Abstract
Arteriovenous fistula (AVF) is the preferred vascular access for hemodialysis (HD). However, many AVFs fail before starting dialysis. To assess the optimal time for AVF placement in the elderly, we linked data from the US Renal Data System with Medicare claims data to identify 17,511 patients≥67 years old on incident HD who started dialysis between January 1, 2005, and December 31, 2008, with an AVF placed as the first predialysis access. AVF success was defined as dialysis initiation using the AVF, with time between AVF placement and dialysis start as our primary variable of interest. The mean age was 76.1±6.0 years, and 58.3% of subjects were men. Overall, 54.9% of subjects initiated dialysis using an AVF, and 45.1% of subjects used a catheter or graft. The success rate increased as time from AVF creation to HD initiation increased from 1–3 months (odds ratio [OR], 0.49; 95% confidence interval [95% CI], 0.44 to 0.53) to 3–6 months (OR, 0.93; 95% CI, 0.85 to 1.02) to 6–9 months (OR, 0.99; 95% CI, 0.88 to 1.11) but stabilized after that time. Furthermore, the number of interventional access procedures increased over time starting at 1–3 months, with a mean of 0.64 procedures/patient for AVFs created 6–9 months predialysis compared with 0.72 for AVFs created >12 months predialysis (P<0.001). Although limited by the observational nature of this study, our results suggest that placing an AVF>6–9 months predialysis in the elderly may not associate with a better AVF success rate.
Keywords: hemodialysis, vascular access, arteriovenous fistula
Arteriovenous fistula (AVF) is the preferred access for hemodialysis (HD), because it is considered to have the lowest risk of complications, lowest need for interventions, best long-term patency, and superior patient survival.1–3 This preference has led to the Fistula First initiative as the general recommendation for all patients on HD,4–6 which resulted in a rapid rise in AVF prevalence rate.7 However, many AVFs fail to mature,8,9 necessitating interventions, such as angioplasty and stent placement, that are often ineffective. Unsuccessful AVF placements result in a high incidence of central venous catheter (CVC) use at HD initiation, which is associated with increased morbidity and mortality.1–3,10 This finding is especially true with regard to the older dialysis population. Many of these patients have higher comorbidity and insufficient vasculature for AVF maturation, resulting in a reduced rate of AVF patency with two times the failure rate compared with a younger population.11–14 In our previous report, we showed that, in those 67 years old or older, only 50.7% of patients with AVF placement initiated dialysis using the AVF, whereas 43.4% of patients initiated dialysis with a CVC and 5.9% of patients initiated dialysis with an arteriovenous graft (AVG).15 AVFs need time to mature before being usable for dialysis, but the optimal time for access placement before starting HD is open for debate. Cannulation practice differs between countries and facilities, such that first cannulation occurred <2 months after AVF placement in 36% of United States, 79% of European, and 98% of Japanese facilities. Although 99% of fistulae are cannulated within 4 months from creation in the United States,16,17 vascular access teams tend to construct AVFs earlier rather than later before HD initiation. In addition to a lack of evidence to support such a policy, early placement of AVFs might be associated with potential problems. A significant number of older patients who have AVFs placed before dialysis may die, and some may undergo preemptive transplantation before HD initiation, making AVF placement unnecessary. Furthermore, other factors should be considered with early fistula creation, such as the inconvenience of having an unused access for a significant period of time, clotting and stenosis necessitating invasive procedural risks, and the less common chance of AVF injury or developing high cardiac output failure.18,19 Counterbalancing these concerns regarding early AVF placement, there are real concerns about delayed AVF placement leading to increased reliance on CVC, which is associated with increased mortality, morbidity, and cost.
The purpose of this study was to determine an optimal time for AVF placement before starting HD. We hypothesized that placing an AVF in this population too early may not lead to a higher success rate but might add an increased number of invasive procedures to maintain patency. To assess vascular access outcomes in the elderly, we sought to find the time before dialysis AVF creation that achieved the highest percentage of fistulae at HD initiation with the lowest number of interventions.
Results
Baseline Characteristics
The study cohort consisted of 17,511 subjects with a mean age of 76.1±6.0 years at the time of initiation of HD; 58.3% of patients were men, 77.6% of patients were white, and 56% of patients had diabetes. (Table 1). Of 17,511 patients, 9608 (54.9%) patients initiated HD using an AVF (AVF success group), and 7903 (45.1%) patients initiated dialysis using a catheter or an AVG (AVF failure group) for the first dialysis session. In men, AVF success rate was 61%, whereas in women, AVF success rate was only 47% (P<0.001). Similarly, the success rate in whites was significantly higher than in blacks (57% versus 46%, P<0.001).
