Abstract
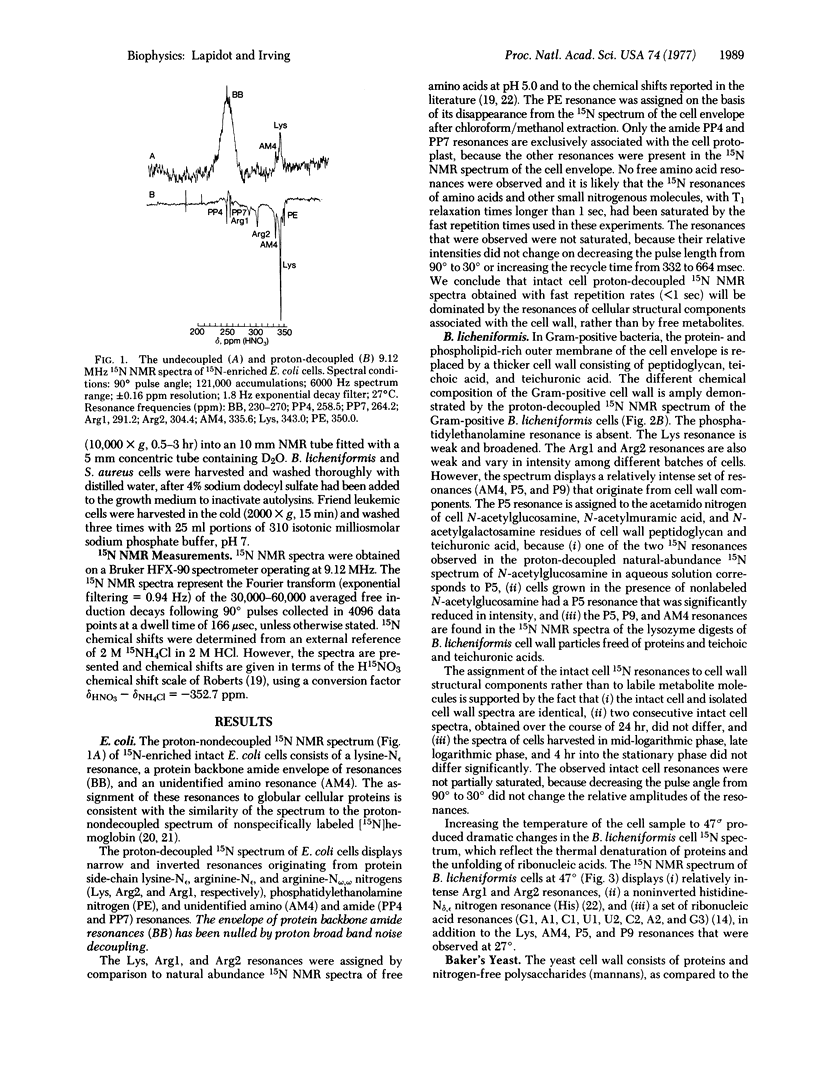
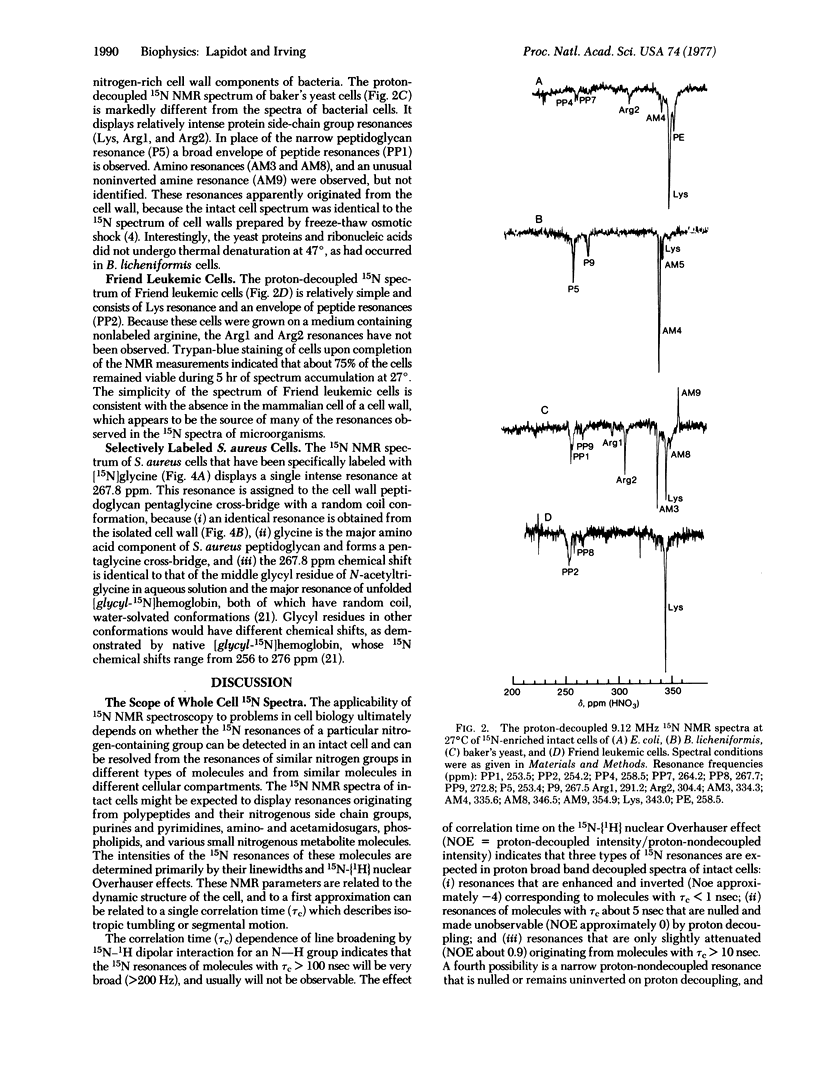
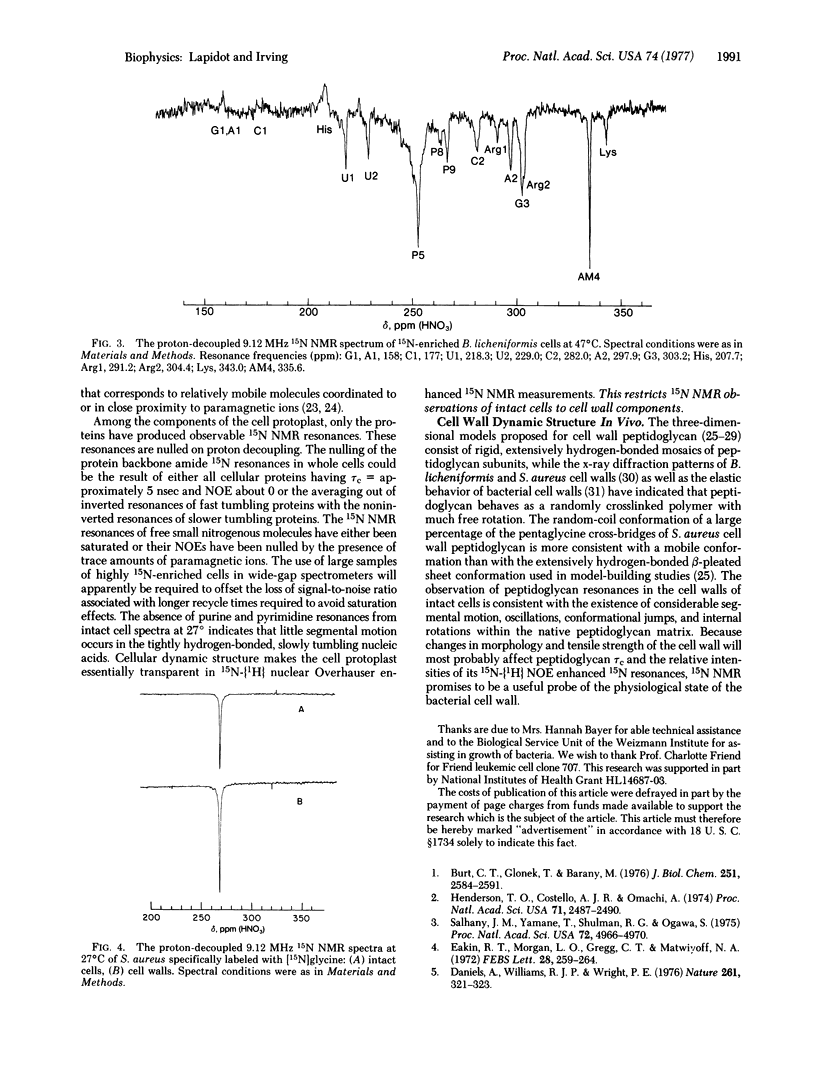
The proton-decoupled 15N Fourier transform nuclear magnetic resonance (NMR) spectra of 15N-enriched Escherichia coli, Bacillus licheniformis, baker's yeast, and Friend leukemic cells were obtained. The 15N NMR spectra of whole cells displayed 15N resonances originating from (i) protein backbones with lysine, arginine, and histidine side chains, (ii) ribonucleic acids, (iii) peptidoglycan, and (iv) phospholipids. Several additional amino and amide resonances were observed but not identified. In bacteria and yeast, the cell wall was found to be the site of a relatively mobile group of molecules, whose resonances dominate the proton-decoupled 15N NMR spectra of whole cells. 15N NMR chemical shifts and nuclear Overhauser effects have provided information on the in vivo structure of cell wall peptidoglycan. In Staphylococcus aureus the pentaglycine cross-bridge of cell wall peptidoglycan was found to have a random coil conformation. In B. licheniformis considerable segmental motional freedom was detected in teichuronic acid and peptidoglycan polysaccharide chains in the wall of the intact cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balyuzi H. H., Reaveley D., Burge R. E. X-ray diffraction studies of cell walls and peptidoglycans from Gram-positive bacteria. Nat New Biol. 1972 Feb 23;235(60):252–253. doi: 10.1038/newbio235252a0. [DOI] [PubMed] [Google Scholar]
