Abstract
AKI is associated with increased morbidity, mortality, and cost of care, and therapeutic options remain limited. Reactive oxygen species are critical for the genesis of ischemic AKI. Stanniocalcin-1 (STC1) suppresses superoxide generation through induction of uncoupling proteins (UCPs), and transgenic overexpression of STC1 inhibits reactive oxygen species and protects from ischemia/reperfusion (I/R) kidney injury. Our observations revealed high AMP-activated protein kinase (AMPK) activity in STC1 transgenic kidneys relative to wild-type (WT) kidneys; thus, we hypothesized that STC1 protects from I/R kidney injury through activation of AMPK. Baseline activity of AMPK in the kidney correlated with the expression of STCs, such that the highest activity was observed in STC1 transgenic mice followed (in decreasing order) by WT, STC1 knockout, and STC1/STC2 double-knockout mice. I/R in WT kidneys increased AMPK activity and the expression of STC1, UCP2, and sirtuin 3. Inhibition of AMPK by administration of compound C before I/R abolished the activation of AMPK, diminished the expression of UCP2 and sirtuin 3, and aggravated kidney injury but did not affect STC1 expression. Treatment of cultured HEK cells with recombinant STC1 activated AMPK and increased the expression of UCP2 and sirtuin 3, and concomitant treatment with compound C abolished these responses. STC1 knockout mice displayed high susceptibility to I/R, whereas pretreatment of STC1 transgenic mice with compound C restored the susceptibility to I/R kidney injury. These data suggest that STC1 is important for activation of AMPK in the kidney, which mediates STC1-induced expression of UCP2 and sirtuin 3 and protection from I/R.
Keywords: renal injury, renal morphology, ischemia-reperfusion, mitochondria, renal, protection, reactive oxygen species
AKI is common and frequently associated with high morbidity and mortality. A study by Chertow et al.1 showed that a small increase in serum creatinine in patients admitted to the hospital is associated with higher mortality, longer hospitalization, and higher cost of care. Ischemic kidney injury may not recover and could lead to end stage kidney failure, or it may exacerbate other underlying disease processes and accelerate the progression to end stage kidney failure.2 For example, a short ischemic episode in the mouse leads to persistent interstitial fibrosis,3 whereas in humans, acute ischemic insult occurring immediately after kidney transplantation may delay graft function and lead to chronic allograft nephropathy.4,5 Experimental mouse ischemia/reperfusion (I/R) kidney injury has been used extensively to study the pathogenesis of ischemic AKI. Increased generation of reactive oxygen species (ROS), endothelial dysfunction, vascular congestion at the corticomedullary junction, and inflammation are critical players in the pathogenesis of I/R kidney injury.6–10 Although the pathogenesis of ischemic kidney injury is better defined, few therapeutic options are currently available.
Mammalian stanniocalcin-1 (STC1) is ubiquitously expressed, and the highest level of expression is in the ovary, kidney, prostate, and thyroid.11 STC1 is an intracellular-acting, extracellular signaling protein (paracrine/intracrine)12; it binds to a cell-surface protein followed by internalization and targeting to the inner mitochondrial membrane.13 Mammalian STC2 is also ubiquitously expressed; cumulative evidence suggests that it also acts in an autocrine/paracrine manner and that its functions are linked to the endoplasmic reticulum.14 STC1 suppresses superoxide generation through induction of uncoupling proteins (UCPs),15,16 stabilizes endothelial barrier function,17 and diminishes macrophage mobility and response to chemoattractants.18 Thus, STC1 may target core pathways in the pathogenesis of I/R kidney injury. Indeed, we have recently shown that transgenic (Tg) overexpression of STC1 protects from I/R kidney injury in mice through inhibition of ROS and inflammation.16 Current observations reveal increased activity of AMP-activated protein kinase (AMPK) in STC1 Tg kidneys. AMPK regulates cellular metabolism and is a key component of the cellular adaptive responses to ischemia in the kidney.19,20 Recent studies suggest that pretreatment with AMPK activators (5-aminoimidazol-4-carboxamide-1-β-d-ribofuranoside and metformin) may have a protective role in renal I/R injury.21 We, therefore, hypothesized that STC1 protects from I/R through activation of AMPK.
Our findings in these experiments are consistent with this hypothesis: baseline activity of AMPK in the kidney correlates with the expression of STC1 and STC2, with the highest activity observed in STC1 Tg followed (in decreasing order) by wild type (WT), STC1 knockout (KO), and STC1/STC2 double KO. I/R in WT kidneys increases AMPK activity and the expression of STC1, UCP2, and silent mating type information regulation 2 homolog 3 (SIRT3; also known as sirtuin 3). UCPs limit free radical production,22,23 whereas SIRT3, a mitochondrial acetyltransferase, decreases ROS through a number of pathways,24 but its functions in the kidney are largely unknown. Pretreatment of WT mice with 6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a] pyrimidine (compound C [CC]), a pharmacologic inhibitor of AMPK,25,26 abolishes I/R-induced AMPK activation, diminishes the expression of SIRT3 and UCP2, and is associated with increased ROS production and worse morphologic changes. In agreement with these data, treatment of human embryonic kidney (HEK) cells with recombinant STC1 (rSTC1) activates AMPK and increases the expression of SIRT3 and UCP2, whereas concomitant treatment with CC abolishes the responses to rSTC1. These data are consistent with placement of STC1 upstream of AMPK, whereas UCP2 and SIRT3 appear to be downstream of AMPK. Importantly, STC1 KO mice display greater susceptibility to I/R kidney injury compared with WT mice, and pretreatment of STC1 Tg mice with CC restored susceptibility to I/R kidney injury. The data suggest that (1) STC1 is important for AMPK activation in the kidney and (2) STC1-induced protection from I/R and expression of UCP2 and SIRT3 are AMPK-dependent.
Results
STC1 Regulates AMPK Activity in the Kidney
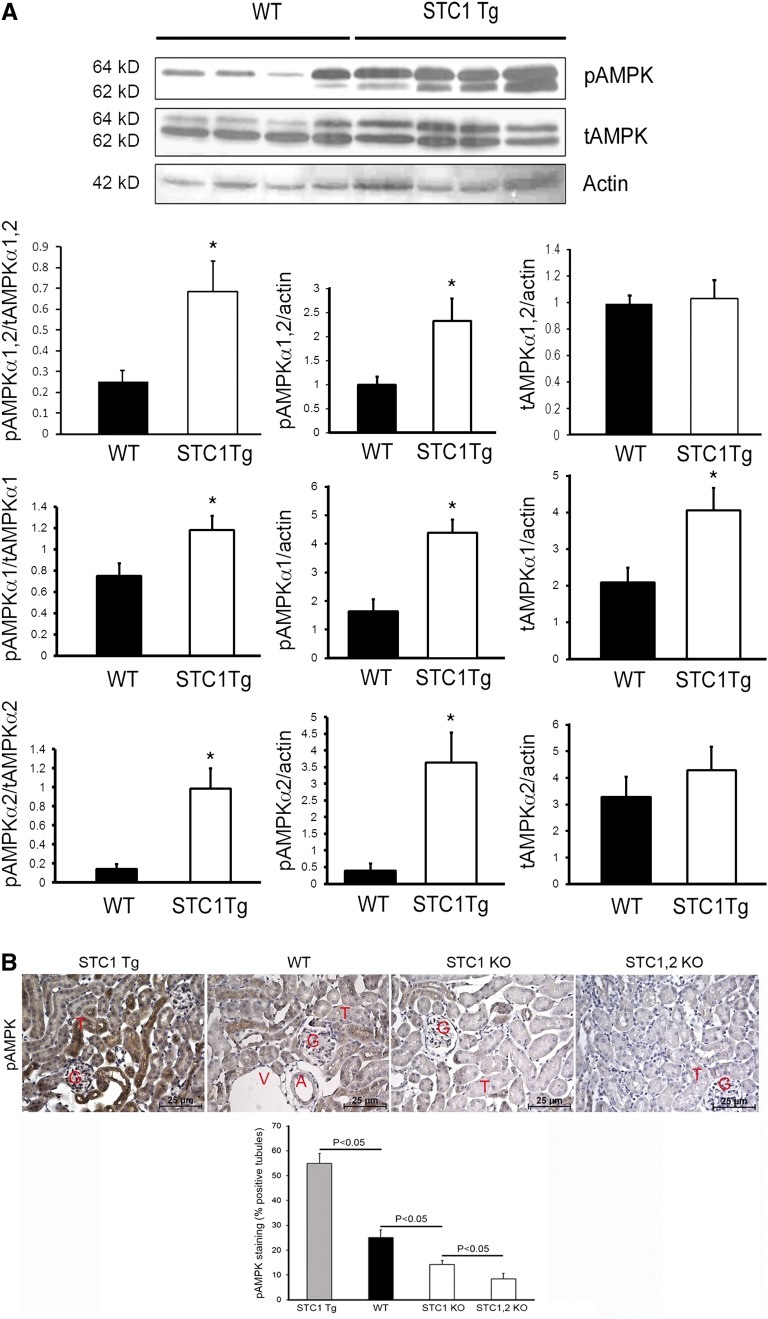
To determine whether AMPK activity in the kidney correlates with the expression of STC1, we compared whole-kidney lysates from WT and STC1 Tg mice and found higher-level activity of AMPK in STC1 Tg kidneys compared with WT kidneys (Figure 1A). Quantitatively, STC1 Tg kidneys displayed 2.5-fold higher baseline activity of AMPK relative to WT (Figure 1A). Staining for phospho-AMPK (pAMPK) revealed the highest activity of AMPK in STC1 Tg27 kidneys followed (in decreasing order) by WT, STC1 KO,28 and STC1/STC2 double-KO29 kidneys (Figure 1B). Staining for pAMPK is observed in the entire kidney, including tubules, glomeruli, and arterioles; tubular staining appears diffusely cytoplasmic, with some tubule segments showing stronger staining than others. Our data suggest that STC1 is an important regulator of AMPK in the kidney. Using antibodies that recognize both α1 and α2 isoforms of the catalytic subunits of AMPK, we observe the phosphorylated α1 (larger-sized band) consistently and phosphorylation of the α2 (smaller-sized band) intermittently in WT mice; however, in STC1 Tg mice, we detect phosphorylation of both isoforms consistently (albeit at varying intensity) (Figure 1A). STC1 shows preferential activation of the α2 isoform; however, the significance of these observations remains to be determined.