Table 1.
Baseline characteristics of the study population at the time of onset of ESRD
| Variable | Entire Study Populationa (n=17,511) | AVF Successa (n=9608) | AVF Failurea (n=7903) | P Value | |
|---|---|---|---|---|---|
| Absolute Number | Percent of Total for Categorical Variables | ||||
| Age (yr) | 76.1 (6.0) | 76.2 (6.0) | 76.0 (6.0) | 0.04 | |
| Age categories (yr) | |||||
| 67–79 | 12,293 | 70.2% | 54% | 46% | 0.09 |
| 80–89 | 4991 | 28.5% | 56% | 44% | |
| ≥90 | 228 | 1.3% | 54% | 46% | |
| Sex | |||||
| Men | 10,209 | 58.3% | 61% | 39% | <0.001 |
| Women | 7302 | 41.7% | 47% | 53% | |
| Race/ethnicity | |||||
| Non-Hispanic white | 13,589 | 77.6% | 57% | 43% | <0.001 |
| Non-Hispanic black | 3222 | 18.4% | 46% | 54% | |
| Asian | 566 | 3.23% | 59% | 41% | |
| Native American | 140 | 0.8% | 55% | 45% | |
| Other | 5 | 0.03% | 40% | 60% | |
| BMI (kg/m2) | 27.8 (6.5) | 27.6 (6.3) | 28.2 (6.8) | <0.001 | |
| Underweight (<18.5) | 718 | 4.1% | 56% | 44% | <0.001 |
| Normal (18.5–24.9) | 5656 | 32.3% | 52% | 48% | |
| Overweight (25–29.9) | 5866 | 33.5% | 53% | 47% | |
| Obese (≥30) | 5271 | 30.1% | 60% | 40% | |
| Duration of pre-ESRD nephrology care | |||||
| None | 876 | 5.0% | 27% | 73% | <0.001 |
| <6 mo | 2346 | 13.4% | 55% | 45% | |
| 6–12 mo | 5026 | 28.7% | 54% | 46% | |
| >12 mo | 8265 | 47.2% | 61% | 39% | |
| Missing | 1016 | 5.8% | 32% | 68% | |
| Employment status | |||||
| Unemployed | 1716 | 9.8% | 48% | 52% | <0.001 |
| Retired | 15,094 | 86.2% | 55% | 45% | |
| Working part time | 263 | 1.5% | 62% | 38% | |
| Working full time | 438 | 2.5% | 59% | 41% | |
| Primary cause of ESRD | |||||
| Diabetes | 7845 | 44.8% | 51% | 49% | <0.001 |
| Hypertension | 6514 | 37.2% | 56% | 44% | |
| Cystic disease | 1016 | 5.8% | 65% | 35% | |
| GN | 403 | 2.3% | 60% | 40% | |
| Other | 1734 | 9.9% | 60% | 40% | |
| Presence of diabetes | |||||
| Yes | 9806 | 56.0% | 51% | 49% | <0.001 |
| No | 7705 | 44.0% | 59% | 41% | |
| Presence of CHF | |||||
| Yes | 6094 | 34.8% | 50% | 50% | <0.001 |
| No | 11,417 | 65.2% | 57% | 43% | |
| Presence of malignancy | |||||
| Yes | 1804 | 10.3% | 57% | 43% | 0.05 |
| No | 15,707 | 89.7% | 55% | 45% | |
| Presence of PVD | |||||
| Yes | 3169 | 18.1% | 54% | 46% | 0.22 |
| No | 14,342 | 81.9% | 55% | 45% | |
| Presence of cerebrovascular disease | |||||
| Yes | 1979 | 11.3% | 51% | 49% | 0.003 |
| No | 15,532 | 88.7% | 55% | 45% | |
| Geographic location | |||||
| Rural | 4133 | 23.6% | 55% | 45% | 0.82 |
| Urban | 13,098 | 74.8% | 55% | 45% | |
| Unknown | 280 | 1.6% | 55% | 45% | |
| Income (annual per family) | $43,571.8 ($17,246.2) | $44,332.3 ($17,395.4) | $42,647.4 ($17,018.3) | <0.001 | |
| Smoking | |||||
| Yes | 595 | 3.4% | 55% | 45% | 0.67 |
| No | 16,916 | 96.6% | 55% | 45% | |
| Alcohol dependence | |||||
| Yes | 70 | 0.4% | 68% | 32% | 0.12 |
| No | 17,441 | 99.6% | 55% | 45% | |
| Drug use | |||||
| Yes | 18 | 0.1% | 55% | 45% | 0.16 |
| No | 17,493 | 99.9% | 55% | 45% | |
| Time between access placement and HD initiation (mo) | 8.5 (7.2) | 9.09 (7.3) | 7.75 (7.1) | <0.001 | |
| Mean no. of procedures/patient | 0.5 (1.6) | 0.41 (1.6) | 0.52 (1.68) | <0.001 | |
Continuous variables are presented as means (SDs), and categorical variables are presented as percentage of the entire study population in each category and percentage of AVF success versus failure in the category.
