Abstract
Children with sickle cell anemia have a high prevalence of silent cerebral infarcts (SCIs) that are associated with decreased full-scale intelligence quotient (FSIQ). While the educational attainment of parents is a known strong predictor of the cognitive development of children in general, the role of parental education in sickle cell anemia along with other factors that adversely affect cognitive function (anemia, cerebral infarcts) is not known. We tested the hypothesis that both the presence of SCI and parental education would impact FSIQ in children with sickle cell anemia. A multicenter, cross-sectional study was conducted in 19 US sites of the Silent Infarct Transfusion Trial among children with sickle cell anemia, age 5–15 years. All were screened for SCIs. Participants with and without SCI were administered the Wechsler Abbreviated Scale of Intelligence. A total of 150 participants (107 with and 43 without SCIs) were included in the analysis. In a multivariable linear regression model for FSIQ, the absence of college education for the head of household was associated with a decrease of 6.2 points (P=0.005); presence of SCI with a 5.2 point decrease (P=0.017); each $1000 of family income per capita with a 0.33 point increase (P=0.023); each increase of 1 year in age with a 0.96 point decrease (P=0.023); and each 1% (absolute) decrease in hemoglobin oxygen saturation with 0.75 point decrease (P=0.030). In conclusion, FSIQ in children with sickle cell anemia is best accounted for by a multivariate model that includes both biologic and socioenvironmental factors.
Introduction
Children with sickle cell anemia are at significant risk for impairment in cognitive function.[1] Silent cerebral infarct (SCI) is the most frequently identified neurological morbidity among children with this disease.[2] SCI occurs in approximately 30% of children with sickle cell anemia younger than 6 years[3] and has been associated with lower intelligence quotients and specific cognitive deficits.[4-7] The Cooperative Study of Sickle Cell Disease provided the first rigorous evidence that SCIs were associated with a decrement in full-scale intelligence quotient (FSIQ), with FSIQ lowest in children with overt stroke, intermediate with SCI and highest with normal magnetic resonance imaging (MRI) of the brain. Subsequently, others confirmed and expanded these findings by demonstrating deficits in specific cognitive domains that are strongly correlated with the location of the SCIs.[8,9] Furthermore, when children with SCIs were compared with sibling controls without sickle cell disease, a difference in FSIQ and specific cognitive functions also was present.[7,10,11] These studies provide compelling evidence that children with sickle cell anemia and SCIs have a lower general and specific cognitive performance when compared with children without SCIs.
Despite the consistent observation that SCIs are a morbid condition and associated with a decrease in cognitive function, several studies have provided evidence that other factors are also associated with impaired cognition in children with sickle cell anemia, including more severe anemia,[7] lower nocturnal and daytime hemoglobin oxygen saturation,[12] older age,[13,14] and social environment.[15] The family financial status is often strained, as evidenced by 60% of the children receiving health coverage from public programs.[16,17] Until recently, no study has been sufficiently large or comprehensive to determine the relative importance of home environment and biological factors related to sickle cell anemia on cognition.
The Silent Infarct Transfusion (SIT) Trial (ClinicalTrials. gov:#NCT00072761) is a randomized, two-arm, controlled clinical trial designed to determine whether the intervention of regular blood transfusion therapy can decrease the rate of recurrent cerebral infarct and prevent the cognitive decline associated with progressive cerebral infarcts.[18] During screening, over 1000 children between 5 and 15 years of age with sickle cell anemia (hemoglobin SS or sickle-β0-thalassemia) had MRI of the brain, with central adjudication for assessment of presence of cerebral infarcts. Participants with SCIs were randomly allocated to monthly transfusion or observation for 36 months. We postulated that household environmental factors, such as head of household educational status and household per capita income would be significant contributors to cognition in this population; therefore, we included specific questions, based on the National Health and Nutrition Examination Survey III questionnaire,[19] to assess the association socioeconomic parameters and cognitive. The primary objective was to identify significant determinants of FSIQ in children with sickle cell anemia. Specifically, we tested the hypothesis that both the presence of an SCI and home environment would be associated with a clinically significant decline in FSIQ.