Figure 1.
STC1 regulates AMPK activity in the kidney. (A) Increased AMPK activity in STC1 Tg kidneys. Lysates from WT and STC1 Tg kidneys were resolved on SDS-PAGE, and blots reacted consecutively with antibodies that recognize pAMPKα1,α2, AMPKα1,α2, or actin. Representative Western blot is shown; bar graphs represent the mean±SEM of n=7 mice/group and depict the ratio of indicated proteins in WT and STC1 Tg mice. *P<0.05 versus WT. tAMPK, total AMPK. (B) Baseline activity of AMPK in the kidney correlates with STCs. Representative immunohistochemistry staining of kidney sections is shown from STC1 Tg, WT, STC1 KO, and STC1/STC2 double-KO mice using anti-pAMPKα1,α2. Bar graph represents quantification of pAMPKα1,α2 staining from four mice for each group, and data are presented as mean±SEM. A, arterioles; G, glomeruli; T, tubules; V, venules.
Inhibition of AMPK Exacerbates I/R Kidney Injury
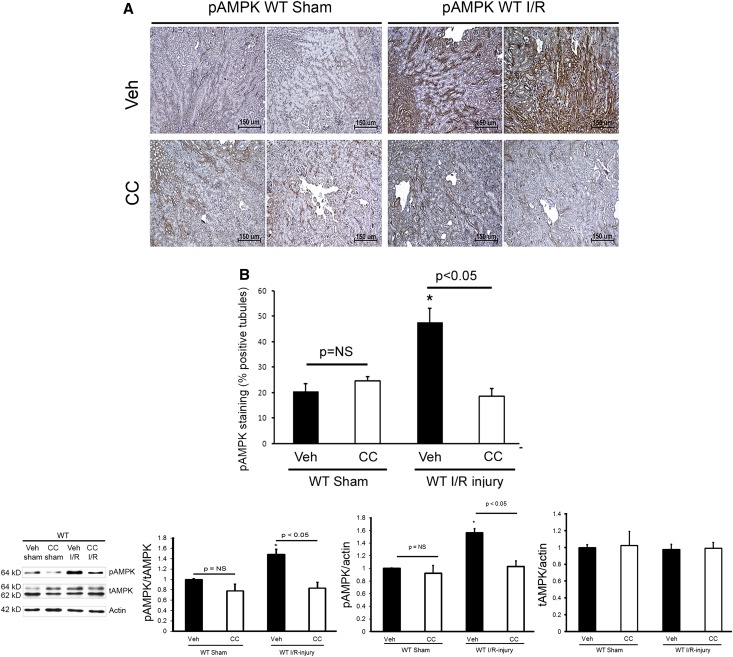
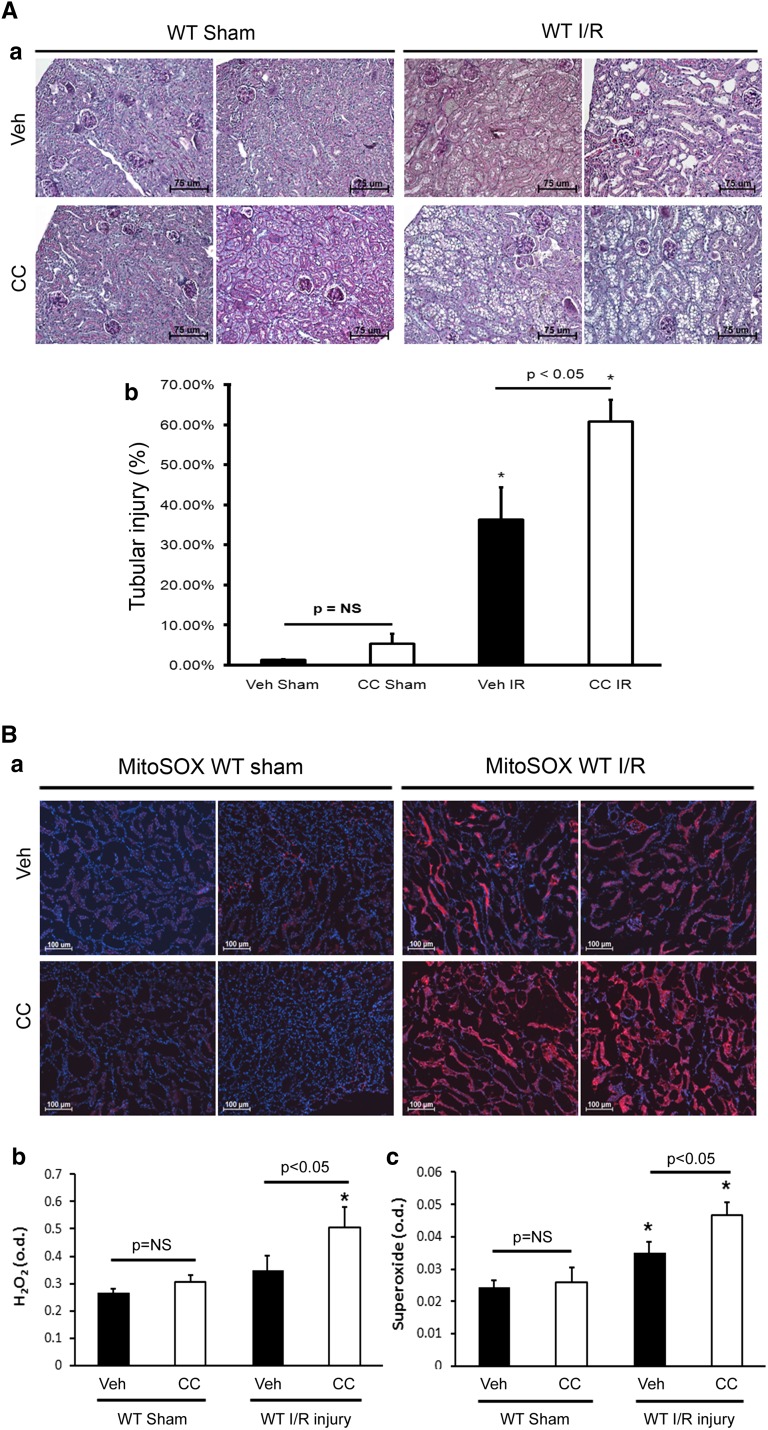
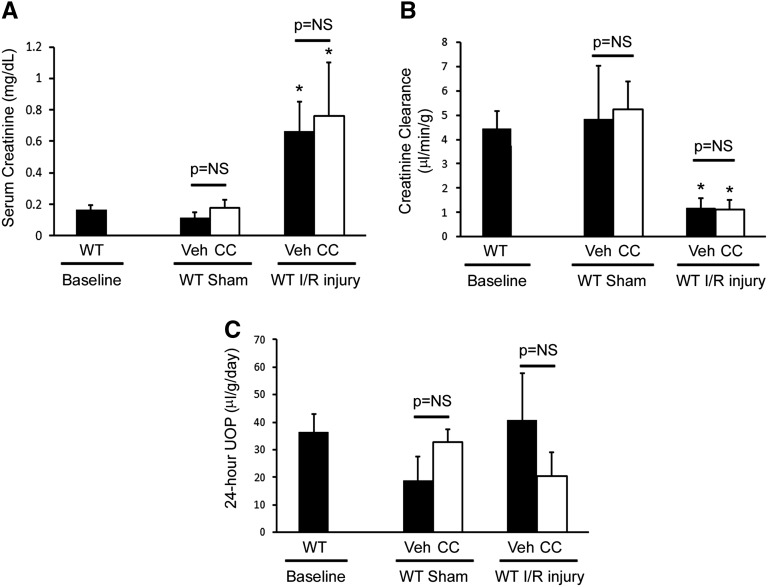
Tg overexpression of STC1 confers resistance to I/R kidney injury through suppression of oxidant stress and inflammation,16 whereas acute knockdown of STC1 in the kidney leads to severe proximal tubule injury and kidney failure.30 AMPK regulates cellular metabolism, and recent studies suggest that pretreatment with AMPK activators (5-aminoimidazol-4-carboxamide-1-β-d-ribofuranoside and metformin) may protect from I/R injury.20,21,31 To determine whether AMPK is important for protection from I/R kidney injury and test whether STC1 acts upstream of AMPK, we pretreated WT mice with CC before I/R. Pretreatment with CC decreased I/R-induced activation of AMPK by one half, compared with responses in vehicle-pretreated mice (Figure 2). Seventy-two hours after I/R, we observed increased cellular vacuolization and tubular dilation, predominantly in the cortex (Figure 3A), coupled with significant elevation in both H2O2 and superoxide in CC-pretreated mice compared with vehicle-treated WT controls (Figure 3B) but no significant differences in serum creatinine, creatinine clearance (CrCl), or urine output (Figure 4). The apparent discrepancy between the severe morphologic changes observed at the 72-hour time point but no apparent difference in CrCl in CC- and I/R-treated WT mice compared with vehicle- and I/R-treated WT mice may be explained by the fact that urine collection for CrCl began 48 hours after clamping (i.e., 24 hours before tissue harvest for histology) and before the full injury had been established in CC- and I/R-treated mice. Treatment of sham-operated WT mice with CC did not increase H2O2 or superoxide (Figure 3B) and did not lead to morphologic or functional changes in the kidney (Figures 3A and 4).