There was a significant difference between the success and failure rates in relation to duration of nephrology care before HD initiation. AVF success rate was significantly higher in those patients who had pre-ESRD nephrology care for >12 months as opposed to those who were not seen by a nephrologist before HD initiation (61% versus 27%, P<0.001) whereas those with pre-ESRD nephrology care for <1 year had an intermediate rate of AVF success (54%–55%).
AVF success rate was significantly lower in patients with diabetes compared with patients without diabetes (51% versus 59%, P<0.001) as well as patients with congestive heart failure (CHF) compared with patients without CHF (50% versus 57%, P<0.001). The presence of malignancy or peripheral vascular disease (PVD) did not significantly affect AVF success rates. The effect of body mass index (BMI) was unclear; whereas the BMI was slightly but significantly lower in the total AVF success group than in the AVF failure group (27.6 versus 28.2, P<0.001), the AVF success rates were higher in the highest and lowest BMI groups.
The number of access procedures between AVF creation and HD initiation was significantly different (P<0.001) between the two groups, such that the mean number of interventions per patient was lower in the AVF success group (0.41±1.6) compared with the AVF failure group (0.52±1.68). Other baseline characteristics and their distribution in the study groups are presented in Table 1.
Logistic Regression Analysis of the Entire Study Population
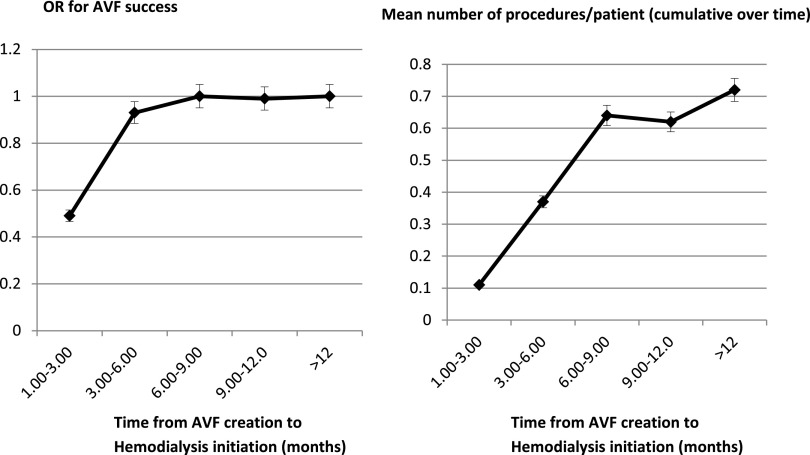
We performed logistic regression analysis to establish the odds ratio (OR) for AVF success in relation to the time from AVF creation to the beginning of HD. A priori, we divided the time into five categories (1–3, 3–6, 6–9, 9–12, and ≥12 months) and compared the success rate in each category with a reference time point of >12 months from AVF creation to HD initiation. The OR for success increased as time from AVF creation to HD initiation increased in the categories of 1–3, 3–6, and 6–9 months (OR, 0.49, 95% confidence interval [95% CI], 0.44 to 0.53; OR, 0.93; 95% CI, 0.85 to 1.02; and OR, 0.99; 95% CI, 0.88 to 1.11, respectively) but stabilized after that time. Therefore, in this overall cohort of elderly patients, placing an AVF >6–9 months predialysis was not associated with greater success. Rather, the number of access procedures continued to rise, such that the average number of procedures per patient was 0.64 (64 procedures per 100 patients) when an AVF was inserted 6–9 months before HD compared with 0.72 (72 procedures per 100 patients) when an AVF was created >12 months before HD (Figure 1, Table 2).
Figure 1.
Stabilization of OR for AVF success at 6–9 months pre-HD. OR for success and mean number of procedures per patient with relation to time from AVF placement to HD initiation. The OR for success reaches its maximum at 6–9 months from AVF creation to the beginning of HD, whereas the number of procedures continues to rise as the duration between placement and HD initiation increases.
Table 2.
