Abstract
The process of amyloid formation by the normally soluble hormone islet amyloid polypeptide (IAPP) contributes to β-cell death in type 2 diabetes and in islet transplants. There are no clinically approved inhibitors of islet amyloidosis, and the mode of action of existing inhibitors is not well-understood. Resveratrol, a natural polyphenol, has been reported to inhibit amyloid formation by IAPP and by the Alzheimer’s disease Aβ peptide. The mechanism of action of this compound is not known, nor is its mode of interaction with IAPP. In this study, we use a series of IAPP variants to examine possible interactions between resveratrol and IAPP. Fluorescence assays, transmission electron microscopy, and mass spectrometry demonstrate that resveratrol is much less effective as an inhibitor of IAPP amyloid formation than the polyphenol (−)-epigallocatechin 3-gallate (EGCG) and, unlike EGCG, does not significantly disaggregate preformed IAPP amyloid fibrils. Resveratrol is also shown to interfere with thioflavin-T assays. His-18 mutants, a truncation mutant, mutants of each of the aromatic residues, and mutants of Arg-11 of IAPP were examined. Mutation of His to Gln or Leu weakens the ability of resveratrol to inhibit amyloid formation by IAPP, as do mutations of Arg-11, Phe-15, or Tyr-37 to Leu, and truncation to form the variant Ac 8−37-IAPP, which removes the first seven residues to eliminate Lys-1 and the N-terminal amino group. In contrast, replacement of Phe-23 with Leu has a smaller effect. The data highlight Phe-15, His-18, and Tyr-37 as being important for IAPP–resveratrol interactions and are consistent with a potential role of the N-terminus and Arg-11 in polypeptide–resveratrol interactions.
Islet amyloidosis, caused by the pathological aggregation of human islet amyloid polypeptide (IAPP, amylin) in the pancreatic islets of Langerhans, contributes to β-cell dysfunction in type 2 diabetes.1−6 Amyloid formation by IAPP also plays a role in the failure of islet transplants, while the prevention of islet amyloidosis prolongs graft survival.7−9 IAPP is produced as a pro-hormone, is processed in parallel with insulin, and is stored in the insulin secretory granule from which it is released by the same stimuli that lead to insulin secretion.10 The mature polypeptide is 37 residues long and contains an amidated C-terminus and a disulfide bridge between residues 2 and 7 (Figure 1). IAPP normally acts as a partner to insulin in glucose metabolism but forms amyloid in type 2 diabetes.11 There are no clinically approved inhibitors of islet amyloidosis despite its therapeutic relevance, and the mode of action of existing inhibitors of in vitro toxicity is not well-understood.
Figure 1.
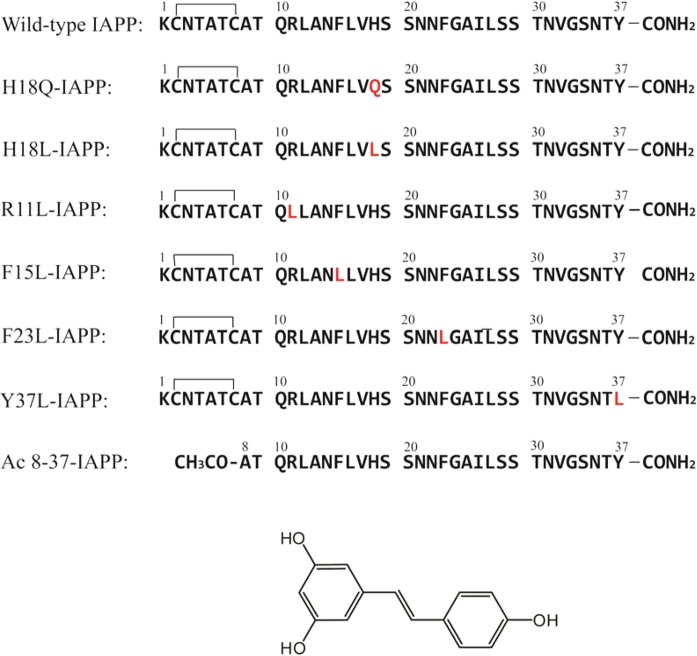
Primary sequences of IAPP and the IAPP variants studied here. The residues that differ from those of wild-type IAPP are colored red. Wild-type IAPP and IAPP variants, with the exception of Ac 8–37-IAPP, all contain a disulfide bond between Cys-2 and Cys-7. All peptides contain an amidated C-terminus. The structure of resveratrol is also shown.
Polyphenols, a class of organic compounds with aromatic phenolic rings, have drawn particular attention as inhibitors of amyloid formation, including the inhibition of IAPP amyloid. For example, (−)-epigallocatechin 3-gallate (EGCG), the most abundant catechin in green tea, inhibits amyloid formation by Aβ, α-synuclein, IAPP, and other polypeptides and protects cultured β-cells against the toxic effects of human IAPP.12−19 EGCG is one of the most effective inhibitors of amyloid formation by IAPP known and disaggregates and remodels IAPP amyloid fibrils to smaller species.13,16,18 The compound is believed to divert amyloidogenic peptides into off-pathway aggregates that are incapable of further assembly to form amyloid.14,17,19 Resveratrol, a polyphenol present in red wine, has received considerable attention in the context of neurodegenerative diseases because of its antineuroinflammatory activity and because of its ability to inhibit amyloid formation by Aβ, the peptide linked to extracellular amyloid plaques in Alzheimer’s disease (Figure 1).20−23 Resveratrol has been reported to inhibit IAPP amyloid formation and to protect against in vitro toxicity in cell culture, although it is not known if its ability to protect cells is due to the direct inhibition of interactions between IAPP toxic species and cells.24−26 Little is known about the mode of interaction of resveratrol with IAPP and its efficacy compared to those of EGCG. Indeed, little is known about the mechanism of any anti-IAPP amyloid agent.
Recent nuclear magnetic resonance (NMR) studies, which made use of a nonphysiological analogue of IAPP that lacks the normal amidated C-terminus, have led to the proposal that Lys-1 and His-18 are involved in the binding of resveratrol.27 The NMR spectra revealed that resonances from the side chain of His-18 exhibited the largest changes during a titration with resveratrol. On the basis of this work His-18 was proposed to be critical for resveratrol–IAPP interactions. Chemical shift changes for Lys-1 were also detected, suggesting that this residue could be a second site for resveratrol–IAPP interactions. However, variants of IAPP with a free C-terminus exhibit behavior different from that of the physiological amidated form and assemble into amyloid on different time scales.28 Other work has highlighted the possible role of interactions between aromatic side chains and amyloid inhibitors,29 but this has not been examined for IAPP and resveratrol. π–cation interactions are important in stabilizing globular proteins and IAPP contains three or four positive charges depending on the pH: the N-terminus, Lys-1, Arg-11, and His-18. Thus, there is also the potential for interactions of the aromatic rings of polyphenols with the positively charged sites in IAPP. In this study we compare the ability of resveratrol and EGCG to inhibit IAPP amyloid formation, examine the interaction of resveratrol with a series of IAPP variants designed to test the role of the aromatic residues and the possible role of Arg-11, His-18, and the N-terminal disulfide-bridged loop, and critically examine the ability of resveratrol to remodel preformed amyloid fibrils.