Methods
Study design and selection of participants
We performed a multicenter, cross-sectional study at 19 of 25 sites in the SIT Trial. Institutional review boards at each institution approved the study, and all participants and their guardians provided assent and consent, respectively.
To assess the determinants of cognition in children with sickle cell anemia, but without SCIs, the study leadership and the Data Safety Monitoring Board approved two SIT Trial ancillary studies. The site investigators at Washington University and Johns Hopkins University collected data from children who were screened for the SIT Trial, but not eligible for random allocation because of the absence of SCIs. We elected to only include participants in the trial from the United States, primarily because of the concern that language assessment with the cognitive tests and home environmental factors for participants in Europe and Canada would not be similar to those in the United States. Children in the ancillary studies were assessed with the same study procedures and cognitive measures as the children who were randomly allocated in the SIT Trial.
Among 950 English-speaking participants screened by MRI, 151 children were randomized and 138 children completed cognitive testing. A total of 51 children without SCIs completed cognitive testing via ancillary studies and were included in the primary analysis. Of these two groups, 107 children with silent cerebral infarction who completed cognitive testing and 43 of the children from ancillary studies had no missing data on key covariates, leading to a final sample size of 150 (see Fig. 1 for the flow diagram).
Figure 1.
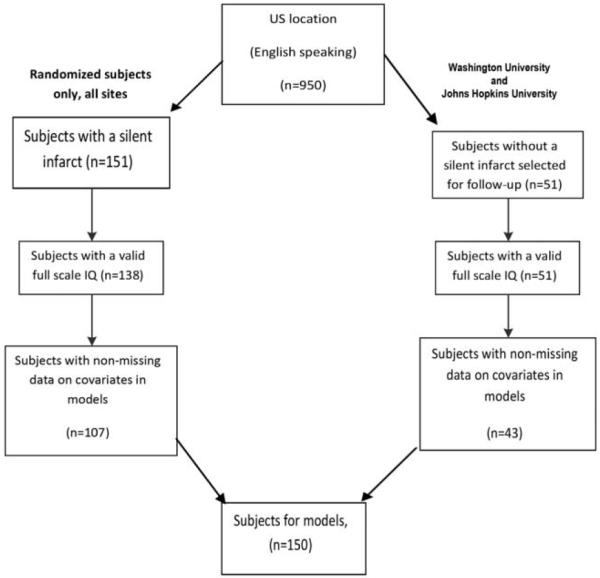
CONSORT diagram of children from the SIT Trial included in this study.
Primary outcomes
FSIQ was a predetermined secondary outcome in the SIT Trial and was measured by the Wechsler Abbreviated Scale of Intelligence (WASI).[20] The WASI is a brief (30 min) and reliable measure of FSIQ that includes the following subtests, Vocabulary, Similarities, Block Design, and Matrix Reasoning. If children were less than 6 years (n=11), they completed the Vocabulary, Matrix Reasoning, Similarities, and Block Design of Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III).[21] Cognitive testing was performed when the patient was at baseline state of health. No opioids should have been taken within 24 h prior to testing, and psychometricians had to rate and approve the child’s general health and function the day of testing.
Potential predictors of outcomes
Baseline laboratory values and medical history
At study entry, a comprehensive review of medical records was completed, including hospitalization for pain, along with collection of laboratory data. White blood cell count, hemoglobin concentration, systolic blood pressure, and daytime hemoglobin oxygen saturation by pulse oximetry were measured at study entry and used as covariates.