Odds of AVF success using multivariate logistic regression model for the entire study population
| Variable | Entire Study Population (n=17,511) | |
|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | |
| Age at onset of ESRD (yr) | 0.99 (0.99 to 1.00) | 0.02 |
| Women (versus men) | 0.61 (0.57 to 0.65) | <0.001 |
| Race/ethnicity | ||
| White | Reference | |
| Black | 0.72 (0.66 to 0.79) | <0.001 |
| Asian | 1.14 (0.95 to 1.37) | 0.16 |
| Native American | 0.90 (0.62 to 1.30) | 0.57 |
| Other | 0.50 (0.09 to 2.84) | 0.43 |
| Time between access placement and HD initiation (mo) | ||
| >12 (n=4374; 25.0%) | Reference | |
| 1–3 (n=4519; 25.8%) | 0.49 (0.44 to 0.53) | <0.001 |
| 3–6 (n=4300; 24.6%) | 0.93 (0.85 to 1.02) | 0.10 |
| 6–9 (n=2579; 14.7%) | 1.00 (0.90 to 1.11) | 0.96 |
| 9–12 (n=1739; 9.9%) | 0.99 (0.88 to 1.11) | 0.85 |
| Number of proceduresa | 0.93 (0.92 to 0.96) | <0.001 |
| BMI (kg/m2)b | 0.99 (0.99 to 0.99) | 0.02 |
| Primary cause of ESRD | ||
| Diabetes | Reference | |
| Hypertension | 1.01 (0.90 to 1.13) | 0.87 |
| GN | 1.24 (1.04 to 1.46) | 0.01 |
| Cystic disease | 1.02 (0.80 to 1.29) | 0.88 |
| Other | 1.17 (0.95 to 1.43) | 0.14 |
| Presence of diabetes | 0.83 (0.74 to 0.92) | <0.001 |
| Presence of CHF | 0.78 (0.73 to 0.83) | <0.001 |
| Presence of PVD | 0.94 (0.86 to 1.03) | 0.17 |
| Presence of malignancy | 0.96 (0.86 to 1.07) | 0.45 |
| Presence of cerebrovascular disease | 0.88 (0.79 to 0.97) | 0.01 |
| Duration of pre-ESRD nephrology care (mo) | ||
| 0 | Reference | |
| <6 | 3.26 (2.72 to 3.89) | <0.001 |
| 6–12 | 3.23 (2.73 to 3.82) | <0.001 |
| >12 | 3.96 (3.36 to 4.66) | <0.001 |
| Missing data | 1.23 (1.00 to 1.52) | 0.05 |
| Income (annual per family) | 1.00 (1.00 to 1.00) | 0.22 |
| Employment status | ||
| Unemployed | Reference | |
| Retired | 1.04 (0.94 to 1.17) | 0.44 |
| Working part time | 1.34 (1.00 to 1.79) | 0.05 |
| Working full time | 1.18 (0.94 to 1.48) | 0.16 |
| History of smoking (no versus yes) | 0.96 (0.80 to 1.15) | 0.64 |
| Alcohol dependence (no versus yes) | 0.83 (0.50 to 1.37) | 0.47 |
| Drug dependence (no versus yes) | 0.45 (0.12 to 1.75) | 0.25 |
| Geographic location | ||
| Rural | Reference | |
| Urban | 0.95 (0.87 to 1.03) | 0.20 |
| Unknown | 1.08 (0.80 to 1.47) | 0.60 |
The multivariate logistic regression model was adjusted for the following covariates: age at HD initiation, race, sex, comorbidities, primary cause of ESRD, number of procedures, BMI, duration of nephrology care, geographic location, substance abuse (history of smoking, alcohol, or street drugs), employment index, and race-stratified annual median income.
The success rate is reduced by 7% for each procedure performed (defined in Concise Methods).
The success rate is reduced by 1% for every 1-unit increase in BMI (kilograms per meter2).
In a secondary analysis, we included in the model the number of procedures and the interaction term between the number of procedures and the time from AVF placement to dialysis initiation. When the interaction term was not included in the model, the number of procedures had a significant negative association with the success of the AVF (OR, 0.93 per procedure performed; P<0.001; 95% CI, 0.92 to 0.96). With the interaction term included in the model, the negative association was quite similar (OR, 0.90; P<0.001; 95% CI, 0.87 to 0.94). Therefore, including this analysis to look for a possible interaction of the number of procedures in the model had no significant effect on our primary outcome results.