Materials and Methods
Peptide Synthesis, Purification, and Sample Preparation
Peptides were synthesized on a 0.1 or 0.25 mmol scale using 9-fluoronylmethoxycarbonyl (Fmoc) chemistry on a CEM Liberty microwave peptide synthesizer. 5-(4′-Fmoc-aminomethyl-3′,5-dimethoxyphenol)valeric acid (Fmoc-PAL-PEG-PS) resin was used to incorporate an amidated C-terminus. Acetic anhydride was used to generate an acetylated N-terminus for the truncated 8–37 IAPP fragment. Fmoc-protected pseudoproline dipeptide derivatives were incorporated at positions 9, 10, 19, 20, 27, and 28 to facilitate the synthesis.30 β-Branched residues, Arg, and all pseudoproline dipeptide derivatives were doubly coupled. A maximal temperature of 50 °C was used for the coupling of His and Cys to reduce the possibility of racemization.31 Peptides were cleaved from the resin by standard trifluoroacetic acid (TFA) methods. Crude peptides were partially dissolved in 20% acetic acid (v/v) and lyophilized. The dry peptide was redissolved in pure dimethyl sulfoxide (DMSO) at room temperature to promote the formation of the disulfide bond.32 Peptides were purified by reverse-phase high-performance liquid chromatography (HPLC) using a Vydac or Proto 300 C18 preparative column (10 mm × 250 mm). A two-buffer gradient system was used. Buffer A consisted of 100% H2O and 0.045% HCl (v/v), and buffer B consisted of 80% acetonitrile, 20% H2O, and 0.045% HCl. HCl was used as the counterion because residual TFA can influence amyloid formation. Analytical HPLC was used to check the purity of peptides before their use. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry confirmed the correct molecular weight: IAPP, expected 3903.3, observed 3902.8; H18Q-IAPP, expected 3894.3, observed 3894.4; R11L-IAPP, expected 3860.3, observed 3860.9; H18L-IAPP, expected 3879.3, observed 3879.3; F15L-IAPP, expected 3871.3, observed 3872.0; F23L-IAPP, expected 3871.3, observed 3871.3; Y37L-IAPP, expected 3855.4, observed 3853.8; Ac 8–37 IAPP, expected 3225.5, observed 3225.1. Pure peptides were first dissolved in 100% hexafluoroisopropanol (HFIP) at a concentration of 1.6 mM and filtered to remove preformed aggregates. For kinetic studies, aliquots were lyophilized for 20–24 h to remove HFIP. Resveratrol stock solutions were freshly prepared in pure DMSO.
Thioflavin-T Fluorescence Assays
Lyophilized peptides were dissolved in a pH 7.4, 20 mM Tris buffer solution containing thioflavin-T and resveratrol at a final peptide concentration of 16 μM; 1% (v/v) DMSO was present in all solutions. Measurements were taken at 25 °C using a Beckman Coulter DTX880 plate reader. An excitation wavelength of 430 nm and an emission wavelength of 485 nm were used. For amyloid fibril remodeling studies, preformed fibrils were produced in advance. Thioflavin-T fluorescence was continuously monitored after the addition of resveratrol.
Transmission Electron Microscopy (TEM)
TEM was performed at the Life Science Microscopy Center at Stony Brook University. Aliquots removed from the fluorescence experiments were used for TEM analysis. Five microliters of the peptide solution was placed on a carbon-coated Formvar 300 mesh copper grid for 1 min and then negatively stained with saturated uranyl acetate for an additional 1 min.
Electrospray Ionization Mass Spectrometry (ESI-MS)
Lyophilized peptides were dissolved in a pH 7.4, 50:50 20 mM ammonium acetate/20 mM ammonium bicarbonate buffer solution containing EGCG or resveratrol at a final peptide concentration of 32 μM; 1% (v/v) DMSO was present in all solutions. A Synapt HDMS quadrupole time-of-flight mass spectrometer (Micromass UK Ltd., Waters Corp., Manchester, U.K.), equipped with a Triversa (Advion Biosciences, Ithaca, NY) automated nano-ESI interface, was used for these analyses. A sampling cone voltage of 30 V was used, and an instrumental backing pressure of 2.0 mbar was applied to preserve protein–ligand interactions. Data were acquired over the range of m/z 400–6000.
Results and Discussion
The primary sequences of wild-type IAPP and the IAPP variants studied here are shown in Figure 1. The His-18 to Gln mutant (H18Q-IAPP) and the His-18 to Leu mutant (H18L-IAPP) allow us to test the role of His-18 in IAPP–resveratrol interactions. A Gln substitution was chosen because Gln has a volume and a hydrophobicity roughly similar to that of a neutral His residue, while Leu represents a more hydrophobic substitution. We used an IAPP fragment, residues 8–37 with an acetylated N-terminus (denoted Ac 8–37-IAPP), to test the importance of potential interactions with the positively charged Lys side chain and N-terminal amino group. This peptide is known to form amyloid with a morphology similar to that observed for wild-type IAPP.16 An Arg-11 to Leu mutant allows us to probe the role of this residue. Interactions between phenolic compounds and aromatic residues in amyloidogenic proteins have been suggested to be important for inhibitory effects, and aromatic cation interactions can be energetically favorable.29,33 IAPP contains three aromatic residues, two Phe’s and one Tyr. We prepared three point mutants, F15L-IAPP, F23L-IAPP, and Y37L-IAPP, to test the possible importance of interactions between resveratrol and aromatic amino acid side chains. The His and Arg mutants together with the truncation mutant allow us to probe potential π–cation interactions between the aromatic rings of the polyphenol and the positively charged sites in IAPP.
We first examined the stability of resveratrol at pH 7.4 as there have been differing reports about its stability in solution. NMR studies suggest that it can degrade in solution with a half-life on the order of 5 h. We used ESI-MS to examine the effects of a 24 h incubation of the compound at pH 7.4. No change in the m/z ratio was observed, and the value of (m + H), 229.04, corresponding to a molecular weight of 228.04, indicates that the compound had not degraded in this time frame (Supporting Information).41,42
The rate of amyloid formation was monitored initially using thioflavin-T fluorescence assays. Thioflavin-T is a small dye that undergoes an increase in fluorescence intensity upon binding to amyloid fibrils.34 Thioflavin-T can be used to monitor the kinetics of IAPP amyloid formation without altering the rate of aggregation under the conditions used.35 However, the use of the dye can be problematic for inhibition studies, because some compounds can compete for thioflavin-T binding or quench thioflavin-T fluorescence and thereby bias the detection of amyloid fibrils.36,37 Resveratrol, the compound tested in these studies, has no significant absorption within the range of thioflavin-T fluorescence, but this does not mean that it will not interfere with thioflavin-T assays. One earlier study has shown that resveratrol reduces thioflavin-T fluorescence when it is added to preformed Aβ fibrils, but does not affect the thioflavin-T intensity of carboxymethylated κ-casein fibrils, suggesting that resveratrol might displace the bound thioflavin-T from Aβ fibrils.36 However, it is not known if resveratrol interferes with the binding of thioflavin-T to IAPP amyloid fibrils. Thus, complementary TEM studies were performed to ensure that the results were not biased by interference between thioflavin-T and resveratrol.