- Blomberg F., Maurer W., Rüterjans H. 15N nuclear magnetic resonance investigations on amino acids. Proc Natl Acad Sci U S A. 1976 May;73(5):1409–1413. doi: 10.1073/pnas.73.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. F., Keiser H. Carbon-13 nuclear magnetic resonance study of chondroitin 4-sulfate in the proteoglycan of bovine nasal cartilage. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3421–3423. doi: 10.1073/pnas.72.9.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- COUTINHO C. B., NUTINI L. G. Correlation between the essential amino-acid requirements of Staphylococcus aureus, their phage types and antibiotic patterns. Nature. 1963 May 25;198:812–813. doi: 10.1038/198812a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Horsley W., Sternlicht H. The isolation of carbon-13 enriched amino acids. Biochim Biophys Acta. 1970 Nov 24;222(2):521–523. doi: 10.1016/0304-4165(70)90144-3. [DOI] [PubMed] [Google Scholar]
- Daniels A., Williams R. J., Wright P. E. Nuclear magnetic resonance studies of the adrenal gland and some other organs. Nature. 1976 May 27;261(5558):321–323. doi: 10.1038/261321a0. [DOI] [PubMed] [Google Scholar]
- Eakin R. T., Morgan L. O., Gregg C. T., Matwiyoff N. A. Carbon-13 nuclear magnetic resonance spectroscopy of living cells and their metabolism of a specifically labeled 13C substrate. FEBS Lett. 1972 Dec 15;28(3):259–264. doi: 10.1016/0014-5793(72)80726-9. [DOI] [PubMed] [Google Scholar]
- Formanek H., Formanek S., Wawra H. A three-dimensional atomic model of the murein layer of bacteria. Eur J Biochem. 1974 Jul 15;46(2):279–294. doi: 10.1111/j.1432-1033.1974.