Figure 2.
Treatment with CC blocks I/R injury-induced activation of AMPK in WT mice. (A) Immunohistochemistry of kidney sections using anti-pAMPKα1,α2. Representative images from two mice per group are shown. (B) Bar graph represents quantification of pAMPKα1,α2 staining. Data represent the mean±SEM from four mice for each group. (C) Total kidney lysates from vehicle sham, CC sham, vehicle I/R, or CC I/R mice were resolved on SDS-PAGE, and Western blots were reacted with anti-pAMPKα1,α2, anti-AMPKα1,α2, and anti-actin; representative Western blots are shown. Bar graphs depict the ratio of pAMPKα1,α2/total AMPKα1,α2, pAMPKα1,α2/actin, and total AMPKα1,α2/actin. Data represent the mean±SEM from four mice for each group. tAMPK, total AMPK; Veh, vehicle. *P<0.05 versus vehicle sham.
Figure 3.
Inhibition of AMPK exacerbates I/R kidney injury in WT mice. (A, a) Periodic acid–Schiff staining of kidney sections for morphology showed severe cellular vacuolization (predominantly in the cortex) 72 hours after I/R in WT mice pretreated with CC. Representative images from two mice per group are shown. (A, b) Bar graph represents quantification of tubular injury and depicts the mean±SEM of the percentage of tubules with cellular vacuolization, dilation, and/or cast formation (n=4 mice/group). (B) AMPK inhibition in WT mice subjected to I/R kidney injury increases H2O2 and superoxide production. (B, a) WT mice were treated with CC or vehicle before I/R or sham surgery, and superoxide (MitoSOX red fluorescence) was measured in tubular epithelial cells 72 hours after I/R or sham surgery. Representative images from two mice per group are shown; blue fluorescence corresponds to 4′,6-diamidino-2-phenylindole. Bar graphs show (B, b) H2O2 and (B, c) superoxide 72 hours after I/R or sham surgery; data represent mean±SEM from four mice per group. Veh, vehicle. *P<0.05 versus vehicle sham.
Figure 4.
AMPK inhibition in WT mice exacerbates kidney injury post-I/R. (A) Serum creatinine, (B) CrCl normalized to weight, and (C) timed urine output were carried out as detailed in Concise Methods. Bar graphs depict the mean±SEM (n=9 mice for WT baseline; n=3 mice for CC sham; n=4 mice/group for Veh sham, Veh I/R, or CC I/R). UOP, urine output; Veh, vehicle. *P<0.05 versus WT baseline.
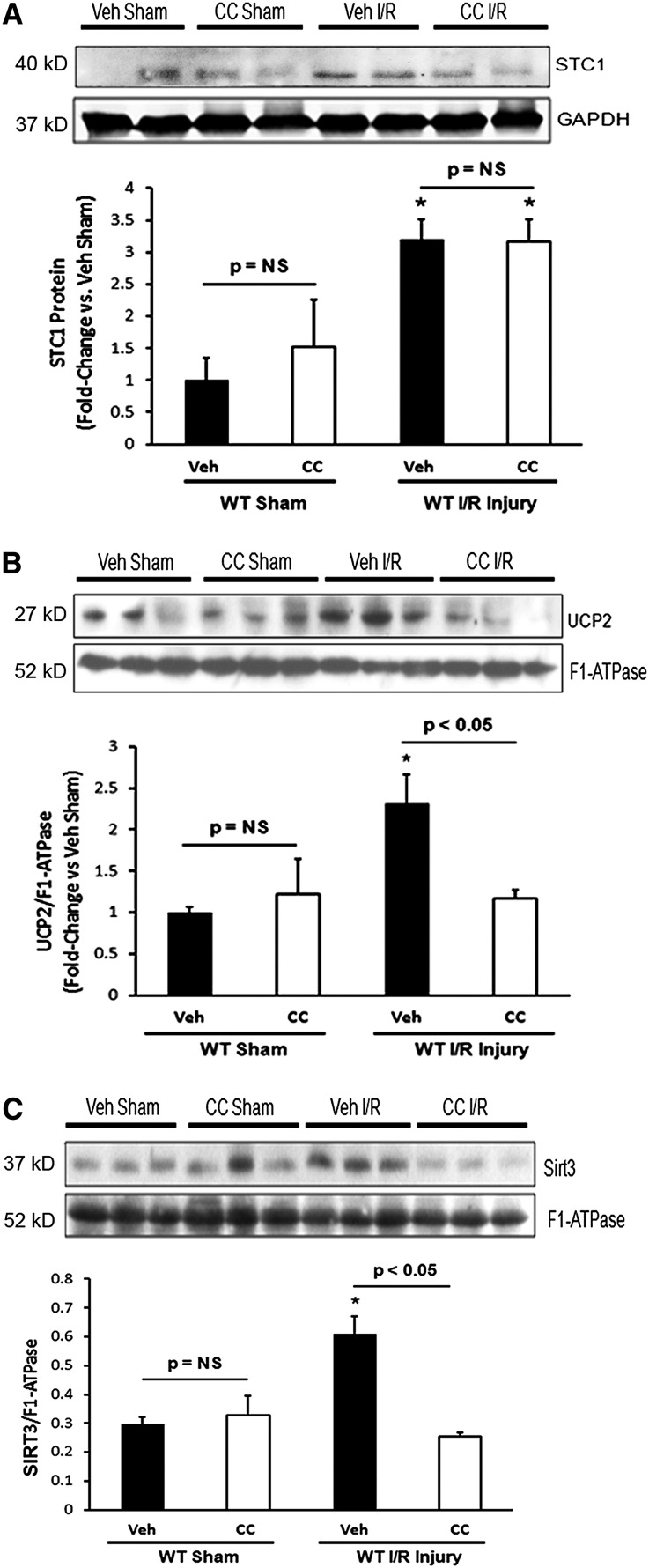
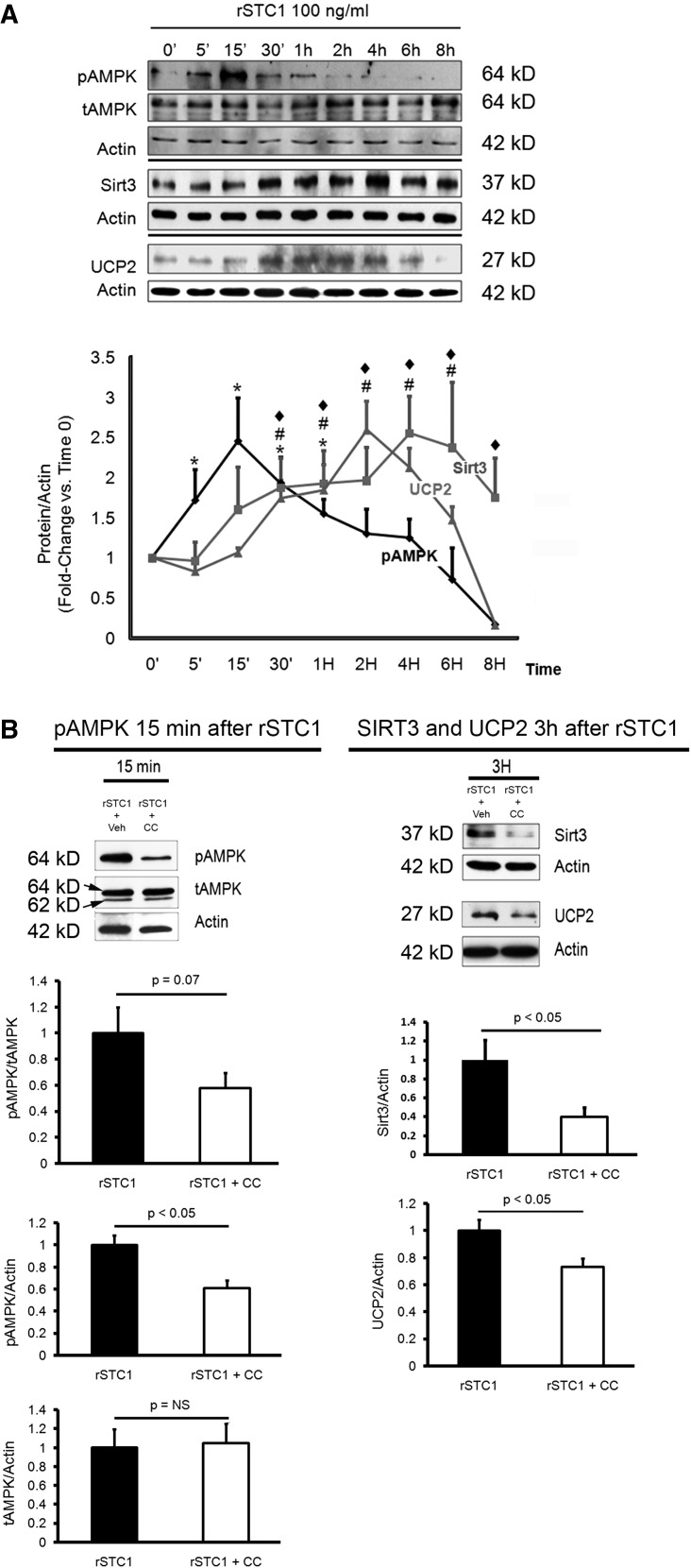
As shown in Figure 5, I/R induced the expression of STC1, UCP2, and SIRT3. UCPs limit free radical production,15,22,23 whereas SIRT3 is a mitochondrial acetyl transferase known to decrease ROS through a number of pathways.24 Pretreatment with CC prevented I/R-induced increase in UCP2 and SIRT3 (Figure 5, B and C), consistent with AMPK-mediated regulation of UCP2 and SIRT3. However, AMPK inhibition did not affect I/R-induced expression of STC1 protein (Figure 5A). In addition, treatment of cultured HEK cells with rSTC1 activates AMPK, and this activation precedes the increase in UCP2 and SIRT3 expression (Figure 6A), whereas concomitant treatment with CC abolishes the responses to rSTC1 (Figure 6B). These observations support the in vivo data and are consistent with STC1-induced activation of AMPK and AMPK-mediated induction of SIRT3 and UCP2, thus placing STC1 upstream of AMPK, whereas UCP2 and SIRT3 are downstream of AMPK. Taken together, these data suggest that STC1 may protect from I/R kidney injury through activation of AMPK to increase the expression of UCP2 and SIRT3 and decrease ROS production.