Resveratrol Prolongs the Lag Phase of IAPP Amyloid Formation but Interferes with Thioflavin-T Assays
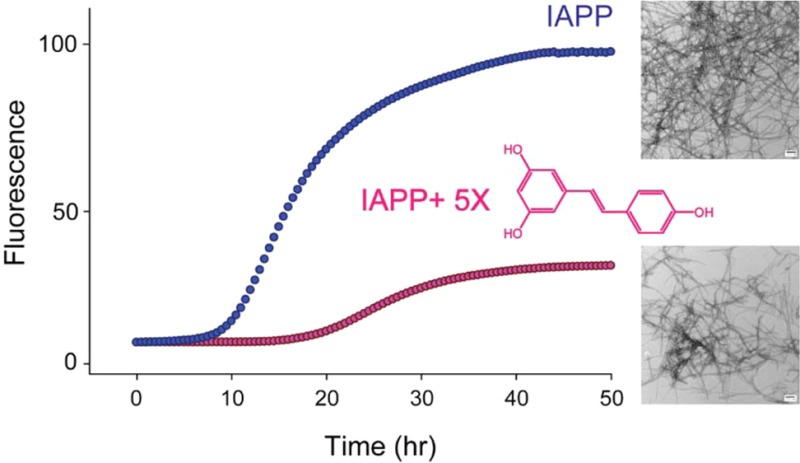
We found that resveratrol slows amyloid formation by wild-type IAPP in a dose-dependent manner, but the effects are very modest (Figure 2A); 1:1 and 1:2 mixtures of wild-type IAPP with resveratrol exhibit a lag phase that is similar to that of wild-type IAPP in the absence of the inhibitor. The final thioflavin-T fluorescence intensity is greatly reduced decreasing by approximately 30 and 60%, respectively. TEM studies, described in detail below, show that at least part of this effect is due to the interference of resveratrol with thioflavin-T assays. The lag phase is increased by a factor of only 1.5 relative to the control for a 1:5 mixture of wild-type IAPP and resveratrol. The effect of resveratrol on the lag phase is very modest compared with that observed for EGCG, which essentially abolishes amyloid formation by IAPP at a 1:1 molar ratio, for experiments conducted at 16 μM IAPP.16,17 When the resveratrol concentration is increased to a 10- or 20-fold excess there is no detectable thioflavin-T fluorescence intensity. The decrease in fluorescence intensity upon the addition of resveratrol may be due to the formation of fewer fibrils, to the interference of resveratrol with thioflavin-T fluorescence, or to effects of resveratrol on thioflavin-T binding. TEM images recorded after incubation for 50 h for samples with a 10- or 20-fold excess of resveratrol show fewer fibrils, and those that are present appear to be thinner. In contrast, extensive mats of fibrils are observed at this time point when resveratrol is absent (Figure 2B–G). These observations confirm that resveratrol retards IAPP amyloid formation under the conditions examined here. However, large amounts of IAPP fibrils are observed in the 1:10 and 1:20 IAPP/resveratrol samples after incubation for 126 h even though there is no increase in the magnitude of the thioflavin-T signal (Supporting Information). These data conclusively show that resveratrol interferes with thioflavin-T assays of IAPP amyloid formation.
Figure 2.
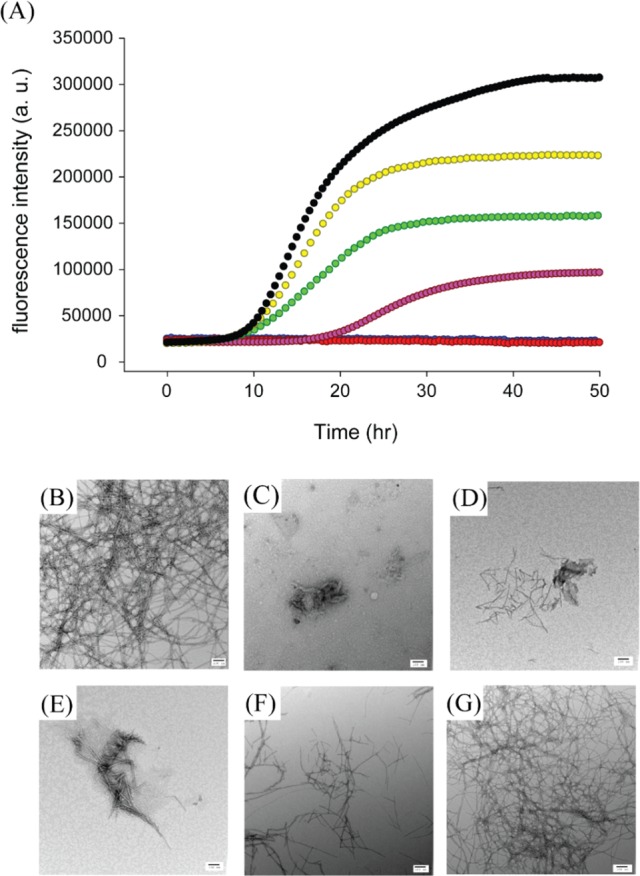
Resveratrol reduces the rate of amyloid formation by wild-type IAPP. (A) Thioflavin-T fluorescence-monitored kinetic experiments for wild-type IAPP (black) and wild-type IAPP and resveratrol at a 1:1 (yellow), 1:2 (green), 1:5 (pink), 1:10 (red), or 1:20 ratio (blue). The red and blue curves overlap. (B) TEM image of wild-type IAPP after incubation for 50 h. (C–G) TEM images of wild-type IAPP with resveratrol at a 1:20 ratio after incubation for 42, 50, 66, 96, and 126 h, respectively. Samples contained 16 μM IAPP in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
ESI-MS Reveals That Resveratrol–IAPP Interactions Are Weaker Than EGCG–IAPP Interactions
We conducted ESI-MS studies to further probe the interaction of resveratrol with IAPP. ESI-MS has been shown to be able to detect interactions between small molecules and monomeric and oligomeric IAPP.17 In the case of EGCG, ESI-MS revealed that the compound binds to monomeric IAPP (Figure 3) and perturbs self-association of the monomeric peptide into higher-order amyloid assemblies.17 EGCG forms covalent Schiff base adducts with some amyloidogenic proteins, but this is not required for the inhibition of amyloid formation by IAPP.16−18 The ESI-MS data indicate that under the conditions of these studies, EGCG interacts with IAPP but does not form a covalent complex. Quite different results were obtained when mixtures of resveratrol and IAPP were examined. In the case of resveratrol, binding to monomeric IAPP was not observed in the mass spectrum, despite the compound being present at a 20-fold molar excess over the peptide. It is clear from these data that EGCG binds either more favorably, more stably, or to a greater extent to monomeric IAPP than resveratrol. These results help to rationalize why EGCG is a much more effective inhibitor of IAPP amyloid formation than resveratrol.
Figure 3.
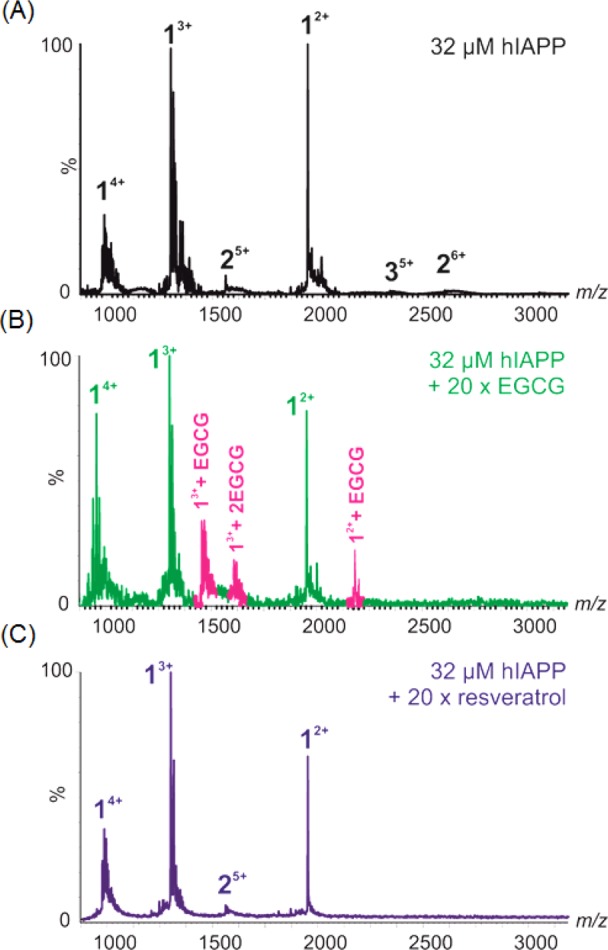
ESI-MS spectra showing (A) wild-type IAPP alone (32 μM, 50:50 20 mM ammonium acetate/20 mM ammonium bicarbonate buffer at pH 7.4) or in the presence of (B) 640 μM EGCG or (C) resveratrol. Numbers adjacent to peaks denote oligomer order, with the positive charge state of each oligomer ions in superscript. Wild-type IAPP monomer exhibits +2, +3, and +4 charge states (labeled 1) and minor amounts of dimer (labeled 2). EGCG binds to both the +2 and +3 charge state ions of IAPP monomer (bound peaks colored pink). No binding of resveratrol to the IAPP monomer is observed.
