Abstract
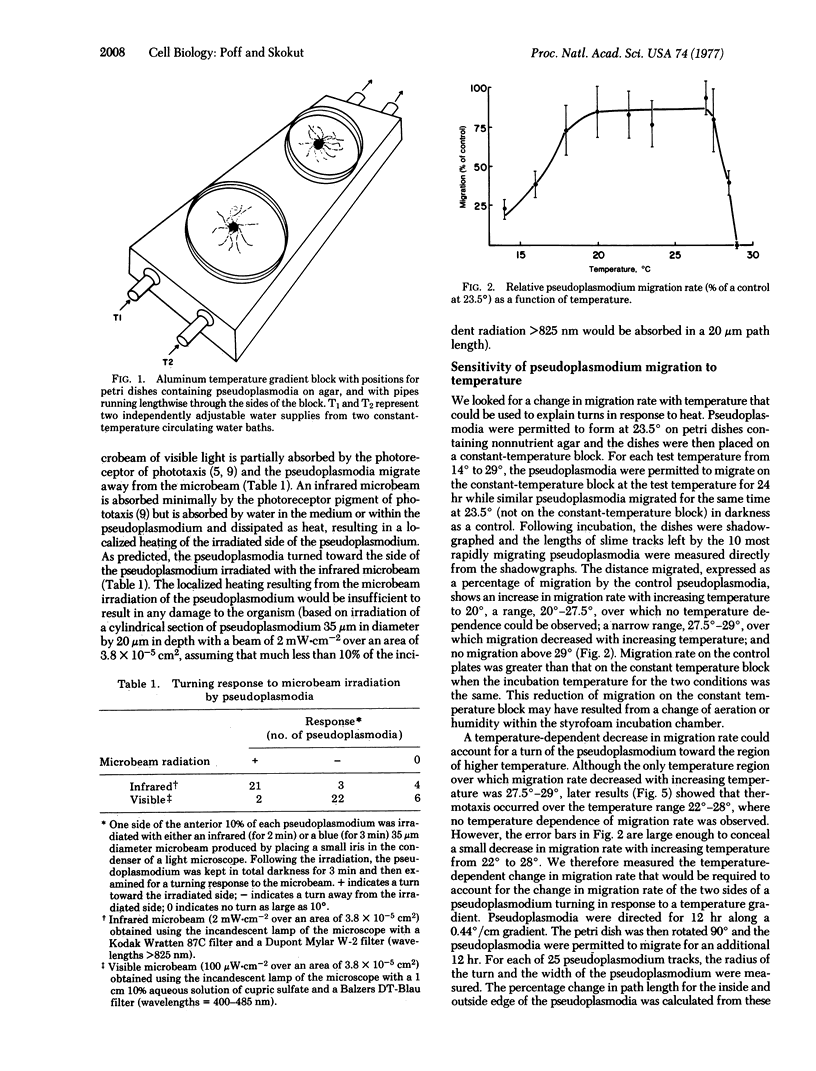
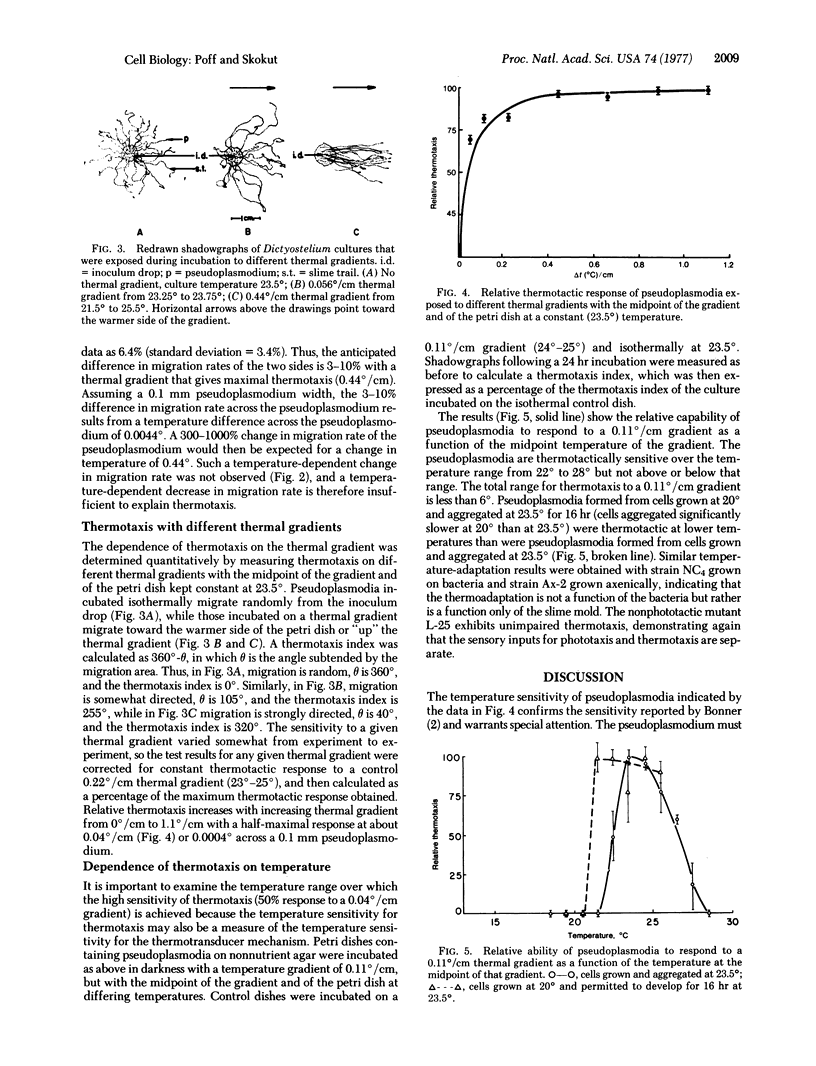
The temperature dependence of migration rate and of the thermotactic sensitivity of pseudoplasmodia of Dictyostelium discoideum has been measured. Migration rate increases with temperature to 20 degrees, is temperature insensitive from 20 degrees to 27.5 degrees, and decreases with temperature to 29 degrees, above which point migration ceases. However, pseudoplasmodia formed from cells grown at 23.5 degrees are thermotactic only from 22 degrees to 27.5 degrees. Thus, a temperature dependence of migration rate is not sufficient to explain thermotaxis. Because random lateral movements by the pseudoplasmodia have not been observed, the measurement of the temperature gradient appears to be spatial rather than temporal, with a half-maximal thermotactic response to a temperature gradient of about 0.04 degree/cm, or 0.0004 degree across an average pseudoplasmodium. Thermotactic sensitivity is adaptive, with pseudoplasmodia formed from cells grown at 20 degrees capable of thermotaxis at temperatures lower than cells grown at 23.5 degrees.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- BONNER J. T., CLARKE W. W., Jr, NEELY C. L., Jr, SLIFKIN M. K. The orientation to light and the extremely sensitive orientation to temperature gradients in the slime mold Dictyostelium discoideum. J Cell Physiol. 1950 Oct;36(2):149–158. doi: 10.1002/jcp.1030360203. [DOI] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- FRANCIS D. W. SOME STUDIES ON PHOTOTAXIS OF DICTYOSTELIUM. J Cell Physiol. 1964 Aug;64:131–138. doi: 10.1002/jcp.1030640113. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Russell R. L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Lorch S. K., Smith G. G., Haug A. Control of membrane lipid fluidity in Acholeplasma laidlawii. FEBS Lett. 1974 Jul 1;43(1):1–5. doi: 10.1016/0014-5793(74)81090-2. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr Mutants in phototaxis of Dictyostelium discoideum. Nature. 1970 Aug 15;227(5259):745–746. doi: 10.1038/227745a0. [DOI] [PubMed] [Google Scholar]
- Poff K. L., Butler W. L., Loomis W. F., Jr Light-induced absorbance changes associated with phototaxis in Dictyostelium. Proc Natl Acad Sci U S A. 1973 Mar;70(3):813–816. doi: 10.1073/pnas.70.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff K. L., Loomis W. F., Jr, Butler W. L. Isolation and purification of the photoreceptor pigment associated with phototaxis in Dictyostelium discoideum. J Biol Chem. 1974 Apr 10;249(7):2164–2167. [PubMed] [Google Scholar]
- Poff K. L., Loomis W. F., Jr Control of phototactic migration in Dictyostelium discoideum. Exp Cell Res. 1973 Nov;82(1):236–240. doi: 10.1016/0014-4827(73)90266-8. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Miyamoto H. Sensitivity of Paramecium thermotaxis to temperature change. J Protozool. 1973 May;20(2):209–292. [PubMed] [Google Scholar]
- Tawada K., Oosawa F. Responses of Paramecium to temperature change. J Protozool. 1972 Feb;19(1):53–57. doi: 10.1111/j.1550-7408.1972.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Tso W. W., Mansour T. E. Thermotaxis in a slime mold, Physarum polycephalum. Behav Biol. 1975 Aug;14(4):499–504. doi: 10.1016/s0091-6773(75)90672-0. [DOI] [PubMed] [Google Scholar]