Figure 5.
Inhibition of AMPK in WT mice blocks I/R-induced expression of SIRT3 and UCP2. (A) Total kidney lysates were obtained 72 hours after I/R. Western blots were reacted with anti-STC1 and anti-GAPDH; bar graph shows mean±SEM of n=3 mice for vehicle sham and n=4 mice for all other groups. Kidney mitochondrial lysates were obtained 72 hours after I/R; Western blots were reacted with (B) anti-UCP2, (C) anti-SIRT3, and (B and C) anti–F1-ATPase. Bar graphs show the mean±SEM from four mice per group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Veh, vehicle. *P<0.05 versus vehicle sham.
Figure 6.
STC1 increases SIRT3 and UCP2 expression in vitro via AMPK. (A) STC1 activates AMPK and increases the expression of SIRT3 and UCP2 in vitro. Cultured HEK cells were treated with rSTC1 for different time points. Western blots were reacted with anti-pAMPKα1,α2, anti-AMPKα1,α2, anti-actin, anti-SIRT3, and anti-UCP2. The graph represents the mean±SEM of three independent experiments depicting densitometry values of pAMPKα1,α2, SIRT3, or UCP2 normalized to actin. P<0.05 versus time 0 minutes (0’) for #SIRT3, ♦UCP2, and *pAMPK. (B) STC1-induced increase in SIRT3 and UCP2 expression is AMPK-dependent. On the basis of the time courses for activation of AMPK and induction of SIRT3 and UCP2 (shown in A), cultured HEK cells were treated with rSTC1 plus vehicle or rSTC1 and CC for the specified time points. Western blots were reacted with anti-pAMPKα1,α2, anti-AMPKα1,α2, anti-actin, anti-SIRT3, and anti-UCP2. AMPK activity (pAMPK) was sampled 15 minutes after treatment with rSTC1, whereas the expressions of SIRT3 and UCP2 were sampled 3 hours after treatment with rSTC1. Graphs represent the mean±SEM of three independent experiments. tAMPK, total AMPK; Veh, vehicle.
Inhibition of AMPK in STC1 Tg Mice Restores Susceptibility to I/R Kidney Injury
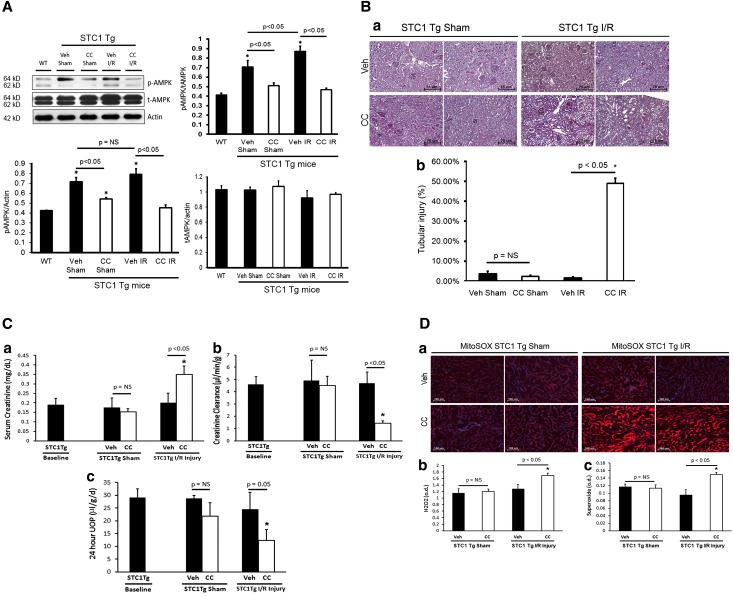
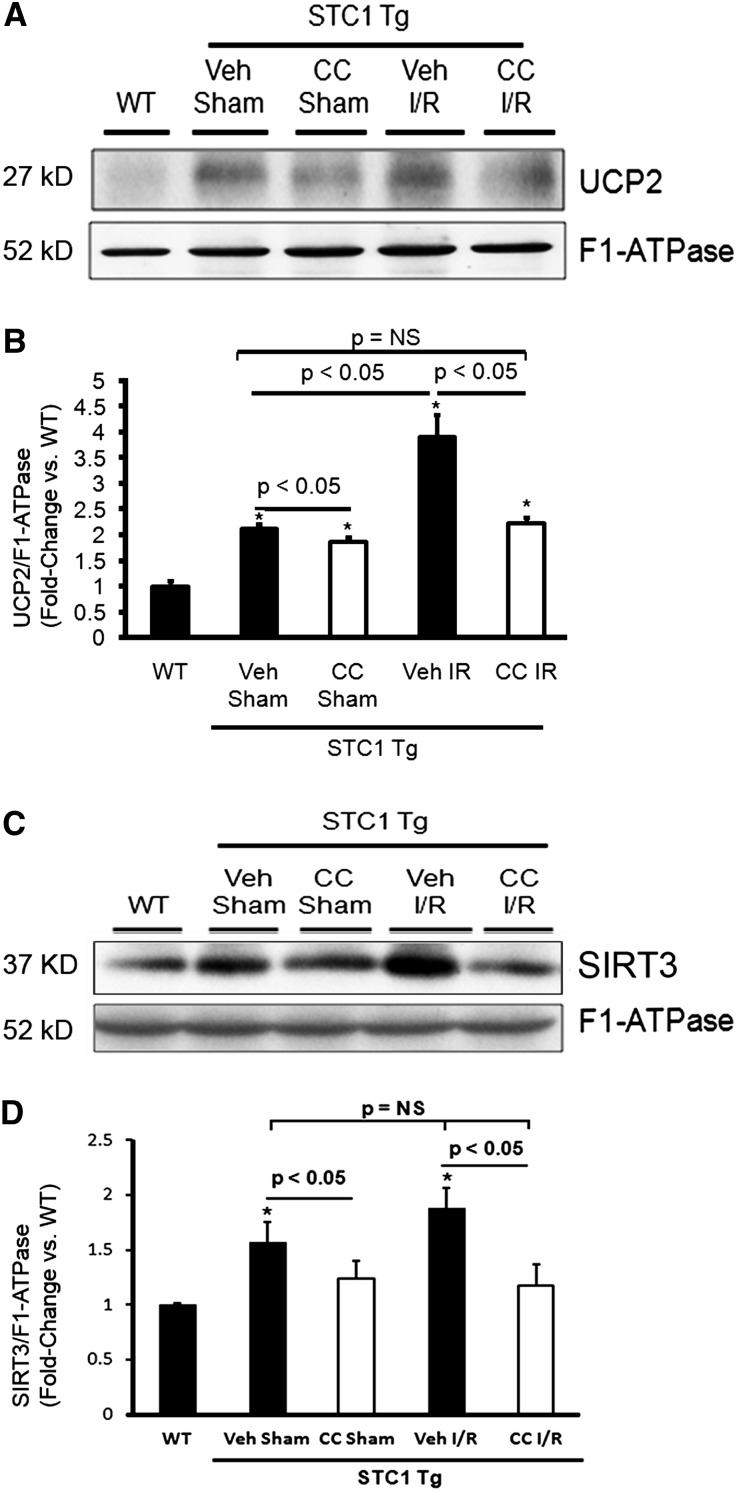
To show that activation of AMPK mediates the renoprotective effects that we observed in STC1 Tg mice,16 we pretreated these mice with CC or vehicle before I/R. Pretreatment of STC1 Tg mice with CC diminished AMPK activity (Figure 7A) and resulted in severe kidney injury after I/R, which was characterized by cellular vacuolization, tubular dilation, and cast formation (predominantly in the cortex) (Figure 7B), similar to those changes observed in WT kidneys after I/R, increased serum creatinine, decreased CrCl and urine output (Figure 7C), and increased superoxide and H2O2 generation (Figure 7D). In addition, pretreatment with CC attenuated the expression of UCP2 and SIRT3 in STC1 Tg kidneys (Figure 8). However, pretreatment of STC1 Tg mice with vehicle did not produce any of these changes. The data suggest that, in STC1 Tg mice, protection from I/R, induction of UCP2 and SIRT3, and suppression of ROS are all AMPK-mediated.