His-18 Is Important for Resveratrol–IAPP Interactions, and Arg-11 Might Play a Role
We next investigated the effects of resveratrol on amyloid formation by two His-18 IAPP variants, H18Q-IAPP and H18L-IAPP. Both of these mutants have been shown to accelerate amyloid formation.28 The compound had a weaker effect on these mutants than it did on wild-type IAPP; 1:1 and 1:2 mixtures of H18Q-IAPP and resveratrol have similar lag times and display the same final fluorescence intensities that were observed for H18Q-IAPP alone (Figure 4A). The lag phase of the 1:5 H18Q-IAPP/resveratrol mixture is only slightly longer than that of the H18Q-IAPP control, and the final intensity is ∼30% lower than that of H18Q-IAPP alone. Addition of either 10- or 20-fold excess of resveratrol also had a weaker effect on H18Q-IAPP aggregation than it did on wild-type IAPP. A 10-fold excess of resveratrol increased the lag phase of H18Q-IAPP by a factor of only 1.8, and dense mats of amyloid fibrils were observed in the TEM images (Figure 4B,C). Although the final thioflavin-T intensity of H18Q-IAPP in the presence of a 20-fold excess of resveratrol is greatly decreased, the kinetic curve is still sigmoidal with a lag phase only 2.4-fold longer than that observed for H18Q-IAPP alone. TEM images confirmed the presence of amyloid fibrils in these samples (Figure 4D). Collectively, the data show that mutation of His-18 significantly affects the ability of the resveratrol to modulate IAPP amyloid formation.
Figure 4.
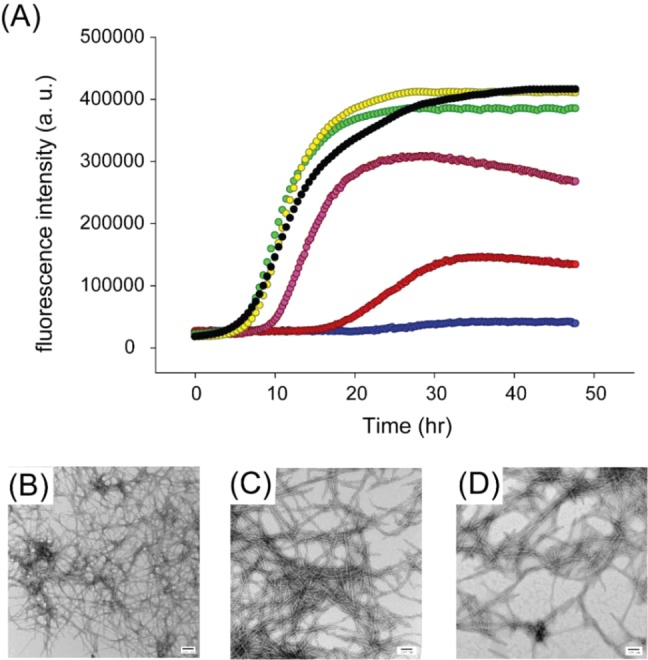
Resveratrol is not an effective inhibitor of amyloid formation by H18Q-IAPP. (A) Thioflavin-T-monitored kinetic experiments with H18Q-IAPP (black) and H18Q-IAPP and resveratrol at a 1:1 (yellow), 1:2 (green), 1:5 (pink), 1:10 (red), or 1:20 ratio (blue). (B) TEM image of the amyloid fibrils formed by H18Q-IAPP. (C) TEM image of the 1:10 mixture of H18Q-IAPP and resveratrol. (D) TEM image of the 1:20 mixture of H18Q-IAPP and resveratrol. Samples were collected for TEM at 48 h and contained 16 μM IAPP in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
Resveratrol is even less effective at inhibiting amyloid formation by H18L-IAPP. The mutant forms amyloid more rapidly than wild-type IAPP, and the kinetic curves quickly reach a plateau for all conditions tested (Supporting Information). TEM images recorded for the 1:20 H18L-IAPP/resveratrol mixture revealed large amounts of amyloid fibrils. The mechanism of IAPP amyloid formation is not known in sufficient detail to offer an atomic level description of the consequences of the H18L mutant on amyloid formation by IAPP or on the binding of IAPP to resveratrol. Nevertheless, the H18L-IAPP data are consistent with the H18Q-IAPP results and with the proposed role of His-18 in resveratrol–IAPP interactions.27
We next examined the R11L mutant of IAPP. This polypeptide rapidly forms amyloid under the conditions of our studies with a lag time on the order of 0.9 h in the absence of resveratrol compound to 10.0 h for wild-type IAPP. The enhanced rate of aggregation can be rationalized by the existing structural modes of IAPP fibrils; they place Arg-11 in the first β-strand, and the parallel, in-register arrangement of the cross-β structure is expected to lead to electrostatic repulsions.43,44
A 5-fold addition of resveratrol increased the lag time of the R11L mutant by a factor of only 1.5–1.6, comparable to the effect observed for resveratrol on wild-type IAPP. The effect on the final fluorescence intensity is less, but as previously noted, there is not a direct relationship between the intensity of a thioflavin-T assay and the amount of amyloid fibrils formed because the final intensity is related to the amount of thioflavin-T bound and its quantum yield. The increased thioflavin-T intensity allows the full curve to be monitored in the presence of a 20-fold excess of the compound. In this case, the lag time is increased only 2.7–2.8-fold relative to that of R11L-IAPP in the absence of resveratrol (Supporting Information). Comparative analysis of TEM images, described in subsequent sections, collected at 2 and 3 times the value of t50, where t50 is the time required to reach 50% of the maximal thioflavin-T signal, suggests that the mutation does modulate the ability of resveratrol to inhibit IAPP amyloid formation.
The Three Aromatic Residues in IAPP Make Different Contributions to Interactions with Resveratrol
Wild-type IAPP contains three aromatic residues, Phe-15, Phe-23, and Tyr-37. Aromatic–aromatic interactions are not required for IAPP amyloid formation, although their removal does slow the process.38,39 Aromatic interactions have been proposed to play an important role in amyloid formation and in IAPP–small molecule interactions,33 but it is not known whether aromatic interactions are important for resveratrol binding. The effects of single aromatic to Leu mutations on IAPP amyloid formation have been previously examined in the absence of resveratrol. All three single aromatic to Leu mutants formed amyloid fibrils, and the mutations did not alter the fibril morphology.39 This collection of mutants provides a convenient tool for testing the potential role of aromatic interactions in the interaction of IAPP with resveratrol.