Presence of SCI
MRI of the brain was performed using a standardized protocol[22] and interpreted by three neuroradiologists who adjudicated the presence of SCIs.[18] The neurology committee reviewed the MRI assessment of the neuroradiology committee and the site neurologist’s history and examination, and by consensus classified the participant as having SCI or stroke. Participants with SCIs were eligible for random allocation to blood transfusion therapy every 3 to 4 weeks of observation. Randomly allocated patients received a cognitive assessment at baseline.[18]
Sociodemographic variables
Parents or guardians reported their own highest level of educational attainment. Grade levels were classified as “high school graduate or less,” and “any amount of college or greater.” Marital status was classified as “married” or “unmarried.” Total household income was reported in $5000 increments. The mid-point of each increment was used to code annual family income as a continuous variable.
Statistical analysis
Analyses were conducted using IBM SPSS Statistics (Version 20, Chicago, IL, IBM). Continuous data were analyzed using t-tests and the Wilcoxon–Mann–Whitney test for variables with unequal variance or those with skewed distributions. Categorical data were analyzed using chi-square tests. Multivariable linear regression was used to model FSIQ. As a part of our primary analysis, we included plausible determinants of FSIQ, including parents’ marital status (married versus single), child gender (male versus female), education of the head of household (college versus less than college education), household income, SCI (present or absent), age in years, baseline measurements of hemoglobin in gram per deciliter, white blood cell count per microliter, and hemoglobin oxygen saturation in percentage. All covariates were tested for multicollinearity prior to entering the covariate into the model (a variance inflation factor<3). The multivariable linear regression was conducted in two steps. An initial model was constructed with all prespecified variables and a significance of P<0.20 was used to select variables for the second, reduced model. We considered P<0.05 to be statistically significant and did not adjust for multiple comparisons.
Missing data
Complete case analysis was used for the primary models because there were few missing data. Rates of missing data for each group were 10.8% for the participants with SCIs and 3.9% for participants from the ancillary control group. Household income was missing for an additional 12.8% of these two groups combined (150 subjects with complete data).
Results
Full Scale IQ is associated with education level of the head of household, SCI, age, and hemoglobin oxygen saturation
Demographic features of the children with and without SCIs are shown in Table I. A total of 150 children with sickle cell anemia were included, of whom 107 had SCIs. Five covariates met the screening criteria of P<0.20: education level of the head of household (P<0.01), income per capita (P=0.06), presence of silent infarct (P=0.02), child age (P=0.02), and hemoglobin oxygen saturation at baseline (P=0.05). In the reduced model (Table II) with only these five covariates, all remained significant (P<0.05). After controlling for the other covariates, if a head of household obtained at least some college education, a child would be predicted to have an FSIQ 6.2 points higher when compared with those with a head of household with no college education (P=0.005). Each $1000 of household income per capita was associated with a 0.33 point increase (P=0.023). After controlling for the other covariates, if a child had SCI, the child's predicted FSIQ is 5.2 points lower than a child without an infarct (P=0.017); an increase of l year of age reduces predicted FSIQ by 0.96 points (P=0.023); and every decrease of 1% (absolute) in the hemoglobin oxygen saturation reduces predicted FSIQ by 0.7 points (P=0.030). The interaction term between age and the presence of SCI was not significant (P=0.25), suggesting that the impact of age on FSIQ was independent of the presence of SCI.
TABLE I.