Logistic Regression Analysis in Patient Subgroups
We repeated analysis in various subgroups as shown in Tables 2 and 3. In the subgroup of patients with CHF, the success rate increased from 1–3 to 3–6 to 6–9 months from AVF creation to HD and stabilized after that time, with a slight decrease in success rate when an AVF was placed >12 months as opposed to only 6–9 months before HD. In patients without CHF, the success rate remained relatively constant from 3–6 months to 1 year but showed an increase of 5% in the rate of success >12 months predialysis.
Table 3.
OR for AVF success with relation to time from AVF placement to HD initiation in subgroup analysis on the basis of diabetes, CHF, sex, and race
| Subgroups | Time from AVF Placement to HD (mo) OR (95% Confidence Interval) | |||
|---|---|---|---|---|
| >1 to <3 | >3 to <6 | >6 to <9 | >9 to <12 | |
| In patients with CHF (n=6095) | 0.51 (0.44 to 0.60) | 0.9 (0.77 to 1.05) | 1.12 (0.93 to 1.34) | 1.14 (0.93 to 1.41) |
| In patients without CHF (n=11,416) | 0.47 (0.42 to 0.53) | 0.95 (0.84 to 1.06) | 0.95 (0.83 to 1.08) | 0.92 (0.79 to 1.07) |
| In patients with diabetes (n=9810) | 0.5 (0.44 to 0.56) | 0.93 (0.82 to 1.05) | 1.08 (0.94 to 1.24) | 1.06 (0.90 to 1.24) |
| In patients without diabetes (n=7701) | 0.47 (0.41 to 0.55) | 0.93 (0.81 to 1.07) | 0.91 (0.78 to 1.07) | 0.91 (0.76 to 1.10) |
| In blacks (n=3224) | 0.49 (0.39 to 0.61) | 0.89 (0.72 to 1.10) | 0.94 (0.74 to 1.20) | 0.93 (0.71 to 1.21) |
| In whites (n=13579) | 0.48 (0.43 to 0.53) | 0.93 (0.84 to 1.03) | 1.01 (0.89 to 1.14) | 1.01 (0.88 to 1.16) |
| In women (n=7300) | 0.46 (0.40 to 0.54) | 0.89 (0.77 to 1.02) | 0.91 (0.78 to 1.07) | 0.87 (0.73 to 1.05) |
| In men (n=10,211) | 0.50 (0.44 to 0.56) | 0.96 (0.85 to 1.08) | 1.07 (0.93 to 1.24) | 1.08 (0.92 to 1.27) |
Time between access placement and HD initiation is taken in months; >12 months is taken as the reference category (i.e., OR, 0.99) in all of the analyses. The results were derived from eight separate logistic regression models; each of them was adjusted for the following covariates: age at HD initiation, race, sex, comorbidities, primary cause of ESRD, BMI, duration of nephrology care, geographic location, substance abuse (history of smoking, alcohol, or street drugs), employment index, and race-stratified annual median income.
In patients with diabetes, the OR for success increased as the time from AVF creation to HD prolonged until 6–9 months, with a minimal decrease in success rate beyond that time. In those without diabetes, the success rate remained relatively constant from 3–6 months to 1 year but showed an increase of 9% in success rate when an AVF was placed >12 months before HD.
In black patients, the success rate increased until 6–9 months from AVF creation to HD but showed another increase in success rate of 6% when an AVF was placed >12 months before HD. In contrary to this finding, the success rate in white patients reached its maximum in 6–9 months and stabilized thereafter.
In men, the success rate reached its maximum when an AVF was placed 6–9 months before HD and did not change with a longer time between AVF placement to HD. However, the success rate in women did not change significantly from 3–6 months to 1 year, but an increase of 9% was observed when the time from AVF placement to HD was >12 months (Table 3).
Discussion
Most experts advocate early placement of AVFs considering that fistulae need about 4–6 weeks to mature for use,20,21 and there is general consensus of the importance of early referral for pre-ESRD care.22–27 Although many AVFs are created in advance of dialysis initiation, 99% of fistulae were cannulated within 4 months of their creation in the United States.16,17 Moreover, time to AVF use was 0.5–6 months with a mean of 1.5 months in 461 patients >65 years of age.28 However, to the best of our knowledge, no previous study has presented data that help address the optimal timing of AVF creation in the elderly patient before ESRD.