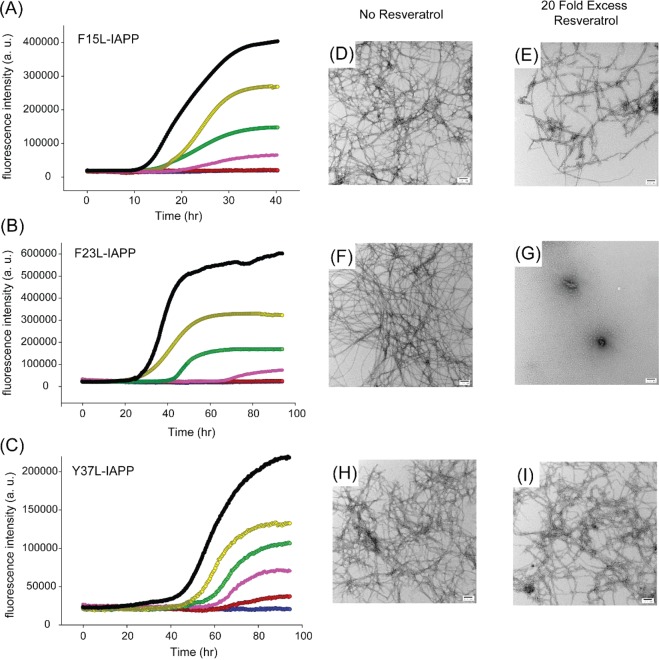
Thioflavin-T fluorescence-monitored kinetic curves were recorded for the set of aromatic to Leu mutants in the absence and presence of different amounts of resveratrol and are displayed in Figure 5. The effects of resveratrol on amyloid formation by F15L-IAPP are similar to those observed for wild-type IAPP at the lower ratios of resveratrol but are different at higher ratios (Figure 5A). The lag phase is slightly increased for the 1:5 mixture compared to that of the F15L-IAPP control. Only a few nonfibrillar aggregates were observed when wild-type IAPP was incubated at 42 h and for 50 h in the presence of a 20-fold excess of resveratrol (Figure 2). In contrast, amyloid fibrils, albeit ones that were shorter and thinner, were found in the 1:20 mixture of F15L-IAPP and resveratrol (Figure 5D,E). This indicates that the Phe-15 mutant affects IAPP–resveratrol interactions and suggests that Phe-15 plays a role in the interaction of resveratrol with IAPP. Different results were observed with the F23L mutant. This substitution did not significantly impact the ability of resveratrol to slow amyloid formation by IAPP, indicating that an aromatic residue at position 23 is not required for IAPP–resveratrol interactions (Figure 5B). Resveratrol slowed F23L-IAPP amyloid formation in a dose-dependent manner. The lag phase of F23L-IAPP increased when a 5-fold excess of resveratrol was present. TEM images recorded for the 1:20 mixture of F23L-IAPP and resveratrol at 96 h reveal that no detectable fibrils were formed, showing that resveratrol retards F23L-IAPP amyloid formation (Figure 5F,G). Further incubation for a total time of 142 h revealed the presence of fibrils (Supporting Information). In contrast, resveratrol had no significant effect on Y37L-IAPP amyloid formation (Figure 5C). Although the final fluorescence intensity of the Y37L-IAPP kinetic experiments gradually decreased upon addition of resveratrol, the length of the lag phase was only 20% longer in the presence of a 10-fold excess of resveratrol. TEM images show that large amounts of Y37L-IAPP fibrils were found even at high ratios of resveratrol as early as 96 h (Figure 5H,I). The data show that Tyr-37 is important for resveratrol–IAPP interactions because mutation of this residue reduces the effect of the compound on IAPP amyloid formation. Mutation of Tyr-37 has a stronger effect on the ability of the compound to slow IAPP amyloid formation than mutation of Phe-15 as judged by the t50 values (Supporting Information).
Figure 5.
Three aromatic residues in IAPP interact differently with resveratrol. Thioflavin-T fluorescence-monitored kinetic experiments with (A) F15L-IAPP, (B) F23L-IAPP, and (C) Y37L-IAPP in the presence of resveratrol. Experiments were conducted at 25 °C and pH 7.4 in 20 mM Tris buffer, 16 μM mutants, and 1% (v/v) DMSO without stirring: (black) peptide alone, (yellow) peptide with resveratrol at a 1:1 ratio, (green) peptide with resveratrol at a 1:2 ratio, (pink) peptide with resveratrol at a 1:5 ratio, (red) peptide with resveratrol at a 1:10 ratio, and (blue) peptide with resveratrol at a 1:20 ratio. The red and blue curves overlap in panels A and B. TEM images of (D) F15L-IAPP fibrils after 42 h, (E) F15L-IAPP fibrils with a 20-fold excess of resveratrol after 42 h, (F) F23L-IAPP fibrils, and (G) F23L-IAPP fibrils with a 20-fold excess of resveratrol. The F23L-IAPP samples were removed from the kinetic experiments at 96 h for TEM. TEM images of (H) Y37L-IAPP fibrils and (I) Y37L-IAPP fibrils with a 20-fold excess of resveratrol. The Y37L-IAPP samples were removed from the kinetic experiments at 96 h for TEM. Scale bars represent 100 nm.
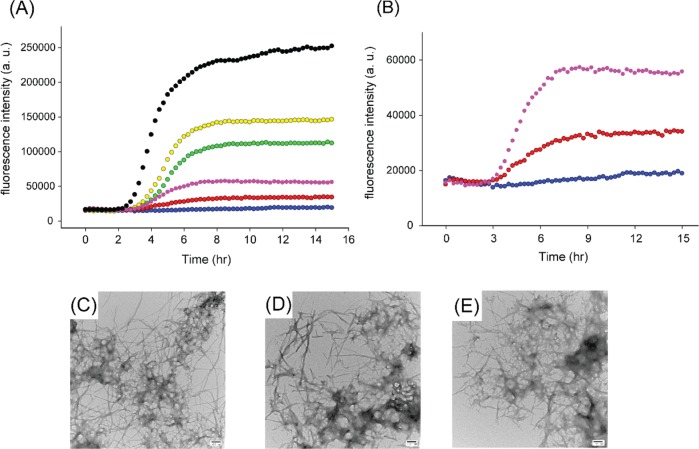
Resveratrol Does Not Inhibit Amyloid Formation by Ac 8–37-IAPP
Previous NMR studies suggested that Lys-1 could be a second binding site for resveratrol.16 We used a truncated acetylated variant of IAPP, Ac 8–37-IAPP, which lacks the seven N-terminal residues to probe the role of the N-terminal region of the polypeptide. This variant has been used in mechanistic studies of IAPP amyloid formation. Resveratrol is not an inhibitor of amyloid formation by Ac 8–37-IAPP. A 10-fold excess of compound had no detectable effect on the lag phase, consistent with the proposed role of Lys-1 (Figure 6). Ac 8–37-IAPP quickly forms amyloid fibrils even when resveratrol is present in a 10- or 20-fold excess. The results are consistent with the proposed role of the N-terminal region of IAPP in resveratrol–IAPP interactions.
Figure 6.