Demographics of Children with Sickle Cell Anemia Screened with Cognitive Assessments in the Silent Infarct Transfusion Trial
| Characteristic | N | No SCI (N = 43) | SCI (N = 107) | P value |
|---|---|---|---|---|
| Age of child (years) | 150 | 8.5 | 9.2 | 0.096a |
| Gender (male, %) | 150 | 53.5 | 53.3 | 0.981b |
| Hemoglobin (g/dl) | 150 | 8.28 | 8.03 | 0.204a |
| WBC per μl | 150 | 12,017 | 13,486 | 0.284a |
| Pain event rate (episodes per 3 years) | 149 | 0.75 | 0.55 | 0.286c |
| 0.33 (median) | 0.33 (median) | |||
| Baseline oximetry (percent saturation) | 150 | 96.9 | 96.3 | 0.230c |
| Individual education plan implemented (%) | 150 | 20.9 | 16.8 | 0.554b |
| Medicaid health care coverage for child (%) | 149 | 53.5 | 67.9 | 0.097b |
| Marital status of parents (married, %) | 150 | 39.5 | 40.2 | 0.941b |
| Age of mother (years) | 140 | 34.8 | 34.1 | 0.548a |
| Age of father (years) | 119 | 37.9 | 36.5 | 0.388a |
| No. people age 16 and younger in household | 150 | 2.65 | 2.74 | 0.737a |
| No. people age 17 and older in household | 150 | 1.81 | 1.78 | 0.811a |
| Head of household education (some college or more, %) | 150 | 60.5 | 57.0 | 0.698b |
| Income per capita in thousand dollars | 150 | 11.04 | 8.36 | 0.123c |
| 7.00 (median) | 7.00 (median) | |||
| Full-scale IQ | 150 | 100.53 | 93.12 | 0.002a |
T-test.
Chi-square test.
Mann–Whitney U test.
TABLE II.
Final Multiple Variable Linear Regression Model for FSIQ that Included 150 Children with Sickle Cell Anemia, With and Without SCIs
| Covariate | B a | SE | 95% CI | P |
|---|---|---|---|---|
| Age | −0.96 | 0.42 | −1.79 to −0.13 | 0.023 |
| Baseline pulse oximetry | 0.75 | 0.34 | 0.07 to 1.42 | 0.030 |
| Head of household completed some college | 6.22 | 2.19 | 1.90 to 10.55 | 0.005 |
| Income per capita (1K) | 0.33 | 0.14 | 0.04 to 0.61 | 0.023 |
| Presence of silent infarct | −5.21 | 2.16 | −9.48 to −0.93 | 0.017 |
Unstandardized regression coefficient
The effects on predicted FSIQ of age, SCI, and head of household education are displayed graphically in Figure 2. Children with a head of household who has some college education have higher predicted FSIQ than those without, no matter their infarct status.
Figure 2.
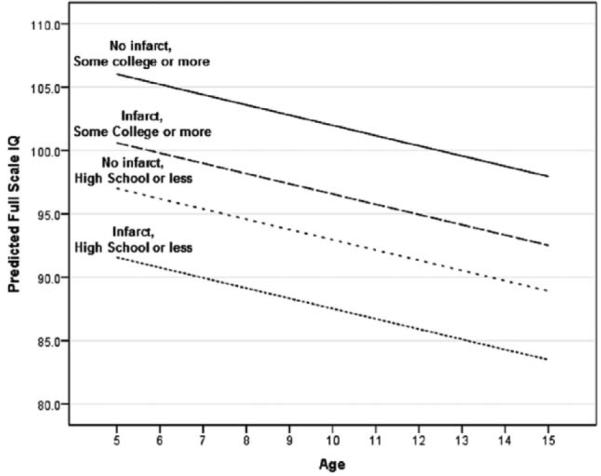
Effect of age, presence of SCI, and head of household education on predicted FSIQ. Model predictions with household per capita income and hemoglobin oxygen saturation fixed at mean value.
The combined effect on FSIQ of all predictors—age, SCI, hemoglobin oxygen saturation, and the socioeconomic factors of head of household education and household per capita income—is large across their full range. For example, setting predictors at the extremes of their range (head of household withou t some college, an SCI, low hemoglobin oxygen saturation of 89.0%, and an age of 15 years) results in a predicted FSIQ of 78.0 (which falls in the borderline range on the WASI). Conversely, setting the predictors at their opposite extremes results in a predicted FSIQ of l08.6.
