Abstract
Podocytes are critically involved in the maintenance of the glomerular filtration barrier and are key targets of injury in many glomerular diseases. Chronic injury leads to progressive loss of podocytes, glomerulosclerosis, and renal failure. Thus, it is essential to maintain podocyte survival and avoid apoptosis after acute glomerular injury. In normal glomeruli, podocyte survival is mediated via nephrin-dependent Akt signaling. In several glomerular diseases, nephrin expression decreases and podocyte survival correlates with increased vascular endothelial growth factor (VEGF) signaling. How VEGF signaling contributes to podocyte survival and prevents apoptosis remains unknown. We show here that Gα–interacting, vesicle-associated protein (GIV)/girdin mediates VEGF receptor 2 (VEGFR2) signaling and compensates for nephrin loss. In puromycin aminonucleoside nephrosis (PAN), GIV expression increased, GIV was phosphorylated by VEGFR2, and p-GIV bound and activated Gαi3 and enhanced downstream Akt2, mammalian target of rapamycin complex 1 (mTORC1), and mammalian target of rapamycin complex-2 (mTORC2) signaling. In GIV-depleted podocytes, VEGF-induced Akt activation was abolished, apoptosis was triggered, and cell migration was impaired. These effects were reversed by introducing GIV but not a GIV mutant that cannot activate Gαi3. Our data indicate that after PAN injury, VEGF promotes podocyte survival by triggering assembly of an activated VEGFR2/GIV/Gαi3 signaling complex and enhancing downstream PI3K/Akt survival signaling. Because of its important role in promoting podocyte survival, GIV may represent a novel target for therapeutic intervention in the nephrotic syndrome and other proteinuric diseases.
Keywords: glomerular disease, podocyte, cell survival, VEGF
Podocytes are highly specialized glomerular epithelial cells uniquely organized into foot processes and filtration slits that are essential for glomerular filtration; podocyte injury often leads to foot process effacement and proteinuria.1–5 Podocyte injury is the initiating cause of many human and experimental glomerular diseases such as minimal change disease (MCD), FSGS, membranous nephropathy, and diabetic nephropathy.5–8 Podocytes have the capability to recover from mild injury; however, if injury is sustained, it can lead to their detachment from the glomerular basement membrane as well as cell death, followed by glomerulosclerosis and ESRD.1,5,7,8 Thus, it is essential to understand how podocytes respond to early injury and to characterize early signaling events that determine the reversibility of podocyte injury in order to develop novel therapeutics that can be used to maintain podocyte viability and to restore kidney function.
Nephrin is currently assumed to act as the key signaling molecule that maintains the filtration slits and podocyte survival by recruitment of phosphoinositide 3-kinase (PI3K) and subsequent activation of prosurvival Akt signaling.3,9–11 In normal podocytes, nephrin interacts with podocin and CD2AP and recruits the p85 subunit of PI3K to initiate Akt activation and inhibit apoptosis in cultured podocytes.4,9,10,12 However, podocytes of nephrin-null mice have rates of apoptosis similar to wild-type mice.13 In addition, nephrin is downregulated and nephrin-p85 interaction12 and tyrosine phosphorylation of nephrin14 (required for activation of Akt) are diminished in patients in early stages of MCD12,14 as well as in puromycin aminonucleoside nephrosis (PAN),15–17 a rat model of MCD, whereas podocytes survive.7,18,19 Furthermore, no significant differences in Akt phosphorylation and podocyte apoptosis were seen in nephrin-null and wild-type mice even though these mice showed foot process effacement, slit diaphragm disruption, and proteinuria.13 Thus, an alternative mechanism must be responsible for podocyte survival during the early, reversible stages of podocyte injury. Up to now, the signaling pathways that compensate for the nephrin-dependent survival pathway have remained unknown.