Resveratrol is not an effective inhibitor of amyloid formation by Ac 8–37-IAPP. (A) Thioflavin-T fluorescence-monitored kinetic experiments with Ac 8–37-IAPP (black) and Ac 8–37-IAPP and resveratrol at a 1:1 (yellow), 1:2 (green), 1:5 (pink), 1:10 (red), or 1:20 (blue). (B) Expansion of the data for Ac 8–37-IAPP with resveratrol at ratios of 1:5, 1:10, and 1:20. TEM images of (C) Ac 8–37-IAPP alone, (D) Ac 8–37-IAPP incubated at a 1:5 ratio with resveratrol, and (E) Ac 8–37-IAPP incubated at a 1:20 ratio with resveratrol. Samples were collected for TEM at 22 h. Samples contained 16 μM IAPP variant in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
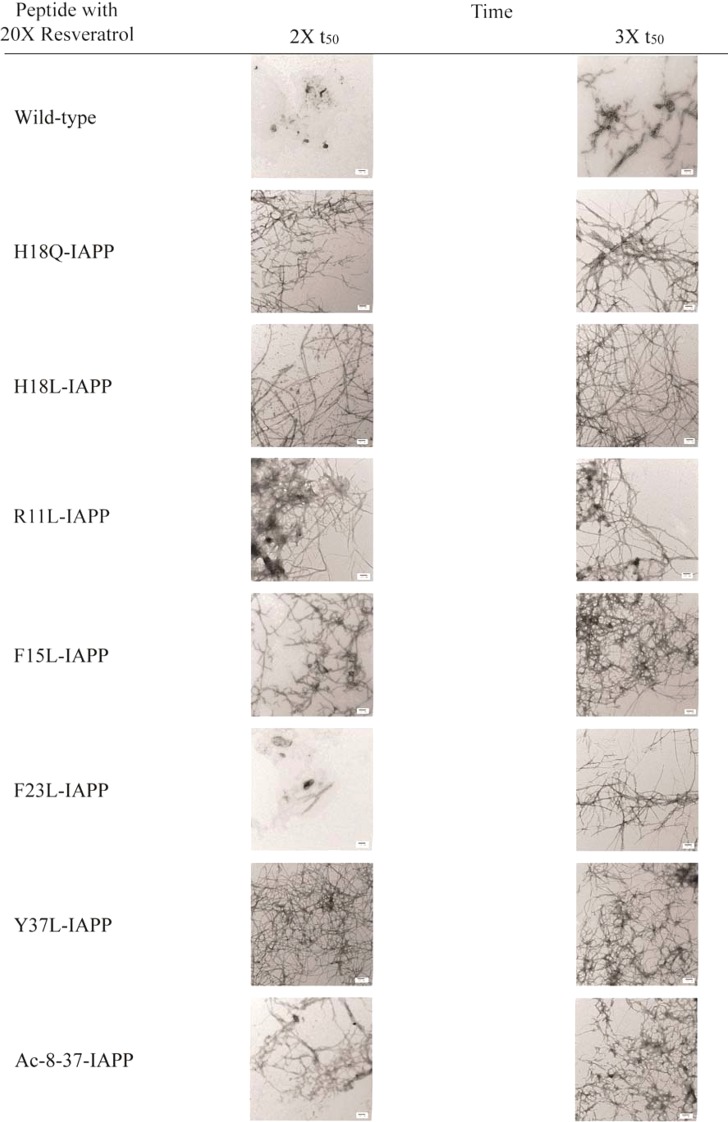
TEM-Based Competitive Analysis of the Effects of Resveratrol on the IAPP Mutants Helps To Reveal the Relative Importance of Different Sites
We collected additional TEM images of all samples in the presence of a 20-fold excess of resveratrol at times corresponding to twice the t50 and thrice the t50, where t50 is the time to reach 50% of the maximal thioflavin-T fluorescence signal for each individual peptide in the absence of inhibitor. These correspond to the wild type (t50 = 16.5 h), H18Q-IAPP (t50 = 12.5 h), H18L-IAPP (t50 = 1.25 h), R11L-IAPP (t50 = 1.6 h), F15L-IAPP (t50 = 20.5 h), F23L-IAPP (t50 = 37.5 h), Y37L-IAPP (t50 = 60.5 h), and Ac 8–37-IAPP (t50 = 4.25 h), under our conditions. The TEM data are shown in Figure 7 and confirm that mutation of Phe-15, Tyr-37, His-18, or Arg-11 or removal of Lys-1 and the N-terminal amino group affects the ability of resveratrol to reduce the rate of IAPP amyloid formation.
Figure 7.
Summary of TEM data collected for each peptide in the presence of resveratrol. Images were collected at twice and thrice the t50 value of the respective peptide in the absence of inhibitor. Samples contained 16 μM peptide and 320 μM resveratrol in pH 7.4, 20 mM Tris buffer with 1% (v/v) DMSO. Scale bars represent 100 nm.
Resveratrol Does Not Effectively Disaggregate IAPP Fibrils
There are conflicting reports on the ability of polyphenols to disaggregate and remodel amyloid fibrils. Resveratrol has been shown to remodel amyloid fibrils formed by Aβ.23 However, the ability of resveratrol to disaggregate amyloid fibrils formed by IAPP has not been examined. Figure 8 displays the results of studies to determine whether resveratrol is able to disaggregate IAPP fibrils. Resveratrol was added when IAPP amyloid formation reached a plateau (Figure 8A, black arrow), and TEM images were recorded before and directly after the addition of resveratrol (Figure 8B,C), as well as after incubation for 3 and 6 days (Figure 8D,E). The thioflavin-T intensity decreases rapidly after addition of resveratrol, suggesting that the compound might interfere with thioflavin-T binding. A slower, additional, loss of signal occurs, but the thioflavin-T intensity does not return to baseline even after incubation for 3 days. However, TEM images recorded at all time points show that numerous amyloid fibrils were present in all samples, and their morphology appears similar at the resolution of these experiments. The origin of the slow second phase of the time-dependent thioflavin-T signal is not understood, but the TEM data show that resveratrol does not disaggregate IAPP amyloid fibrils. In contrast, TEM studies have shown that EGCG disaggregates IAPP fibrils.16
Figure 8.
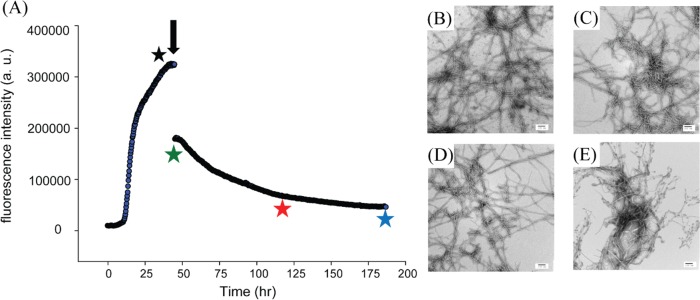
Resveratrol does not effectively remodel amyloid fibrils formed by wild-type IAPP. (A) Thioflavin-T-monitored time course. Amyloid formation was allowed to proceed for 48 h, and then resveratrol was added (black arrow). (B) TEM image recorded before adding resveratrol (black star). TEM images recorded (C) immediately after adding resveratrol (green star), (D) 3 days after addition of resveratrol (red star), and (E) 6 days after addition of resveratrol (blue star). Experiments were conducted at 25 °C in 20 mM Tris buffer, 32 μM thioflavin-T, and 16 μM IAPP. Resveratrol was added to a final concentration of 160 μM. Samples contained 1% (v/v) DMSO after the addition of resveratrol. Scale bars represent 100 nm.