Discussion
The strong relationship between home environment, including parental education, and FSIQ is well documented.[23-25] However, the contribution of the home environment to cognitive function has not been documented in children who have an acquired injury to the brain, such as an SCI. The home environment is affected by parental education in large part by providing stimulation for learning that is essential to optimal cognitive development, contributing more in predictive models of IQ than a biological factor such as preterm birth.[23,24] For the first time, we demonstrate that both the home environment and disease-specific features independently contribute to impaired cognition in children with sickle cell anemia. Specifically, among children with sickle cell anemia, both characteristics of the home environment (i.e., parental education and household income) and biologic factors, including presence of SCIs, hemoglobin oxygen saturation and age, and are significant predictors of FSIQ.
Our finding that home environment predicts FSIQ suggests that targeted intervention could improve FSIQ in the most vulnerable populations. Several studies have shown that measures of intelligence can improve over time with a change in environment.[26,27] Among abused or neglected children who were adopted between 4 and 6 years of age, IQ scores rose significantly by adolescence. However, the IQ scores rose by an average of 19.5 points when adopted by high socioeconomic status (SES) families and 7.7 points by low SES families.[26] Based on the Duyme’s results and our study, evidence-based interventions to increase FSIQ of children should be considered, particularly for children from low income. Such evidence-based strategies have included parental interventions to improve the measure of the children’s cognition.[28,29]
Despite the high prevalence of poverty in the sickle cell anemia population, the magnitude of the influence of poverty on cognition was not previously well defined. Based on the results of this study, observational studies or clinical trials focused on cognition as an outcome, must take into consideration the home environment, particularly since approximately 60% of all children with sickle cell anemia in the United States receive public health insurance,[16,17] as was the case in this study. Another important finding in this study was that in children with and without SCIs, loss of FSIQ was associated with increasing age. Previously, our group demonstrated the presence of acute silent cerebral ischemia in asymptomatic children with sickle cell anemia,[30] and Dowling et al.[31] demonstrated that children with sickle cell anemia with a drop in their hemoglobin to less than 5.5 g/dl can also have asymptomatic acute silent cerebral ischemia. Our group has also shown that subgroups of children with sickle cell anemia with and without SCI have elevated plasma levels of GFAP, a known marker of stroke, suggesting the presence of subclinical brain injury.[32] In the only longitudinal study to date, an association of older age with lower verbal IQ was demonstrated13; however, the versions used for IQ testing were changed during the study, creating an inherent limitation when interpreting the significance of the data on IQ change. A meta-analysis of 15 cross-sectional studies of IQ among children with sickle cell disease also demonstrated a decrease in mean IQ score among children with an increasing age.[14] In addition, Vichinsky et al.[33] found that adults with sickle cell anemia and no neurological events had significantly lower performance IQ than controls and this worsened with older age. Together, these studies suggest a progressive decrease in cognitive abilities with age that may be secondary to ongoing chronic injury to the brain of children and adults with sickle cell anemia.
As was the case in this study, the relationship between low hemoglobin saturation and decreased cognitive performance in sickle cell anemia has been reported in at least two other studies.[34,35] Further evidence supporting the role of hemoglobin saturation in neurological morbidity are the observations that low hemoglobin oxygen saturation is associated with stroke[36,37] and abnormal cerebral arterial blood flow velocity.[12,38] Additional investigation is warranted to establish the extent of the relationship between arterial oxygen saturation of hemoglobin and cognition in children with sickle cell anemia.
Hemoglobin levels were not associated with FSIQ. The hemoglobin values were obtained upon study entry, and cognitive testing may have lagged by a few months. However, the hemoglobin values (mean 8.1 and 8.3 g/dl) represent the child at their most well state.
The observed five-point difference associated with SCI was as expected from the literature. Figure 3 summarizes the results of previous studies and their aggregate expected influence of FSIQ on children with and without SCIs. Based on the findings from longitudinal studies that have followed samples from adolescence into adulthood, a loss of IQ of five points is related to a significant decrease in educational attainment and a loss of 5% to 8.5% in annual income.[39] Further, a five-point loss in IQ is comparable with the effects of chronic cannabis use during adolescence on adult IQ[40] and is greater than the effect of prenatal and perinatal lead exposure on childhood IQ.[41] Given these findings and the well-established relationship between FSIQ and school achievement and career attainment,[42,43] the implications of lower FSIQ in this population of vulnerable children are even more significant. Functional status of the participants with a lower FSIQ was not assessed in the SIT Trial, but could be a component of a follow-up study.