It has recently become evident that vascular endothelial growth factor receptor (VEGFR) 2 is the main receptor responsible for PI3K/Akt regulation and podocyte survival in response to autocrine vascular endothelial growth factor (VEGF)-A in cultured human and mouse podocytes.20 Akt functions as a critical regulator of growth factor–dependent survival and ameliorates cell apoptosis induced by toxic stimuli of a wide variety of cell types. There is growing evidence that VEGF signaling protects glomeruli from injury in numerous glomerular diseases, including MCD, FSGS, and diabetic nephropathy.21–24 In MCD, VEGF levels are upregulated in podocytes and correlate with decreased nephrin levels,25,26 and transgenic mice overexpressing VEGF-A in podocytes show proteinuria, loss of slit diaphragms, increased VEGFR2 phosphorylation, and downregulation of nephrin in glomeruli, but podocytes survive.27 The mechanism by which VEGF-VEGFR2 prosurvival signaling is maintained in spite of reduced nephrin levels after podocyte injury remains to be elucidated.
We recently identified Gα–interacting, vesicle-associated protein (GIV) as a multidomain signal transducer that enhances PI3 kinase/Akt signaling downstream of growth factor receptors.28 It does so by directly binding activated growth factor receptor tyrosine kinases (RTKs), G proteins, Akt, and actin.28–30 GIV enhances PI3K-Akt signaling via two independent mechanisms that synergistically mount the maximal response: (1) activation of Gαi via GIV’s guanine nucleotide exchange factor (GEF) motif releases “free” Gβγ subunits, which bind and activate class 1B PI3Ks31; (2) GIV is phosphorylated by RTKs at tyrosines 1764 and 1798, which directly bind and activate class 1A PI3Ks.30 Here we show that GIV similarly potentiates podocyte survival signaling early after podocyte injury in PAN rats. During early stages of MCD, GIV binds VEGFR2 and mediates podocyte survival via activation of Gαi3 and enhancement of downstream PI3K/Akt/mammalian target of rapamycin (mTOR) signaling. Thus, as nephrin is downregulated, GIV takes over, prevents apoptosis, and maintains cell survival early after podocyte injury.
Results
GIV Is Induced in Podocytes of PAN Rats
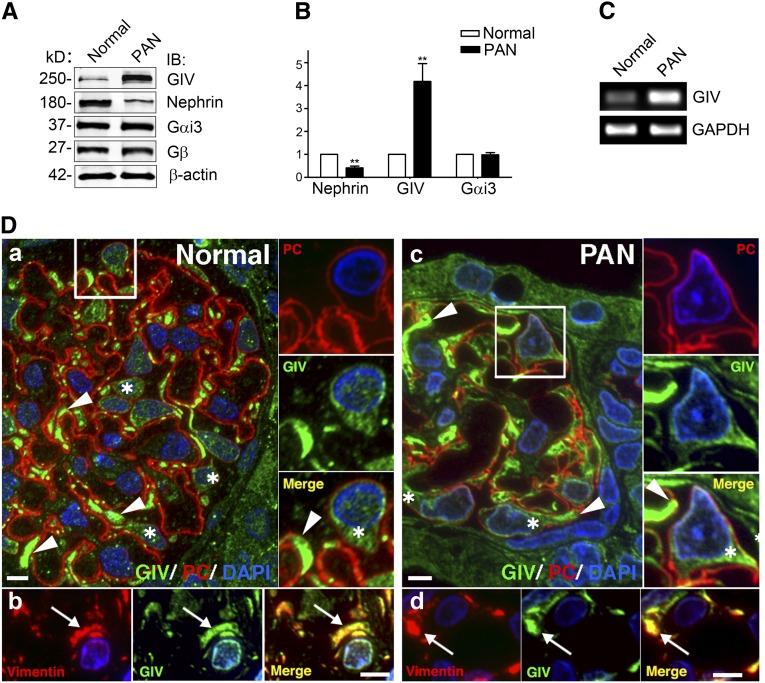
PAN is a well established animal model to study reversible podocyte injury.32 Because GIV is known to enhance Akt survival signaling in other cells,28 we first assessed the expression of GIV in glomeruli isolated from normal and 7-day PAN rats. We found by quantitative immunoblotting that expression of GIV protein is increased approximately 4-fold (Figure 1, A and B) and by RT-PCR that GIV mRNA is increased 10-fold (Figure 1C) in 7-day PAN rats compared with normal controls, whereas Gαi3, a GIV effector, and Gβ showed little change (Figure 1, A and B). By contrast, nephrin was decreased (42%) (Figure 1, A and B) as previously reported.16 These results indicate that both GIV transcript and GIV protein levels are significantly upregulated in PAN.