Figure 7.
AMPK inhibition in STC1 Tg mice restores susceptibility to I/R kidney injury. (A) Treatment with CC blocks activation of AMPK in STC1 Tg mice. Total kidney lysates of WT (control) and STC1 Tg mice treated with vehicle sham, CC sham, vehicle I/R, or CC I/R were resolved on SDS-PAGE, and Western blots were reacted with anti-pAMPKα1,α2, anti-AMPKα1,α2, and anti-actin. Bar graphs represent the mean±SEM of data obtained from four mice per group. *P<0.05 versus WT. (B) STC1 Tg mice were pretreated with CC or vehicle, subjected to I/R or sham, and euthanized 72 hours later. (B, a) In CC-pretreated STC1 Tg mice, periodic acid–Schiff staining of kidney sections for morphology showed severe kidney injury after I/R characterized by cellular vacuolization, tubular dilation, and cast formation (predominantly in the cortex). Representative images from two mice per group are shown. (B, b) Bar graph represents quantification of tubular injury and depicts the mean±SEM of the percentage of tubules with cellular vacuolization, dilation, and/or cast formation; n=4 mice/group for vehicle or CC sham, and n=5 mice/group for vehicle or CC I/R. *P<0.05 versus vehicle sham. (C) Inhibition of AMPK in STC1 Tg results in severe kidney injury after I/R. STC1 Tg mice were pretreated with CC or vehicle and subjected to I/R or sham surgery and serum creatinine (C, a), CrCl was normalized to weight (C, b), and timed urine output (C, c) were carried out as detailed in Concise Methods. Bar graphs depict the mean±SEM (n=9 mice for baseline in STC1 Tg; n=4 mice/group for vehicle or CC sham; n=5 mice/group for vehicle or CC I/R). *P<0.05 versus STC1 Tg baseline. (D) Inhibition of AMPK in STC1 Tg mice increases H2O2 and superoxide after I/R. (D, a) STC1 Tg mice were treated with CC or vehicle before I/R or sham surgery, and superoxide (MitoSOX red fluorescence) was measured in tubular epithelial cells 72 hours after I/R or sham surgery. Representative images from two mice per group are shown; blue fluorescence corresponds to 4′,6-diamidino-2-phenylindole. Bar graphs show (D, b) H2O2 and (D, c) superoxide 72 hours post-I/R or sham surgery; data represent mean±SEM (n=4 mice/group for vehicle or CC sham; n=5 mice/group for vehicle or CC I/R). UOP, urine output; tAMPK, total AMPK; Veh, vehicle. *P<0.05 versus vehicle sham.
Figure 8.
Inhibition of AMPK in STC1 Tg mice attenuates UCP2 and SIRT3 expression. Kidney mitochondrial lysates were obtained 72 hours after I/R or sham surgery; Western blots were reacted with (A) anti-UCP2, (C) anti-SIRT3, or (A and C) anti–F1-ATPase. Bar graphs depict the ratios of (B) UCP2/F1-ATPase and (D) SIRT3/F1-ATPase normalized to WT control. Data represent the mean±SEM from four mice per group. Veh, vehicle.*P<0.05 versus WT.
STC1 KO Mice Display Increased Susceptibility to I/R
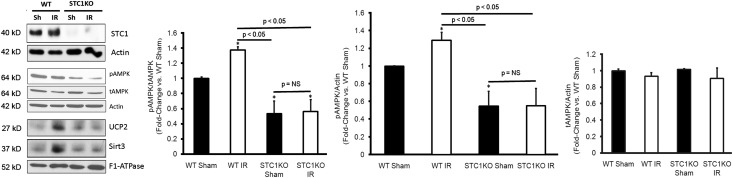
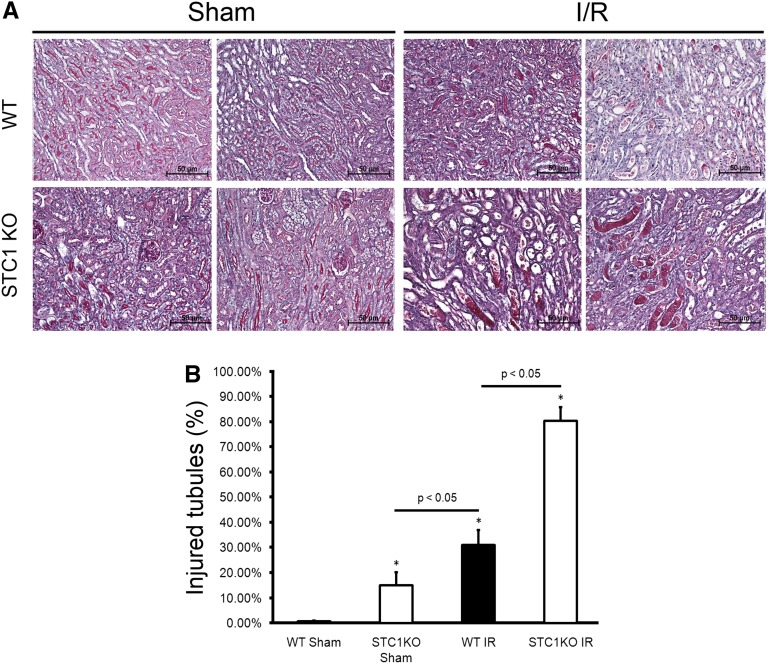
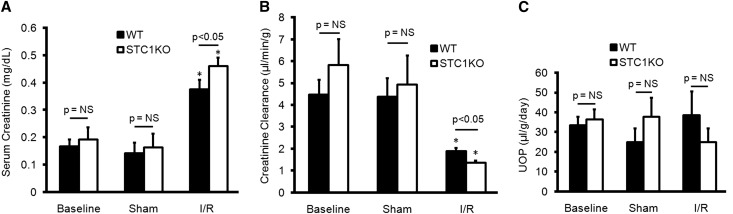
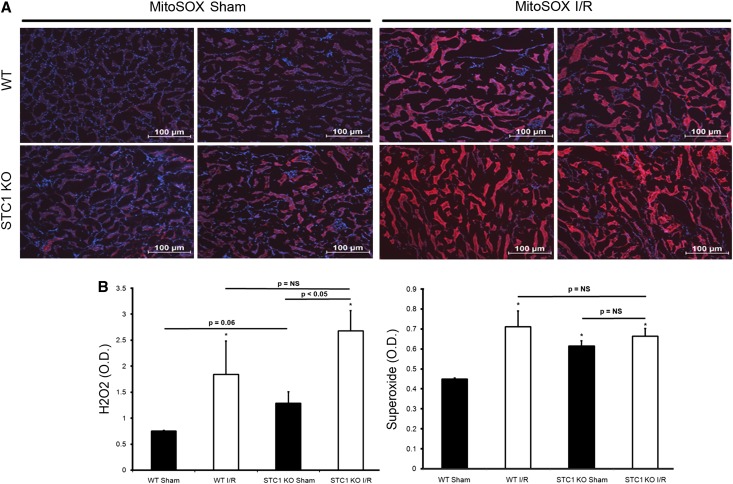
To lend additional support for the above data, we carried out I/R experiments in STC1 KO mice, an alternate model of low AMPK activity (Figure 9). Of note, baseline expression of UCP2 and SIRT3 is low in STC1 KO kidneys, and we observe no change in SIRT3 and UCP2 levels after I/R (Figure 9), consistent with STC1-induced and AMPK-mediated expression of both SIRT3 and UCP2. Of interest, histology revealed mild kidney injury in sham-treated STC1 KO mice compared with sham-treated WT mice. The data suggest that, absent STC1 expression and hence, diminished AMPK activity at baseline, the kidneys manifest injury after the stress of sham operation; these observations are consistent with recent findings from our laboratory, where acute and kidney-specific knockdown of STC1 leads to AKI.30 I/R produced greater kidney injury in STC1 KO mice compared with WT mice (Figure 10). This injury was characterized by higher serum creatinine, lower CrCl (Figure 11), greater ROS generation (Figure 12), diffuse cellular vacuolization, and marked tubular dilation as well as cast formation that involved approximately 30% of the tubules in the cortex (Figure 10). Morphologically, the injury appeared worse than that observed in WT or STC1 Tg mice after CC treatment and I/R. These data suggest that STC1 expression and preactivation of AMPK before I/R are critical for the protection of the kidney from I/R (see Discussion).