The earlier the better—is it a myth or a truth? In an attempt to address this question, we studied a large national dataset of elderly patients on HD. Our definition of outcome (i.e., access used for the initial dialysis treatment after placement of an AVF first) was chosen on the basis of its association with patient survival.1,3 This outcome is different from most previous studies (e.g., those looking at access type used at HD initiation [rather than first placed], AVF failure to mature rates, or primary and secondary AVF survival). In an analysis of the entire study population, we found that placing an AVF >6–9 months before first HD did not improve the success rate at HD initiation but was associated with an increased number of interventional procedures. Of note, the total number of interventions would be even greater had we not excluded those patients with placement of a second AVF after failure of the initial fistula in this study.
Given the observational nature of the study, we cannot determine the mechanism of the reported associations. The main finding that the association between the time of AVF placement and odds of success was nonlinear but rather, peaked at 6–9 months makes sense. It is intuitive that an AVF will need time to mature, but there is probably an optimal time, after which time little additional improvement occurs. Moreover, with longer time, the odds of developing stenosis or clotting may be higher.
The subgroup analysis revealed that the trends seen were even more pronounced in those with certain comorbidities. In patients with CHF, placing an AVF >6–9 months pre-HD was not associated with an increase in the success rate; rather, there was a slight decrease in success when the time from AVF insertion to first HD was >12 months. Similar results were observed in the subgroup of patients with diabetes, whereas those without CHF or diabetes showed small benefits (5% and 9%, respectively) when an AVF was placed >12 months before dialysis initiation. One potential explanation is that, in patients with comorbidities that may lead to endothelial dysfunction and vascular wall abnormalities, the hemodynamic shear stress of an AVF might cause increased neointimal hyperplasia with a lack of vascular dilation.29 Because a significant degree of neointimal hyperplasia has been found at the site of the vascular access placement in patients with CKD before AVF creation,30,31 placing an AVF too early in patients with CHF or diabetes may increase the exposure time to neointimal hyperplasia and thereby, AVF failure.
The opposite relationship was seen in the association of timing of AVF placement and success rate on the basis of race and sex. Other reports have noted that women and black race are independent risk factors for AVF failure.32–37 Given the higher failure rate in women and blacks as opposed to men and whites, it is possible that placing a fistula earlier in these high-risk subgroups conferred a better outcome by enabling the longer time needed for maturation. However, the longer time before HD initiation may necessitate the performance of endovascular or surgical interventions to achieve adequate patency.
Certain limitations should be considered when interpreting the results of this study. The study is an observational study performed retrospectively using the data from a large national data registry. Observational analysis of a data registry shows the association (but not the causative relationships) between the primary variables of interest and the outcome. The quality of data is always a concern because of a significant amount of missing and potentially erroneous information (misclassification bias) and misreporting. In particular, potential underreporting of comorbidities by the Centers for Medicare and Medicaid Services (CMS) Form 2728 might have minimized the potential effect of comorbidities. Specifically, PVD surprisingly was not associated with AVF failure, perhaps because of a selection bias of placing an AVF only in those patients with limited PVD that were considered more suitable for AVF creation. Furthermore, the power of large amounts of data may lead to small and clinically insignificant associations that show statistical significance in the analysis. Therefore, the associations with borderline P value (<0.05), although technically significant, should be interpreted with caution. In addition, the standard definition of success that we used of AVF use at dialysis initiation for the first session lacks information about how effective or how long the AVF was functional. Finally, because our multivariate models depend on the variables available in the dataset, certain independent variables were not included in the analysis (e.g., location of AVF). Survival bias is another limitation relevant to our study, because only people who survived to initiate dialysis were included; data regarding those who did not survive are absent.
This study has found that placing an AVF at 6–9 months before initiation of dialysis in elderly patients with CKD was associated with a relatively higher rate of fistulae for HD with a smaller number of interventions. However, the potential cost of this tradeoff were one to wait to achieve placement at about 6–9 months pre-HD versus earlier AVF placement is the increased risk of a rapid deterioration of renal function precipitating the need for dialysis that might require a CVC. Because predicting dialysis initiation time is difficult, any goal predialysis AVF timing may be impractical, but our study suggested that markedly early AVF creation offered little, if any, advantage to this elderly cohort. However, our data found that earlier placement of an AVF seemed to be advantageous in certain subgroups of patients, such as blacks, women, and those without CHF or diabetes.