Conclusions
The data presented here are consistent with previous studies24 that concluded that resveratrol slows, but does not prevent, human IAPP amyloid formation. This work also shows that resveratrol is much less effective than EGCG and reveals that resveratrol interferes with thioflavin-T assays. In fact, this is a widespread issue with thioflavin-T-based assays of IAPP amyloid formation.36,37,40 The structures of EGCG and resveratrol are very different, and there are several features that might contribute to their different levels of effectiveness. EGCG is larger, which may facilitate more extensive hydrophobic interactions with amino acid side chains. EGCG is also more extensively hydroxylated, containing three adjacent hydroxyl groups on two of its aromatic rings and two on the third. Structure–function studies have shown that the gallate ester group of EGCG contributes to its ability to inhibit IAPP amyloid formation as does the integrity of the trihydroxyl features.16 In addition, EGCG has been shown to form covalent linkages with some polypeptides,15 although this is not required for inhibition of IAPP amyloid formation and no cross-linking was detected in our ESI-MS studies.16
Our data are consistent with previous NMR studies with a nonphysiological variant of IAPP that lacks the amidated C-terminus. That work concluded that His-18 in IAPP makes contacts with resveratrol and Lys-1 is a secondary binding site.27 The work presented here also reveals the importance of Phe-15 and Tyr-37 in IAPP–resveratrol interactions and the potential role of Arg-11. The observation that the vast majority of the mutations have modest effects on the ability of resveratrol to modulate IAPP amyloid formation is consistent with relatively nonspecific interactions between IAPP and the compound. The observed importance of Phe-15 and Tyr-37 is consistent with the hypothesis that aromatic residues are a possible site for inhibitor protein interactions, but this observation is not general for IAPP because EGCG effectively inhibited variants of IAPP that lacked aromatic residues.16
This work also highlights the difficulty of using fluorescence-based thioflavin-T assays in inhibition studies. Even though small molecules may not absorb at the same wavelength as thioflavin-T fluorescence, they may alter the binding of thioflavin-T to amyloid fibrils; resveratrol is such a case. The use of complementary methods such as electron microscopy, atomic force microscopy, or mass spectrometry is strongly recommended to support thioflavin-T data in inhibition studies.
In summary, we have shown that resveratrol retards IAPP amyloid formation, but does not completely abolish fibril formation under the conditions used here. Interactions between resveratrol and monomeric IAPP are not observed by ESI-MS, in contrast to the case with other polyphenols,17 and changes in the lag time are modest. The study highlights the role of Arg-11, His-18, Phe-15, and Tyr-37 but indicates that Phe-23 is not as important for IAPP–resveratrol interactions. There is very little structural information available about pre-amyloid oligomers formed by IAPP, and it is currently not possible to offer a structural explanation for the different effects observed at position 23 relative to those detected for residues 15 and 37. The mutations may modulate IAPP–resveratrol interactions by altering residues that make direct contacts with the compound, or they may exert their effects indirectly by modulating the properties of IAPP oligomers. Studies with the nongenetically encoded amino acid p-cyanophenylalanine argue that the side chains of Phe-15, Phe-23, and Tyr-37 are all exposed to solvent during the lag phase of amyloid formation, suggesting that differential solvent accessible in pre-amyloid oligomers is not responsible for the different role of Phe-23.35 However, residues 15, 23, and 37 are in different environments in the amyloid fibrils. Residues 15 and 37 are part of the parallel β-sheet structure in high-resolution models of IAPP amyloid fibrils, but Phe-23 is in a less well-ordered loop that connects the N- and C-terminal β-strands.43,44 It may be that the compound interacts with all of the aromatic residues, but interactions with Phe-15 and Tyr-37 have a larger impact on amyloid formation than interactions with Phe-23 because Phe-23 is not part of the core structure of the fibril. Another important feature that other IAPP amyloid inhibitors, such as EGCG and morin hydrate, have is the ability to remodel amyloid fibrils.16,40 Our TEM studies demonstrate that resveratrol does not disaggregate IAPP fibrils, even though it induces a decrease in thioflavin-T fluorescence.
Acknowledgments
We thank Professor David Green and members of the Raleigh, Radford, and Ashcroft groups for helpful discussions.
Glossary
Abbreviations
- Ac 8–37-IAPP
truncated acetylated variant of human islet amyloid polypeptide
- CD
circular dichroism
- EGCG
(−)-epigallocatechin 3-gallate
- F15L-IAPP
Phe-15 to Leu variant of human islet amyloid polypeptide
- F23L-IAPP
Phe-23 to Leu variant of human islet amyloid polypeptide
- H18Q-IAPP
His-18 to Gln variant of human islet amyloid polypeptide
- H18L-IAPP
His-18 to Leu variant of human islet amyloid polypeptide
- IAPP
human islet amyloid polypeptide
- NMR
nuclear magnetic resonance
- R11L-IAPP
Arg-11 to Leu variant of human islet amyloid polypeptide
- t50
time required for 50% of the total signal change in a kinetic experiment
- TEM
transmission electron microscopy
- Y37L-IAPP
Tyr-37 to Leu variant of human islet amyloid polypeptide.
Supporting Information Available
A figure showing mass spectra of resveratrol after incubation for 24 h in mass spectra buffer, a figure showing thioflavin-T-monitored kinetic experiments for wild-type IAPP alone and wild-type IAPP in the presence of a 20-fold excess of resveratrol assayed from 0 to 126 h, a figure showing thioflavin-T-monitored kinetic experiments and TEM studies for H18L-IAPP and H18L-IAPP with resveratrol, additional TEM image of a sample of F23L-IAPP with a 20-fold excess of resveratrol collected after incubation for 142 h, a figure comparing the effects of different amounts of resveratrol on the t50 value for amyloid formation by wild-type IAPP and three aromatic to Leu point mutants, and a figure showing the effect of different amounts of resveratrol on amyloid formation by R11L-IAPP, which includes thioflavin-T kinetic curves and TEM data. This material is available free of charge via the Internet at http://pubs.acs.org.