Figure 9.
Low AMPK activity and diminished expression of UCP2 and SIRT3 in STC1 KO kidneys. Total kidney lysates were obtained 72 hours after I/R or sham surgery; Western blots were reacted with anti-STC1, anti-pAMPKα1,α2, anti-AMPKα1,α2, and anti-actin. Kidney mitochondrial lysates were obtained 72 hours after I/R or sham surgery; Western blots were reacted with anti-UCP2, anti-SIRT3, and anti–F1-ATPase. Bar graphs represent the mean±SEM of data obtained from three mice per group. Sh, sham; tAMPK, total AMPK. *P<0.05 versus WT sham.
Figure 10.
STC1 KO mice display severe histologic injury post-I/R. STC1 KO and WT mice were subjected to I/R or sham surgery and euthanized 72 hours later. (A) Periodic acid–Schiff staining of kidney sections for morphology showed greater injury in sham-treated STC1 KO mice compared with WT mice, and after I/R, STC1 KO mice displayed severe kidney injury characterized by diffuse cellular vacuolization, marked tubular dilation, and cast formation involving the cortex and corticomedullary junction. Representative images from two mice per group are shown. (B) Bar graph represents quantification of tubular injury and depicts the mean±SEM of the percentage of tubules with cellular vacuolization, dilation, and/or cast formation (n=3 mice/group). *P<0.05 versus WT sham.
Figure 11.
STC1 KO mice display increased susceptibility to I/R kidney injury. (A) STC1 KO or WT mice were subjected to I/R or sham surgery and serum creatinine, (B) CrCl was normalized to weight, and (C) timed urine output was carried out as detailed in Concise Methods. UOP, urine output. Bar graphs depict the mean±SEM from three mice per group. *P<0.05 versus WT baseline.
Figure 12.
STC1 KO mice display increased H2O2 and superoxide levels after I/R. (A) WT or STC1 KO mice were subjected to I/R or sham surgery, and superoxide (MitoSOX red fluorescence) was measured in tubular epithelial cells 72 hours after I/R or sham surgery. Representative images from two mice per group are shown; blue fluorescence corresponds to 4′,6-diamidino-2-phenylindole. (B) Bar graphs show H2O2 and superoxide 72 hours post-I/R or sham surgery; data represent mean±SEM from three mice per group. *P<0.05 versus WT sham.
SIRT3 Induces UCP2 in the Kidney and Decreases Superoxide Generation
The mitochondria account for the majority of cellular ROS production. Recent published works suggest that mitochondrial SIRT3 activates several enzymes that are critical for the regulation of cellular ROS.24,32,33 In brown adipose tissue, increased expression of SIRT3 upregulates the expression of UCP1.34 Because SIRT3 expression is induced by I/R and attenuated by pretreatment with CC (Figure 5), we hypothesized that SIRT3 is involved in the regulation of UCP2 and superoxide in the kidneys. Indeed, SIRT3 Tg kidneys express higher baseline levels of UCP2 relative to WT, which correlates with lower baseline superoxide generation (Figure 13), similar to observations that we made in STC1 Tg kidneys.16 As observed in WT mice, where pretreatment with CC attenuated I/R-induced UCP2 and SIRT3 expression, pretreatment of STC1 Tg mice with CC decreases the expression of UCP2 and SIRT3 at both baseline and post-I/R (Figure 8). Similarly, in cultured HEK cells, treatment with CC abolishes STC1-induced expression of UCP2 and SIRT3 (Figure 6B). Collectively, our data suggest that STC1 confers resistance to I/R kidney injury through activation of AMPK, leading to induction of the mitochondrial proteins SIRT3 and UCP2 and suppression of superoxide generation.
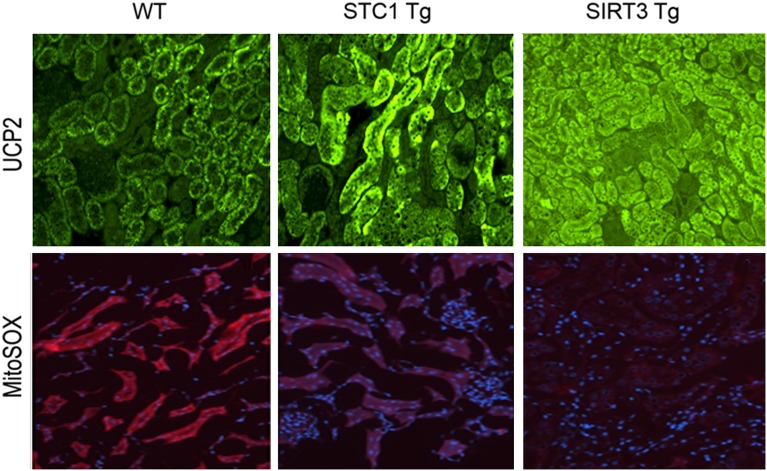
Figure 13.
Increased expression of UCP2 in tubular epithelium of STC1 Tg or SIRT3 Tg kidneys correlates with decreased superoxide generation. (Upper panel) Kidney sections from WT, STC1 Tg, and SIRT3 Tg mice were reacted with anti-UCP2 followed by FITC-labeled secondary antibody. Representative immunofluorescence images show higher-level UCP2 in tubular epithelial cells of STC1 Tg and SIRT3 Tg kidneys. (Lower panel) Decreased superoxide (MitoSOX red fluorescence) in tubular epithelial cells of STC1 Tg and SIRT3 Tg kidneys corresponding to higher levels of UCP2 expression; blue fluorescence corresponds to 4′,6-diamidino-2-phenylindole.
Discussion
Our findings identify STC1 as a regulator of AMPK, UCP2, and SIRT3 in the kidney and bring new insight into the role of STC1 in kidney physiology. Baseline activity of AMPK in the kidney correlates with the expression of STCs, with the highest activity observed in STC1 Tg followed (in decreasing order) by WT, STC1 KO, and STC1/STC2 double-KO mice. On the basis of the relatively small number of tubules that display AMPK activity in STC1 KO mice, which is absent in STC1/STC2 double-KO mice and thus, attributable to STC2, we conclude that STC2 has a minor role in AMPK activation in the kidney. We have previously shown that Tg overexpression of STC1 increases the expression of UCP2, suppresses superoxide generation, and confers resistance to I/R kidney injury.16 We found that treatment with CC does not affect I/R-induced STC1 protein expression but abolishes the increase in UCP2 and SIRT3 in response to I/R. Moreover, STC1-induced expression of UCP2 and SIRT3 in HEK cells is AMPK-mediated. These data place STC1 upstream of AMPK, whereas UCP2 and SIRT3 appear to be downstream of AMPK. Current observations suggest that inhibition of AMPK in STC1 Tg mice diminishes the expression of SIRT3 and UCP2, increases the generation of ROS, and restores the susceptibility to I/R kidney injury. Consistently, STC1 KO mice, an alternate model of low AMPK activity, display greater susceptibility to I/R kidney injury. Thus, activation of AMPK in the kidney seems to be critical for STC1-induced suppression of superoxide and cytoprotection, and the protective effects of STC1/AMPK are possibly mediated through UCP2 and SIRT3.
STC1 is a stress gene; it responds to various stimuli, including hypoxia35 and cytokines.36 The expression of STC1 after I/R is likely a response to the resulting hypoxia and/or release of cytokines; however, previous work showed that stress-induced expression of STC1 may lag considerably,36 as does the expression of UCPs in response to STC1.15 Thus, effective protection by STC1 requires pre-exposure of the cells to STC1 or preactivation of AMPK.21 Indeed, Tg overexpression of STC1 (pre-exposure) inhibits ROS and renal I/R injury in mice16; AMPK activity is elevated in STC1 Tg mice at baseline (relative to WT) and does not change after I/R (Figure 7A). In contrast, STC1 KO mice are more susceptible to I/R kidney injury (relative to WT); consistently, AMPK activity is low in STC1 KO mice at baseline and does not change after I/R (Figure 9). Thus, the timing of STC1 expression and/or AMPK activation before I/R is important for kidney protection rather than the level of AMPK activity in response to stress or injury.