Concise Methods
Data Sources and Study Population
Our study cohort consists of patients with ESRD on HD started between January 1, 2005, and December 31, 2008, in whom an AVF was the initial access placed before dialysis initiation (Current Procedural Terminology 4 [CPT-4] claim for AVF creation 36818, 36819, 36820, 36825, and 36821). We used the US Renal Data System (USRDS) linked with Medicare claims data to identify our retrospective cohort of interest. The USRDS dataset provided patients’ clinical data that described baseline characteristics and comorbidities (as derived from CMS Form 2728), vascular access actually used at HD initiation, and time of death or transplantation. We used a minimum age of 67 years old, because we combined Medicare data from 2003 to make all study patients potentially Medicare eligible 2 years preceding dialysis initiation. Geographic population distribution divided into metropolitan, micropolitan, and rural areas was determined by the Rural–Urban Commuting Area database linked to USRDS by the zip code of the patient’s residence. In addition, we used information from the US Census Bureau of median income stratified by race, which was linked to the study dataset by patient’s zip codes.
Patients were excluded from the study if information regarding the outcome (dialysis access during the first outpatient treatment) was missing. In addition, those patients who changed to peritoneal dialysis or received transplantation before initiation of dialysis were also excluded. Patients who died after AVF placement but before HD initiation are not included in the USRDS, and therefore, they were not a part of this study. Also, 1067 patients in whom the initially placed AVF had failed and a new AVF had been created and used for dialysis were also excluded. That decision was on the basis of uncertainty of how to classify the successful outcome of the consequent AVF, and because the initial AVF did, in fact, fail, including this group might be potentially misleading. Finally, because there is a minimal time needed for AVF maturation, patients in whom the AVF was created <1 month before HD initiation were excluded as well.
Primary Outcome
In this study, AVF success was defined as initiation of HD using the AVF initially placed, regardless of the functionality and durability of the AVF. AVF failure was defined as, despite an AVF being the initial access placed, dialysis initiated using access other than the AVF. In this retrospective project, we aimed to identify the preferential time for AVF placement in the elderly that is associated with the highest rate of successful AVF first approach but the lowest number of predialysis access procedures used to maintain patency.
It is noted that AVF success might depend on the timing of dialysis initiation, which is somewhat subjective (i.e., nephrologists might tend to wait until the AVF is ready to be used, which might increase the likelihood of success).
Primary Variables of Interest and Covariates
The time between AVF placement and dialysis initiation was our primary variable of interest, which we defined a priori into five categories of 1–3, 3–6, 6–9, 9–12, and ≥12 months pre-HD initiation. Independent variables included in the model were age at HD initiation, race, sex, comorbidities, primary cause of ESRD, BMI, and duration of nephrology care.22,23,36,38,39
Clinical information at the time of dialysis initiation was derived from the CMS Form 2728. In addition, we included the variables describing geographic location (categorized as macropolitan, micropolitan, and rural) and average race-stratified income associated with a patient’s zip code. Access complication-related procedural data were obtained from the Medicare claims. The procedures considered for our project were thrombectomy, angioplasty, revisions of AVF/AVG, ligation of AVF, fistulogram, and distal revascularization–interval ligation. We calculated the number (mean per patient) of these procedures between first AVF placed and HD initiation.
Statistical Analyses
Summary statistics are presented as percentages for categorical data or mean (±SD) for continuous variables. Differences in baseline characteristics between the groups were tested using the chi-squared test for categorical variables or t test for continuous variables. A logistic regression model was used to compare the odds of success associated with different times of AVF placement. We also analyzed subgroups divided by sex, race, and specific comorbidities (i.e., diabetes and CHF) to evaluate the potential effect of the characteristic on the vascular access outcome.3,40 The logistical regression model was adjusted for the following covariates: age at HD initiation, race, sex, comorbidities, primary cause of ESRD, BMI, duration of nephrology care, geographic location, substance abuse (history of smoking, alcohol, or street drugs), employment index, race-stratified annual median income, and testing of the interaction term between the number of procedures and time.
We considered using the propensity scores method to better control for confounding, but we decided that using propensity scores would add very little to our analysis. In most studies, the results were not significantly different when the propensity scores method was used along with traditional multivariate analysis.41,42
All analyses were performed with SAS Software, version 9.2 (SAS Institute, Inc., Cary, NC).
Disclosures
None.
Acknowledgments
The study was funded from departmental funds and did not have any outside sponsor or funding agency.
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Timing of Arteriovenous Fistula Placement: Keeping It in Perspective,” on pages 241–243.