Figure 3.
Meta-analyses for all studies in children with sickle cell anemia that included FSIQ for those with and without SCIs. The meta-analyses include a total of nine published studies and the current analysis that compares the mean difference in FSIQ between those children with sickle cell anemia with and without an SCI. The x-axis reflects the mean FSIQ difference between those with and without an SCI. The horizontal lines represent the upper and lower boundaries of the 95% confidence interval. If the 95% confidence interval overlaps zero or crosses the zero threshold then no statistical differences were observed in that study. The black and gray diamonds represent the results of the fixed and random effect models. The edges of the diamonds represent the 95% confidence interval of the meta-analyses for the fixed and random effect models.
Several limitations exist in this observational cross-sectional study. The SIT Trial cohort represents the largest group of children with sickle cell anemia who have been evaluated for neurological disease, with over 1000 children enrolled and screened with MRI of the brain; however, despite its size, children who participated in the trial did not represent the general population of children with sickle cell anemia in the United States. Participants were excluded if they were on or considering hydroxyurea therapy, had strokes or epilepsy, or were receiving regular blood transfusion therapy. The controls were children from only two of the 19 centers, one on the East coast and one in the Midwest. When comparing the current study controls (n=47) with controls from the SIT Trial screening phase (n=471), there were two statistically significant differences in the demographic variables. The controls from the SIT trial were more likely to have Medicaid for health coverage (72.6% vs. 53.5%, P=0.008) and had lower mean income per capita ($8,400 vs. $11,000, P=0.035). All other demographics were similar. From the original potential participants in the current analysis, 39 cases were excluded for missing data in key variables. When compared with the remaining 150, there were a few differences. Those dropped have heads of household with less college and slightly more unmarried parents. Since these differences make the remaining 150 cases more homogeneous on these variables, this is a conservative bias, as it should make the effects of education and income in the models less pronounced. Nevertheless, we believe that our results reflect the importance of both home and biological factors on FSIQ in sickle cell anemia.
In summary, among children with sickle cell anemia, the home environment is a major predictor of FSIQ and a suitable target for interventions to improve cognition and functional status, including parenting skills and early childhood education. While it is known that the educational attainment of parents is a strong predictor of the cognitive development of children in general, sickle cell disease also adversely affects the structure and function of the brain because of anemia and progressive ischemic injury starting in infancy. Our novel and important finding is that parents’ educational attainment is a stronger correlate of a child’s FSIQ even in the setting of cerebral infarcts. Future studies of children with sickle cell anemia and other children with acquired injury to the brain should consider characteristics of the home environment for both assessment and interventions.
Supplementary Material
Acknowledgments
AAK conducted the literature review, contributed to the study design, data collection, data interpretation, and wrote and edited the first draft of the manuscript with JJS. JJS contributed to the study design, data collection and interpretation, wrote the first draft of the manuscript with AAK and edited the manuscript. MJR and JPM conducted the data analysis, and participated in manuscript writing and editing. BEC contributed to the data analysis, data interpretation, and manuscript writing. JFC participated in the design of the SIT Trial, recruited patients, collected data, participated in data analysis and critically reviewed and edited the manuscript. RCM contributed in the study design of the SIT Trial, revision of the manuscript, and chaired the committee that adjudicated the image interpretation. MJN participated in study design, data interpretation, and writing of the manuscript. CTQ participated in collection of data, interpretation of data, and writing of the manuscript. RI participated in study design and review of the manuscript. MMD contributed data to the study, participated in the interpretation of the data, and critical review of the manuscript. MRD is the PI of the SIT Trial; he recruited patients, collected data, participated in data interpretation, and critically reviewed and edited the manuscript.