The metabolic sensor AMPK regulates energy-generating and -consuming pathways; it is highly expressed in the kidney and involved in kidney physiology and pathophysiology.20 Sodium transport consumes large amounts of energy, and AMPK couples ion transport to cellular energy metabolism. AMPK is activated in response to high dietary salt37; it regulates several ion transporters, including the Na+,K+-ATPase,38,39 the epithelial sodium channel, the Na+,K+2Cl− cotransporter, and the vacuolar hydrogen pump H+,ATPase.19,20,40,41 AMPK has also been reported to play a role in the regulation of podocyte function and the pathogenesis of diabetic nephropathy. Activation of AMPK by adiponectin in podocytes reduces albuminuria,42 whereas reduced AMPK activity in the diabetic kidney is associated with accumulation of triglycerides and glycogen and results in kidney hypertrophy.43 Kidney ischemia activates AMPK, and cumulative data suggest that preactivation of AMPK protects from I/R kidney injury. Here, we identify STC1 as an important activator and regulator of AMPK in the kidney; by extrapolation, our data suggest an important role for STC1 in the control of transport processes in the kidney from Cl− to Na+ and H+ as well as the regulation of podocyte function and diabetes.
The observation that STC1 induces SIRT3 highlights the importance of STC1 even further. Null mutations of silent information regulator 2 shorten, whereas an extra copy of silent information regulator 2 extends lifespan in yeast by 50%.44–47 The mammalian family of sirtuins consists of seven members that catalyze an NAD+-dependent ADP ribosylation or acetyl transfer from lysine moieties on proteins to inhibit or activate proteins, respectively.48 Sirtuins localize to the nucleus (SIRT1, SIRT6, and SIRT7), mitochondria (SIRT3, SIRT4, and SIRT5), and cytoplasm (SIRT2).49 STC1 localizes to the inner mitochondrial membrane13; SIRT3 also localizes to the inner mitochondrial membrane, induces UCPs, and decreases superoxide generation in adipocytes.34 We, therefore, hypothesized that UCP2 expression in the kidney is SIRT3-dependent. Indeed, our observations reveal increased expression of UCP2 concomitant with lower-level superoxide (red mitochondrial superoxide indicator [MitoSOX] fluorescence) in SIRT3 Tg kidneys. Moreover, STC1 and AMPK regulate the expression of SIRT3 in the kidney. It is, therefore, possible that the cytoprotective effects of STC1 are mediated, in part, through changes in SIRT3-induced responses that may not be limited to upregulation of UCP2 and reduction in mitochondrial superoxide. Thus, our data pave the way for the discovery of novel mitochondrial pathways for cytoprotection.
In summary, our data suggest that (1) STC1 is important for AMPK activation in the kidney and that (2) STC1-induced protection from I/R and expression of UCP2 and SIRT3 are AMPK-dependent.
Concise Methods
Materials
rSTC1 was purchased from MyBioSource (San Diego, CA). Goat anti-ATP5B subunit of F1-ATPase, goat anti-UCP2, and goat anti-STC1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-AMPKα1/α2 was purchased from Signalway (Atlanta, GA). Rabbit anti-pAMPKα1/α2 (recognizes phosphorylated Thr172) was purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-SIRT3 was purchased from EMD Millipore (Billerica, MA). Rabbit anti-actin was purchased from Sigma-Aldrich (St. Louis, MO). MitoSOX was purchased from Invitrogen (Carlsbad, CA). Creatinine measurement kit was purchased from BioAssay Systems (Hayward, CA).
Mice
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and animal experiments were approved by university ethics review boards. STC1 Tg mice were generated by Varghese et al.27 and made available for our studies (derived from line 2). STC1 Tg is driven by the metallothionein I minimal promoter over C57B/6 genetic background,27 and their phenotype has been described in previous studies.16,27,50 SIRT3 Tg is driven by the cytomegalovirus early enhancer/chicken actin promoter on C57B/6 background; mice display no overt phenotype (unpublished data). STC1 KO and STC1/STC2 double KO are also on C57B/6 genetic background and have been described elsewhere.28,29 All studies are carried out using mice homozygous for the transgene or WT mice derived from heterozygous breeders of the respective lines. Mice are maintained in air-conditioned rooms under pathogen-free conditions with 12-/12-hour light/dark cycles and given free access to food and water during the experiments.
AKI Model
We used an established mouse model of kidney I/R injury induced by clamping of bilateral renal pedicles.16 Briefly, male mice (10–25 weeks, 19–30 g; the proportion of young-to-old mice was evenly distributed between the treatment groups) are anesthetized with an intraperitoneal injection of 2 ml/kg combination anesthetic (contains 37.5 mg ketamine, 1.9 mg xylazine, and 0.37 mg acepromazine per 1 ml). After abdominal incision, left and right renal pedicles are bluntly dissected, and a nontraumatic vascular clamp (Roboz Surgical Instruments, Gaithersburg, MD) is placed on each renal pedicle for 30 minutes. During the procedure, animals are kept under heating lamp and hydrated with warm saline. After 30 minutes of ischemia, the clamps are removed, the wounds are sutured, and the animals are allowed to recover. Sham-treated animals undergo similar surgical procedures without clamping of the renal pedicles. Mice are killed at 72 hours after surgical procedure; blood samples are obtained for creatinine measurement. Kidneys are harvested for histology, immunohistochemistry, Western blotting, superoxide, and H2O2 measurements. Histologic injury was analyzed by one member of the team (J.S.-C.P.), determined based the number of tubules showing vacuolization, dilation, and cast formation, and expressed as the mean±SEM of the percentage of tubules with cellular vacuolization, dilation, and/or cast formation.
CC Treatment
CC (Sigma-Aldrich) is dissolved in methanol and diluted in saline (final concentration was 1.25 mg/ml CC and 20% methanol). WT and STC1 Tg mice are given a single intraperitoneal injection of CC at 20 mg/kg or vehicle (20% methanol) 2 hours before clamping/sham surgery.
Assessment of Renal Function
For baseline CrCl measurement, mice are placed in metabolic cages for urine collection starting 24 hours before I/R or sham operation, and blood samples are collected for creatinine measurement before I/R or sham surgery. For CrCl measurement post-I/R or sham operation, mice are placed in metabolic cages for 24 hours of timed urine collection beginning 48 hours post-I/R or sham surgery and ending at the time of euthanasia (72 hours post-I/R); blood samples are taken at the time of euthanasia. Serum and urine creatinine are measured using the QuantiChrom Creatinine Assay Kit (Jaffe method) as per the manufacturer’s instructions. CrCl is calculated and normalized to weight. Please note that this method overestimates serum creatinine (almost by a factor of two) compared with capillary electrophoresis-16 or HPLC-based51 measurement of serum creatinine and thus, yields lower estimates of CrCl.
Cell Culture
HEK cells were grown in DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C under 5% CO2/95% air. For time-course experiment, cells were treated with rSTC1 (100 ng/ml) or PBS for the designated time periods, scraped, collected by centrifugation, lysed in radioimmunoprecipitation assay buffer containing phosphatase inhibitors and complete mini protease inhibitor (Roche Diagnostics, Indianapolis, IN), and stored until used. In some experiments, cells were treated with rSTC1 plus CC (2 μM in 20% methanol) or rSTC1 (100 ng/ml) plus vehicle (20% methanol) for predetermined periods followed by harvesting as above.
Western Blotting
Lysates representing whole kidney or kidney mitochondrial fraction from WT, STC1 Tg, or STC1 KO mice are suspended in radioimmunoprecipitation assay buffer containing complete mini protease inhibitor (Roche Diagnostics), phosphatase inhibitors, trichostatin A, and nicotinamide (Sigma-Aldrich). Nicotinamide and trichostatin A are class III and class I histone deacetylase inhibitors, respectively, and they are added to prevent the deacetylation of proteins in vitro. Lysates are centrifuged at 8000×g for 10 minutes at 4°C to remove cell debris. Equal amounts of protein are separated on 12% SDS-PAGE, transferred onto nitrocellulose membranes, and incubated overnight at 4°C with primary antibodies for pAMPK, AMPK, STC1, F1-ATPase (loading control for mitochondrial proteins), actin, SIRT3, or UCP2. After washing with PBS containing 0.1% Tween-20, the membrane is incubated with horseradish peroxidase-conjugated secondary antibody. The bound antibodies are visualized using chemiluminescence. Band densities of target proteins are quantified using National Institutes of Health ImageJ Software.
AMPK Activities
Whole-kidney lysates are resolved on 12% SDS-PAGE, and Western blots are reacted with pAMPK antibodies followed by stripping and reaction with AMPK antibodies. Bands representing pAMPK are normalized to the corresponding bands representing total AMPK (pAMPK/total AMPK) or actin (pAMPK/actin) and expressed as mean±SEM.