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Gust D., Moon R. B., Roberts J. D. Applications of natural-abundance nitrogen-15 nuclear magnetic resonance to large biochemically important molecules. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4696–4700. doi: 10.1073/pnas.72.12.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes G. E., Randall E. W., Bradley C. H. Theory and practice for studies for peptides by 15N nuclear magnetic resonance at natural abundance: gramicidin S. Nature. 1975 Oct 30;257(5529):767–772. doi: 10.1038/257767a0. [DOI] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen M. V., Rogers H. J. Three-dimensional molecular models of bacterial cell wall mucopeptides (peptidoglycans). Proc Natl Acad Sci U S A. 1971 May;68(5):992–996. doi: 10.1073/pnas.68.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás M., Wüthrich K., Schwotzer W., Von Philipsborn W. 15N nuclear magnetic resonance of living cells. Nature. 1975 Oct 30;257(5529):817–818. doi: 10.1038/257817a0. [DOI] [PubMed] [Google Scholar]
- Oldmixon E. H., Glauser S., Higgins M. L. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers. 1974;13(10):2037–2060. doi: 10.1002/bip.1974.360131008. [DOI] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Coccal cell-wall compactness and the swelling action of denaturants. Can J Microbiol. 1972 May;18(5):623–629. doi: 10.1139/m72-099. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson K. L., Roberts J. D. Conformational analysis by nuclear magnetic resonance. Nitrogen-15 and carbon-13 spectra of lactams. J Am Chem Soc. 1976 Aug 18;98(17):5082–5086. doi: 10.1021/ja00433a005. [DOI] [PubMed] [Google Scholar]


