use among U.S. women with HIV. J Women's Health. 2007;16(5):657–66. doi: 10.1089/jwh.2006.0204. 15409996. [DOI] [PubMed] [Google Scholar]
- 100.Higgins JA, et al. Dual method use at last sexual encounter: a nationally representative, episode-level analysis of US men and women. Contraception. 2014;90:399–406. doi: 10.1016/j.contraception.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santelli JS, et al. The use of condoms with other contraceptive methods among young men and women. Fam Plan Perspect. 1997;29(6):261–7. [PubMed] [Google Scholar]
- 102.Santelli JS, et al. Combined use of condoms with other contraceptive methods among inner-city Baltimore women. Fam Plan Perspect. 1995;27(2):74–8. [PubMed] [Google Scholar]
- 103.Brown JL, et al. Multiple method contraception use among African American adolescents in four US cities. Infect Dis Obstet Gynecol. 2011;2011 doi: 10.1155/2011/765917. 765917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Credé S, et al. Factors impacting knowledge and use of long acting and permanent contraceptive methods by postpartum HIV positive and negative women in Cape Town, South Africa: a cross-sectional study. BMC Public Health. 2012;12:197. doi: 10.1186/1471-2458-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dhont N, et al. Improved access increases postpartum uptake of contraceptive implants among HIV-positive women in Rwanda. Eur J Contracept Reprod Health Care. 2009;14(6):420–5. doi: 10.3109/13625180903340584. [DOI] [PubMed] [Google Scholar]
- 106.Heffron R, et al. A prospective study of contraceptive use among African women in HIV-1 serodiscordant partnerships. Sex Transm Dis. 2010;37(10):621–8. doi: 10.1097/OLQ.0b013e3181e1a162. [DOI] [PubMed] [Google Scholar]
- 107.Kikuchi K, et al. High rate of unintended pregnancies after knowing of HIV infection among HIV positive women under antiretroviral treatment in Kigali, Rwanda. Biosci Trends. 2011;5(6):255–63. doi: 10.5582/bst.2011.v5.6.255. [DOI] [PubMed] [Google Scholar]
- 108.Laher F, et al. A qualitative assessment of decisions affecting contraceptive utilization and fertility intentions among HIV-positive women in Soweto, South Africa. AIDS Behav. 2009;13(Suppl 1):47–54. doi: 10.1007/s10461-009-9544-z. [DOI] [PubMed] [Google Scholar]
- 109.Nduna M, Farlane L. Women living with HIV in South Africa and their concerns about fertility. AIDS Behav. 2009;13(Suppl 1):62–5. doi: 10.1007/s10461-009-9545-y. [DOI] [PubMed] [Google Scholar]
- 110.Polis CB, et al. Trends and correlates of hormonal contraceptive use among HIV-infected women in Rakai, Uganda, 1994-2006. Contraception. 2011;83(6):549–55. doi: 10.1016/j.contraception.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Ezeanolue EE, et al. Contraception choices in a cohort of HIV+ women in the era of highly active antiretroviral therapy. Contraception. 2011;84(1):94–7. doi: 10.1016/j.contraception.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 112.Carrieri MP, et al. Oral contraception and unprotected sex with occasional partners of women HIV-infected through injection drug use. AIDS Care. 2006;18(7):795–800. doi: 10.1080/09540120500431584. [DOI] [PubMed] [Google Scholar]
- 113.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87(3):183–90. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taiwo B. Understanding transmitted HIV resistance through the experience in the USA. Int J Infect Dis. 2009;13(5):552–9. doi: 10.1016/j.ijid.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 115.Campbell MS, et al. HIV-1 superinfection in the antiretroviral therapy era: are seroconcordant sexual partners at risk? PLoS ONE. 2009;4(5):e5690. doi: 10.1371/journal.pone.0005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brenner B, et al. Persistence of multidrug-resistant HIV-1 in primary infection leading to superinfection. AIDS (London, England) 2004;18(12):1653–60. doi: 10.1097/01.aids.0000131377.28694.04. [DOI] [PubMed] [Google Scholar]
- 117.Bouhlal H, et al. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J Immunol. 2002;169(6):3301–6. doi: 10.4049/jimmunol.169.6.3301. [DOI] [PubMed] [Google Scholar]
- 118.Gutsche S, et al. Seminal plasma induces mRNA expression of IL-1beta, IL-6 and LIF in endometrial epithelial cells in vitro. Mol Hum Reprod. 2003;9(12):785–91. doi: 10.1093/molehr/gag095. [DOI] [PubMed] [Google Scholar]