SIT Trial
5U01-NS042804-07 (MRD)
Doris Duke Charitable Foundation
K23HL079073 (AAK); K23HL078819 (JJS)
American Society of Hematology (JJS)
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Schatz J, Finke R, Kellett J, Kramer J. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol. 2002;27:739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]
- 2.DeBaun MR, Armstrong FD, McKinstry RC, et al. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–4596. doi: 10.1182/blood-2011-02-272682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146:300–305. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong FD, Thompson RJ, Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics. 1996;97:864–870. 6 Pt 1. [PubMed] [Google Scholar]
- 5.Steen RG, Reddick WE, Mulhern RK, et al. Quantitative MRI of the brain in children with sickle cell disease reveals abnormalities unseen by conventional MRI. J Magn Reson Imaging. 1998;8:535–543. doi: 10.1002/jmri.1880080304. [DOI] [PubMed] [Google Scholar]
- 6.Watkins KE, Hewes SKM, Connelly A, et al. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Develop Med Child Neurol. 1998;40:536–543. doi: 10.1111/j.1469-8749.1998.tb15412.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernaudin F, Verlhac S, Freard F, et al. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol. 2000;15:333–343. doi: 10.1177/088307380001500510. [DOI] [PubMed] [Google Scholar]
- 8.Schatz JCS, Koby M, DeBaun MR. A lesion analysis of visual orienting performance in children with cerebral vascular injury. Develop Neuropsychol. 2000;17:49–56. doi: 10.1207/S15326942DN1701_03. [DOI] [PubMed] [Google Scholar]
- 9.Schatz J, Craft S, Koby M, et al. Neuropsychologic deficits in children with sickle cell disease and cerebral infarction: role of lesion site and volume. Child Neuropsychol. 1999;5:92–103. [Google Scholar]
- 10.Schatz J, Brown RT, Pascual JM, et al. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–1111. doi: 10.1212/wnl.56.8.1109. [DOI] [PubMed] [Google Scholar]
- 11.Schatz J, Finke R, Roberts CW. Interactions of biomedical and environmental risk factors for cognitive development: a preliminary study of sickle cell disease. J Dev Behav Pediatr. 2004;25:303–310. doi: 10.1097/00004703-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Hogan AM, Pit-ten Cate IM, Vargha-Khadem F, et al. Physiological correlates of intellectual function in children with sickle cell disease: hypoxaemia, hyperaemia and brain infarction. Dev Sci. 2006;9:379–387. doi: 10.1111/j.1467-7687.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Enos L, Gallagher D, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:391–397. doi: 10.1067/mpd.2001.116935. [DOI] [PubMed] [Google Scholar]
- 14.Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol. 2002;27:739–748. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RJ, Jr., Gustafson KE, Bonner MJ, Ware RE. Neurocognitive development of young children with sickle cell disease through three years of age. J Pediatr Psychol. 2002;27:235–244. doi: 10.1093/jpepsy/27.3.235. [DOI] [PubMed] [Google Scholar]
- 16.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA: J Am Med Assoc. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 17.McCavit TL, Xuan L, Zhang S, Flores G, Quinn CT. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2013;60:823–827. doi: 10.1002/pbc.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27:69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez MA, Winkleby MA, Ahn D, et al. Identification of population subgroups of children and adolescents with high astluna prevalence: finding; from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2002;156:269–275. doi: 10.1001/archpedi.156.3.269. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler lntelligence Scale fur Oilldren Fourth Edition. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 21.Wechsler D. Wechsler Preschool and Primary Scale of IntelligenceTM–Third Edition (WPPSITM - III) The Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- 22.Vendt BA, McKinstry RC, Ball WS, et al. Silent Cerebral Infarct Transfusion (SIT) trial imaging core: application of novel imaging information technology for rapid and central review of MRI of the brain. J Digit Imaging. 2009;22:326–343. doi: 10.1007/s10278-008-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks-Gunn J, Klebanov PK, Duncan GJ. Ethnic differences in children's intelligence test scores: role of economic deprivation, home environment, and maternal characteristics. Child Dev. 1996;67:396–408. [PubMed] [Google Scholar]