D.P.R. acknowledges support from the National Institutes of Health (GM078114). L.M.Y. is funded by a Biotechnology and Biological Sciences Research Council (BBSRC) CASE studentship (Grant BB/I015361/1) sponsored by Micromass UK Ltd./Waters Corp. A.G.W. was supported, in part, by a GAANN fellowship from the U.S. Department of Education. The Synapt HDMS mass spectrometer was purchased with funds from the Biotechnology and Biological Sciences Research Council through its Research Equipment Initiative scheme (BB/E012558/1). S.E.R. also acknowledges funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013; 322408).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kahn S. E.; Andrikopoulos S.; Verchere C. B. (1999) Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48, 241–253. [DOI] [PubMed] [Google Scholar]
- Hull R. L.; Westermark G. T.; Westermark P.; Kahn S. E. (2004) Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643. [DOI] [PubMed] [Google Scholar]
- Konarkowska B.; Aitken J. F.; Kistler J.; Zhang S. P.; Cooper G. J. S. (2006) The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 273, 3614–3624. [DOI] [PubMed] [Google Scholar]
- Cheng F.; Marzban L. (2007) Cytotoxic effects of human islet amyloid polypeptide on primary islet α-cells and β-cells: Implications for understanding the pathogenesis of type 2 diabetes. Diabetes 56, A410–A411. [Google Scholar]
- Haataja L.; Gurlo T.; Huang C. J.; Butler P. C. (2008) Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrinol. Rev. 29, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P.; Andersson A.; Westermark G. T. (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 91, 795–826. [DOI] [PubMed] [Google Scholar]
- Westermark G. T.; Westermark P.; Nordin A.; Tornelius E.; Andersson A. (2003) Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Upsala J. Med. Sci. 108, 193–203. [DOI] [PubMed] [Google Scholar]
- Westermark G. T.; Westermark P.; Berne C.; Korsgren O.; Nordic Network for Clinical Islet Transplantation (2008) Widespread amyloid deposition in transplanted human pancreatic islets. N. Engl. J. Med. 359, 977–979. [DOI] [PubMed] [Google Scholar]
- Potter K. J.; Abedini A.; Marek P.; Klimek A. M.; Butterworth S.; Driscoll M.; Baker R.; Nilsson M. R.; Warnock G. L.; Oberholzer J.; Bertera S.; Trucco M.; Korbutt G. S.; Fraser P. E.; Raleigh D. P.; Verchere C. B. (2010) Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc. Natl. Acad. Sci. U.S.A. 107, 4305–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S. E.; Dalessio D. A.; Schwartz M. W.; Fujimoto W. Y.; Ensinck J. W.; Taborsky G. J.; Porte D. (1990) Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes 39, 634–638. [DOI] [PubMed] [Google Scholar]
- Young D. A.; Deems R. O.; Deacon R. W.; McIntosh R. H.; Foley J. E. (1990) Effects of amylin on glucose-metabolism and glycogenolysis in vivo and in vitro. Am. J. Physiol. 259, E457–E461. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K.; Arendash G. W.; Hou H. Y.; Fernandez F.; Jensen M.; Runfeldt M.; Shytle R. D.; Tan J. (2008) Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 1214, 177–187. [DOI] [PubMed] [Google Scholar]
- Meng F. L.; Abedini A.; Plesner A.; Verchere C. B.; Raleigh D. P. (2010) The flavanol (−)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry 49, 8127–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke J.; Russ J.; Friedrich R. P.; Ehrnhoefer D. E.; Wobst H.; Neugebauer K.; Wanker E. E. (2010) EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U.S.A. 107, 7710–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovych N.; Brender J. R.; Soong R.; Vivekanandan S.; Hartman K.; Basrur V.; Macdonald P. M.; Ramamoorthy A. (2012) Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248–286). J. Phys. Chem. B 116, 3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P.; Raleigh D. P. (2012) Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry 51, 2670–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. M.; Cao P.; Raleigh D. P.; Ashcroft A. E.; Radford S. E. (2014) Ion mobility spectrometry-mass spectrometry defines the oligomeric intermediates in amylin amyloid formation and the mode of action of inhibitors. J. Am. Chem. Soc. 136, 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palhano F. L.; Lee J.; Grimster N. P.; Kelly J. W. (2013) Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 135, 7503–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer D. E.; Bieschke J.; Boeddrich A.; Herbst M.; Masino L.; Lurz R.; Engemann S.; Pastore A.; Wanker E. E. (2008) EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 15, 558–566. [DOI] [PubMed] [Google Scholar]
- Sun A. Y.; Wang Q.; Simonyi A.; Sun G. Y. (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 41, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E.; Olivieri G.; Meier F.; Seifritz E.; Wirz-Justice A.; Muller-Spahn F. (2003) Red wine ingredient resveratrol protects from β-amyloid neurotoxicity. Gerontology 49, 380–383. [DOI] [PubMed] [Google Scholar]
- Marambaud P.; Zhao H. T.; Davies P. (2005) Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 280, 37377–37382. [DOI] [PubMed] [Google Scholar]
- Ladiwala A. R. A.; Lin J. C.; Bale S. S.; Marcelino-Cruz A. M.; Bhattacharya M.; Dordick J. S.; Tessier P. M. (2010) Resveratrol selectively remodels soluble oligomers and fibrils of amyloid A-β into off-pathway conformers. J. Biol. Chem. 285, 24228–24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R.; Sellin D.; Radovan D.; Gohlke A.; Winter R. (2009) Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem 10, 445–449. [DOI] [PubMed] [Google Scholar]
- Evers F.; Jeworrek C.; Tiemeyer S.; Weise K.; Sellin D.; Paulus M.; Struth B.; Tolan M.; Winter R. (2009) Elucidating the mechanism of lipid membrane-induced IAPP fibrillogenesis and its inhibition by the red wine compound resveratrol: A synchrotron X-ray reflectivity study. J. Am. Chem. Soc. 131, 9516–9521. [DOI] [PubMed] [Google Scholar]
- Radovan D.; Opitz N.; Winter R. (2009) Fluorescence microscopy studies on islet amyloid polypeptide fibrillation at heterogeneous and cellular membrane interfaces and its inhibition by resveratrol. FEBS Lett. 583, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Wei L.; Jiang P.; Xu W. X.; Li H.; Zhang H.; Yan L. Y.; Chan-Park M. B.; Liu X. W.; Tang K.; Mu Y. G.; Pervushin K. (2011) The molecular basis of distinct aggregation pathways of islet amyloid polypeptide. J. Biol. Chem. 286, 6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L. H.; Serrano A. L.; Zanni M. T.; Raleigh D. P. (2014) Mutational analysis of preamyloid intermediates: The role of His-Tyr interactions in islet amyloid formation. Biophys. J. 106, 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat Y.; Mazor Y.; Efrat S.; Gazit E. (2004) Inhibition of islet amyloid polypeptide fibril formation: A potential role for heteroaromatic interactions. Biochemistry 43, 14454–14462. [DOI] [PubMed] [Google Scholar]
- Abedini A.; Raleigh D. P. (2005) Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org. Lett. 7, 693–696. [DOI] [PubMed] [Google Scholar]
- Marek P.; Woys A. M.; Sutton K.; Zanni M. T.; Raleigh D. P. (2010) Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org. Lett. 12, 4848–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini A.; Singh G.; Raleigh D. P. (2006) Recovery and purification of highly aggregation-prone disulfide-containing peptides: Application to islet amyloid polypeptide. Anal. Biochem. 351, 181–186. [DOI] [PubMed] [Google Scholar]
- Porat Y.; Abramowitz A.; Gazit E. (2006) Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 67, 27–37. [DOI] [PubMed] [Google Scholar]
- Krebs M. R. H.; Bromley E. H. C.; Donald A. M. (2005) The binding of thioflavin-T to amyloid fibrils: Localisation and implications. J. Struct. Biol. 149, 30–37. [DOI] [PubMed] [Google Scholar]
- Marek P.; Mukherjee S.; Zanni M. T.; Raleigh D. P. (2010) Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: A combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J. Mol. Biol. 400, 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S. A.; Ecroyd H.; Kee T. W.; Carver J. A. (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 276, 5960–5972. [DOI] [PubMed] [Google Scholar]
- Meng F. L.; Marek P.; Potter K. J.; Verchere C. B.; Raleigh D. P. (2008) Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin-T interactions: Implications for mechanistic studies β-cell death. Biochemistry 47, 6016–6024. [DOI] [PubMed] [Google Scholar]
- Marek P.; Abedini A.; Song B. B.; Kanungo M.; Johnson M. E.; Gupta R.; Zaman W.; Wong S. S.; Raleigh D. P. (2007) Aromatic interactions are not required for amyloid fibril formation by islet amyloid polypeptide but do influence the rate of fibril formation and fibril morphology. Biochemistry 46, 3255–3261. [DOI] [PubMed] [Google Scholar]
- Tu L. H.; Raleigh D. P. (2013) Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry 52, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor H.; Cao P.; Raleigh D. P. (2012) Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci. 21, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela B. C.; Waterhouse A. L. (1996) Resveratrol: Isomeric molar absorptivities and stability. J. Agric. Food Chem. 44(5), 1253–1257. [Google Scholar]
- Pineda-Sanabria S. E.; Robertson I. M.; Sykes B. D. (2011) Structure of trans-Resveratrol in Complex with the Cardiac Regulatory Protein Troponin C. Biochemistry 50(8), 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca S.; Yau W.-M.; Leapman R.; Tycko R. (2007) Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry 46(47), 13505–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltzius J. J. W.; Sievers S. A.; Sawaya M. R.; Cascio D.; Popov D.; Riekel C.; et al. (2008) Atomic structure of the cross-β spine of islet amyloid polypeptide (amylin). Protein Sci. 17(9), 1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.