- 119.Sharkey DJ, et al. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 120.Sharkey DJ, et al. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 121.Berlier W, et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod. 2006;21(5):1135–42. doi: 10.1093/humrep/dei496. [DOI] [PubMed] [Google Scholar]
- 122.White HD, et al. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of themenstrual cycle and menopause. J Immunol. 1997;158(6):3017–27. [PubMed] [Google Scholar]
- 123.Plummer FA. Heterosexual transmission of human immunodeficiency virus type 1 (HIV): interactions of conventional sexually transmitted diseases, hormonal contraception and HIV-1. AIDS Res Hum Retrovir. 1998;14(Suppl 1):S5–10. [PubMed] [Google Scholar]
- 124.Cu-Uvin S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14(4):415–21. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 125.Kovacs A, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358(9293):1593–601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 126.Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flynn PM, et al. Long-term observation of adolescents initiating HAART therapy: three-year follow-Up. AIDS Res Hum Retrovir. 2007;23(10):1208–14. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 128.Murphy DA, et al. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157(3):249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 129.Murphy DA, et al. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13(1):27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 130.Roccio M, et al. Low-dose combined oral contraceptive and cervicovaginal shedding of human immunodeficiency virus. Contraception. 2011;83(6):564–70. doi: 10.1016/j.contraception.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Debiaggi M, et al. Viral excretion in cervicovaginal secretions of HIV-1-infected women receiving antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2001;20(2):91–6. doi: 10.1007/s100960000442. [DOI] [PubMed] [Google Scholar]
- 132.Rasheed S. Infectivity and dynamics of HIV type 1 replication in the blood and reproductive tract of HIV type 1-infected women. AIDS Res Hum Retrovir. 1998;14(Suppl 1):S105–18. [PubMed] [Google Scholar]
- 133.Donnell D, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Del Romero J, et al. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:c2205. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neely MN, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(1):38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fiore JR, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS. 2003;17(15):2169–76. doi: 10.1097/00002030-200310170-00004. [DOI] [PubMed] [Google Scholar]
- 137.Andreoletti L, et al. Independent levels of cell-free and cell-associated human immunodeficiency virus-1 in genital-tract secretions of clinically asymptomatic, treatment-naive African women. J Infect Dis. 2003;188(4):549–54. doi: 10.1086/377104. [DOI] [PubMed] [Google Scholar]
- 138.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51(6):725–31. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anton P, Herold BC. HIV transmission: time for translational studies to bridge the gap. Sci Transl Med. 2011;3(77):77ps11. doi: 10.1126/scitranslmed.3002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Day S, et al. A prospective cohort study of the effect of depot medroxyprogesterone acetate on detection of plasma and cervical HIV-1 in women initiating and continuing antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;66(4):452–6. doi: 10.1097/QAI.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Panel on Antiretroviral Guidelines for Adults and Adolescents Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2013 updated 7/31/12; cited 2013 November 14. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 142.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Editor. [Google Scholar]
- 143.Centers for Disease Control and Prevention (CDC) Update to CDC's U.S. medical eligibility criteria for contraceptive use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR. 2012;61(24):449–52. [PubMed] [Google Scholar]
- 144.Rodriguez MI, Reeves MF, Caughey AB. Evaluating the competing risks of HIV acquisition and maternal mortality in Africa: a decision analysis. BJOG. 2012;119(9):1067–73. doi: 10.1111/j.1471-0528.2012.03402.x. [DOI] [PubMed] [Google Scholar]
- 145.Butler AR, et al. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. AIDS. 2013;27(1):105–13. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]