- 24.Cserjesi R, Van Braeckel KN, Timmerman M, et al. Patterns of functioning and predictive factors in children born moderately preterm or at term. Dev Med Child Neurol. 2012;54:710–715. doi: 10.1111/j.1469-8749.2012.04328.x. [DOI] [PubMed] [Google Scholar]
- 25.Aran-Filippelli V, Richaud de Minzi MC. A structural analysis of executive functions and socioeconomic status in school-age children: cognitive factors as effect mediators. J Genetic Psychol. 2012;173:393–416. doi: 10.1080/00221325.2011.602374. [DOI] [PubMed] [Google Scholar]
- 26.Duyme M, Dumaret AC, Tomkiewicz S. How can we boost IQs of "dull children"?: A late adoption study. Proc Natl Acad Sci U S A. 1999;96:8790–8794. doi: 10.1073/pnas.96.15.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana Y, Fukushima A, Saito H, et al. A new mother-child play activity program to decrease parenting stress and improve child cognitive abilities: a clnster randomized controlled trial. PLoS One. 2012;7:e38238. doi: 10.1371/journal.pone.0038238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans GW, Ricciuti HN, Hope S, et al. Crowding and Cognitive Development: The Mediating Role of Maternal Responsiveness Among 36-Month-Old Children. Environ Behav. 2010;42:135–148. [Google Scholar]
- 29.Doan SN, Evans GW. Maternal responsiveness moderates the relationshlp between atic load and working memory. Dev Psychopathol. 2011;23:873–880. doi: 10.1017/S0954579411000368. [DOI] [PubMed] [Google Scholar]
- 30.Quinn CT, McKinstry RC, Dowling MM, et al. Acute silent cerebral ischemic events in children with sickle cell anemia. JAMA Neurol. 2013;70:58–65. doi: 10.1001/jamaneurol.2013.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling MM, Quinn CT, Plwnb P, et al. Acute silent cerebral ischemia and infarction druing acute anemia in children with and without sickle cell disease. Blood. 2012;120:3891–3897. doi: 10.1182/blood-2012-01-406314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savage WJ, Barron-Casella E, Fu Z, et al. Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am J Hematol. 2011;86:427–429. doi: 10.1002/ajh.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vichinsky EP, Neumayr LD, Gold JI, et al. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA: J Am Med Assoc. 2010;303:1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollocks MJ, Kok TB, Kirkham FJ, et al. Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. J Intern Neuropsychologic Soc: JINS. 2012;18:168–173. doi: 10.1017/S1355617711001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan AM, Kirkham FJ, Prengler M, et al. An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br J Haematol. 2006;132:99–107. doi: 10.1111/j.1365-2141.2005.05828.x. [DOI] [PubMed] [Google Scholar]
- 36.Kirkham FJ, Hewes DK, Prengler M, et al. Nocturnal hypoxaemia and central-nervoussystem events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 37.Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor fur overt stroke in children with sickle cell anaemia. Br J Haematol. 2008;140:336–339. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn CT, Variste J, Dowling MM. Haemoglobin oxygen saturation is a determinant of cerebral artery blood fiow velocity in children with sickle cell anaemia. Br J Haematol. 2009;145:500–505. doi: 10.1111/j.1365-2141.2009.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zagorsky JL. Do you have to be smart to be rich? The impact of IQ on weallh, income and flllancial distress. Intelligence. 2007;35:489–501. [Google Scholar]
- 40.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Nat Acad Sci USA. 2012;109:E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environ Heallh Perspect. 2004;112:987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deary IJ. Intelligence. Annu Rev Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- 43.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