Immunohistochemistry
Formalin- or methanol-Carnoy–fixed kidney sections (5 µm) are subjected to periodic acid–Schiff staining (for morphology), anti-pAMPK (active kinase), or anti-UCP2 followed by FITC-labeled secondary antibodies. Detection is carried out using a peroxidase enzyme-based detection system (Vector Laboratories, Burlingame, CA) or fluorescence microscopy (excitation at 488 nm). Photomicrographs were taken, and quantitation of immunostaining was carried out using NIS-elements Br 3.0 software (Nikon); two representative areas of the kidney sections (viewed at magnification of ×100 for pAMPK and ×200 for periodic acid–Schiff) were analyzed.
MitoSOX Fluorescence
Freshly isolated kidneys are sectioned coronally to obtain 1-mm-thick slices that are incubated in DMEM containing 5 μmol/L MitoSOX red reagent for 10 minutes at room temperature. MitoSOX permeates live cells and selectively targets the mitochondria. It is rapidly oxidized by superoxide and emits red fluorescence. After incubation with MitoSOX, kidney slices are rinsed in PBS, fixed in 4% paraformaldehyde overnight at 4°C, and embedded in optimal cutting temperature compound; 5-μm-thick frozen sections are stained with 4′,6-diamidino-2-phenylindole and viewed under fluorescence microscope.
Superoxide Measurements
Kidneys are harvested and homogenized in 250 μL sucrose buffer (composition: 0.31 mol/L sucrose, 10 mmol/L Tris-HCL, pH 7.4) on ice followed by protein quantitation (Bradford method). Kidney lysates (100 μg protein/100 μL) are placed in clear flat-bottom wells (96-well plate) containing excess dihydroethidium (100 μmol/L) in a final volume of 100 μL. The absorbance (at 530 nm) is immediately measured. Absorbance values are normalized to the absorbance of wells containing equimolar concentrations of dihydroethidium alone and expressed as OD.
H2O2 Measurements
Kidneys are harvested and homogenized in 250 μL sucrose buffer on ice followed by protein quantitation (Bradford method). H2O2 is measured as described previously.52 The method takes advantage of the conversion of Fe2+ to Fe3+ in the presence of H2O2 followed by detection of Fe3+-xylenol orange complex. Briefly, kidney lysate (100 μg/20 μL) is added to 180 μL assay buffer (composition: 100 μmol/L xylenol orange, 250 μmol/L ammonium ferrous sulfate, 4 mmol/L butylatedhydroxytoluene, and 25 mmol/L H2SO4 in methanol) and incubated at room temperature for 30 minutes followed by measurement of absorbance at 560 nm. Absorbance values are normalized to the absorbance of wells containing assay buffer alone and expressed as OD.
Isolation and Purification of the Heavy Mitochondrial Fraction from Murine Kidney
Mitochondria are isolated from mouse kidney as previously described.53 Briefly, kidneys are harvested and then transferred to chilled homogenization medium (HM; composition: 0.2 M manitol, 50 mM sucrose, 10 mM KCl, 1 mM EDTA, and 10 mM Hepes, pH 7.4). After rinsing, the kidney is homogenized in HM containing a protease inhibitor cocktail, 500 nM trichostatin A, and 10 mM nicotinamide. The homogenate is centrifuged on a bench-top centrifuge for 10 minutes at 1000×g (4°C). The supernatant is recovered and centrifuged for 10 minutes at 3000×g (4°C). The resulting supernatant is then aspirated, leaving a brown mitochondrial pellet. The mitochondrial pellet is then resuspended in HM and recentrifuged for 10 minutes at 3000×g (4°C); this wash procedure is repeated three times. After the final wash, the pellet is resuspended in 100 μL mitochondria lysis buffer (composition: 1% n-dodecyl-B-D-maltoside, 0.5 mM EDTA, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, protease inhibitor cocktail, 500 nM trichostatin A, and 10 mM nicotinamide) and stored at −80°C until analyzed.
Disclosures
None.
Acknowledgments
This work was supported by Veteran Administration Grant BX002006-01, National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health Grants R01-DK080306 and T32-DK062706, and a generous gift from Dr. and Mrs. Harold Selzman. This project was also supported by the Pathology and Histology Core at Baylor College of Medicine with funding from National Institutes of Health Grant NCI P30-CA125123 and the expert assistance of Michael Ittmann.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Carpenter CB: Long-term failure of renal transplants: Adding insult to injury. Kidney Int Suppl 50: S40–S44, 1995 [PubMed] [Google Scholar]
- 3.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV: Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem 278: 27256–27266, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Gueler F, Gwinner W, Schwarz A, Haller H: Long-term effects of acute ischemia and reperfusion injury. Kidney Int 66: 523–527, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM: The “injury response”: A concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 29: 79–81, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P: Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr 17: 193–199, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Molitoris BA, Sandoval R, Sutton TA: Endothelial injury and dysfunction in ischemic acute renal failure. Crit Care Med 30[Suppl]: S235–S240, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA: Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS: Endothelial dysfunction in ischemic acute renal failure: Rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282: F1140–F1149, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Jang HR, Ko GJ, Wasowska BA, Rabb H: The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 87: 859–864, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Varghese R, Wong CK, Deol H, Wagner GF, DiMattia GE: Comparative analysis of mammalian stanniocalcin genes. Endocrinology 139: 4714–4725, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Re RN, Cook JL: The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol 299: H577–H583, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCudden CR, James KA, Hasilo C, Wagner GF: Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277: 45249–45258, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Yeung BH, Law AY, Wong CK: Evolution and roles of stanniocalcin. Mol Cell Endocrinol 349: 272–280, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R, Poindexter B, Sheikh-Hamad D: Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol 86: 981–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D: Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 82: 867–877, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Jamaluddin MS, Yan S, Sheikh-Hamad D, Yao Q: Human stanniocalcin-1 blocks TNF-alpha-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 28: 906–912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanellis J, Bick R, Garcia G, Truong L, Tsao CC, Etemadmoghadam D, Poindexter B, Feng L, Johnson RJ, Sheikh-Hamad D: Stanniocalcin-1, an inhibitor of macrophage chemotaxis and chemokinesis. Am J Physiol Renal Physiol 286: F356–F362, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Pastor-Soler NM, Hallows KR: AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens 21: 523–533, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Hallows KR, Mount PF, Pastor-Soler NM, Power DA: Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lempiäinen J, Finckenberg P, Levijoki J, Mervaala E: AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 166: 1905–1915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand MD, Esteves TC: Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2: 85–93, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kim-Han JS, Dugan LL: Mitochondrial uncoupling proteins in the central nervous system. Antioxid Redox Signal 7: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Bause AS, Haigis MC: SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 48: 634–639, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE: Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiva MA, Gonçalves LM, Providência LA, Davidson SM, Yellon DM, Mocanu MM: Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther 24: 25–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varghese R, Gagliardi AD, Bialek PE, Yee SP, Wagner GF, Dimattia GE: Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology 143: 868–876, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Chang AC, Cha J, Koentgen F, Reddel RR: The murine stanniocalcin 1 gene is not essential for growth and development. Mol Cell Biol 25: 10604–10610, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR: The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology 149: 2403–2410, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Belousova T, Pan JS, Du J, Ju H, Lu L, Zhang P, Truong LD, Nuotio-Antar A, Sheikh-Hamad D: AKI after conditional and kidney-specific knockdown of stanniocalcin-1 [published online ahead of print April 3, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ: Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol 301: F1346–F1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D: Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA: Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi T, Wang F, Stieren E, Tong Q: SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280: 13560–13567, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CK: Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146: 4951–4960, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC: Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 38: 1025–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA: Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Alves DS, Farr GA, Seo-Mayer P, Caplan MJ: AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol Biol Cell 21: 4400–4408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benziane B, Björnholm M, Lantier L, Viollet B, Zierath JR, Chibalin AV: AMP-activated protein kinase activator A-769662 is an inhibitor of the Na(+)-K(+)-ATPase. Am J Physiol Cell Physiol 297: C1554–C1566, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA: Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 405: 85–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM: Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ: Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cammisotto PG, Londono I, Gingras D, Bendayan M: Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. Am J Physiol Renal Physiol 294: F881–F889, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Kaeberlein M, McVey M, Guarente L: The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13: 2570–2580, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinclair DA, Guarente L: Extrachromosomal rDNA circles—a cause of aging in yeast. Cell 91: 1033–1042, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Sinclair DA, Mills K, Guarente L: Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 277: 1313–1316, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, Pak SM, Laroche T, Gasser SM, Guarente L: Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89: 381–391, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Pereira CV, Lebiedzinska M, Wieckowski MR, Oliveira PJ: Regulation and protection of mitochondrial physiology by sirtuins. Mitochondrion 12: 66–76, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Haigis MC, Guarente LP: Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D: Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol 174: 1368–1378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K: Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Jiang ZY, Woollard AC, Wolff SP: Hydrogen peroxide production during experimental protein glycation. FEBS Lett 268: 69–71, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Hirschey MD, Shimazu T, Huang JY, Verdin E: Acetylation of mitochondrial proteins. Methods Enzymol 457: 137–147, 2009 [DOI] [PubMed] [Google Scholar]