References
- 1.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ: Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 47: 469–477, 2006 [DOI] [PubMed] [Google Scholar]
- 3.DeSilva RN, Sandhu GS, Garg J, Goldfarb-Rumyantzev AS: Association between initial type of hemodialysis access used in the elderly and mortality. Hemodial Int 16: 233–241, 2012 [DOI] [PubMed] [Google Scholar]
- 4.NKF-K/DOQI : III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: Update 2000. Am J Kidney Dis 37[Suppl 1]: S137–S181, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Roy-Chaudhury P, Thakar CV: Improving incident fistula rates: A process of care issue. Am J Kidney Dis 57: 814–817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker TF, 3rd, Glassock RJ, Steinman TI: Conclusions, consensus, and directions for the future. Clin J Am Soc Nephrol 4[Suppl 1]: S139–S144, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Ishani A, Collins AJ, Herzog CA, Foley RN: Septicemia, access and cardiovascular disease in dialysis patients: The USRDS Wave 2 study. Kidney Int 68: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Richardson AI, 2nd, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, Glickman MH: Should fistulas really be first in the elderly patient? J Vasc Access 10: 199–202, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN: A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg 45: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lok CE, Allon M, Moist L, Oliver MJSH, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Desilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first: Not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW: Timing of first cannulation and vascular access failure in haemodialysis: An analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19: 2334–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Goodkin DA, Mapes DL, Held PJ: The dialysis outcomes and practice patterns study (DOPPS): How can we improve the care of hemodialysis patients? Semin Dial 14: 157–159, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Wasse H, Singapuri MS: High-output heart failure: How to define it, when to treat it, and how to treat it. Semin Nephrol 32: 551–557, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Miller GA, Hwang WW: Challenges and management of high-flow arteriovenous fistulae. Semin Nephrol 32: 545–550, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Asif A, Leon C, Orozco-Vargas LC, Krishnamurthy G, Choi KL, Mercado C, Merrill D, Thomas I, Salman L, Artikov S, Bourgoignie JJ: Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol 2: 1191–1194, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Asif A, Roy-Chaudhury P, Beathard GA: Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol 1: 332–339, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, Coresh J: Timing of nephrologist referral and arteriovenous access use: The CHOICE Study. Am J Kidney Dis 38: 494–501, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T, Bragg-Gresham JL, Yamazaki S, Fukuhara S, Akizawa T, Kleophas W, Greenwood R, Pisoni RL: Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol 4: 595–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora P, Obrador GT, Ruthazer R, Kausz AT, Meyer KB, Jenuleson CS, Pereira BJ: Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 10: 1281–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Ravani P, Marcelli D, Malberti F: Vascular access surgery managed by renal physicians: The choice of native arteriovenous fistulas for hemodialysis. Am J Kidney Dis 40: 1264–1276, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Winkelmayer WC, Owen WF, Jr., Levin R, Avorn J: A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 14: 486–492, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Vargas PA, Craig JC, Gallagher MP, Walker RG, Snelling PL, Pedagogos E, Gray NA, Divi MD, Gillies AH, Suranyi MG, Thein H, McDonald SP, Russell C, Polkinghorne KR: Barriers to timely arteriovenous fistula creation: A study of providers and patients. Am J Kidney Dis 57: 873–882, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Jennings WC, Landis L, Taubman KE, Parker DE: Creating functional autogenous vascular access in older patients. J Vasc Surg 53: 713–719, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Riella MC, Roy-Chaudhury P: Vascular access in haemodialysis: Strengthening the Achilles’ heel. Nat Rev Nephrol 9: 348–357, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A: Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access 13: 168–174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hod T, Desilva RN, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Factors predicting failure of AV “fistula first” policy in the elderly. Hemodial Int 18: 507–515, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Obialo CI, Tagoe AT, Martin PC, Asche-Crowe PE: Adequacy and survival of autogenous arteriovenous fistula in African American hemodialysis patients. ASAIO J 49: 435–439, 2003 [PubMed] [Google Scholar]
- 34.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Allon M, Ornt DB, Schwab SJ, Rasmussen C, Delmez JA, Greene T, Kusek JW, Martin AA, Minda S, Hemodialysis (HEMO) Study Group : Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study. Kidney Int 58: 2178–2185, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Stehman-Breen CO, Sherrard DJ, Gillen D, Caps M: Determinants of type and timing of initial permanent hemodialysis vascular access. Kidney Int 57: 639–645, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Salmela B, Hartman J, Peltonen S, Albäck A, Lassila R: Thrombophilia and arteriovenous fistula survival in ESRD. Clin J Am Soc Nephrol 8: 962–968, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W, Jr.: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Xi W, MacNab J, Lok CE, Lee TC, Maya ID, Mokrzycki MH, Moist LM: Who should be referred for a fistula? A survey of nephrologists. Nephrol Dial Transplant 25: 2644–2651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S: A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59: 437–447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkelmayer WC, Kurth T: Propensity scores: Help or hype? Nephrol Dial Transplant 19: 1671–1673, 2004 [DOI] [PubMed] [Google Scholar]