Figure 1.
GIV protein and mRNA are induced in glomeruli from PA-treated rats. (A) Expression of GIV protein is greatly increased (4-fold) and nephrin is decreased (to 40% of normal) in glomeruli from PAN rats. Gαi3 and Gβ remain unchanged. Equal aliquots (50 µg) of glomerular lysate from normal or PAN (7-day) rats are immunoblotted for GIV, nephrin, Gαi3, Gβ, and β-actin, and analyzed by Li-Cor Odyssey Infrared Imaging. (B) Quantification of data in A (normalized to β-actin). Data are expressed as the fold change in PAN compared with normal controls (**P<0.001; mean±SD n=6). (C) GIV mRNA is increased 10-fold in glomeruli after PA injury. RT-PCR is carried out on mRNA isolated from glomeruli prepared from normal and PAN rats using specific primers for rat GIV and GAPDH as a control. (D) GIV is highly expressed in podocytes in glomeruli from both normal and PAN rats. GIV is concentrated in primary processes (arrowheads) and cell bodies (asterisks) of podocytes in both normal (Da) and PAN (Dc) rats. Podocytes are identified by location and podocalyxin staining. We are unable to detect differences in the level of GIV staining between podocytes in glomeruli from normal and PAN rats. The boxed regions in A and C are enlarged (×2) to the right. Within podocytes, GIV is concentrated in primary processes (arrows) that stain for vimentin, a marker for podocytes in both normal (Db) and PAN (Dd) glomeruli. Semithin cryosections of kidney from normal and PAN-treated rats are stained for PC (red) or vimentin (red), and GIV-CT (green) and DAPI (blue), and examined by immunofluorescence. IB, immunoblot; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PC, podocalyxin; DAPI, 4,6-diamidino-2-phenylindole. Bar, 10 μm.
Glomeruli contain several cell types, including podocytes, endothelial cells, and mesangial cells. To determine which cell types express GIV, we carried out immunofluorescence for GIV and the podocyte markers podocalyxin33,34 and vimentin35 on semithin cryosections obtained from kidneys of normal and PAN rats. In glomeruli from both normal and PAN rats, GIV is expressed at the highest levels in podocytes (Figure 1D). It also codistributes with the intermediate filament protein vimentin, which is a specific marker for the primary processes of podocytes.35
Akt/mTOR Survival Signaling Is Maintained in PAN Rats
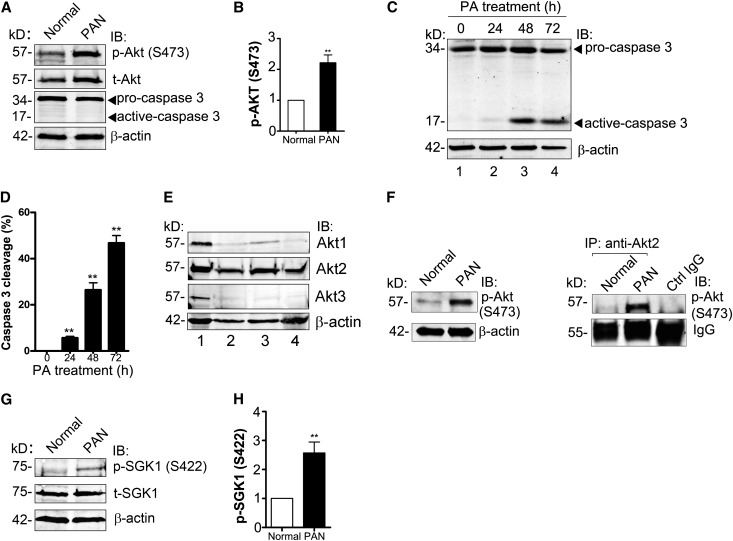
In normal podocytes, survival signaling is mediated through the well defined nephrin/CD2AP-dependent, PI3K/Akt pathway,9 but how cell survival is maintained after podocyte injury when nephrin expression decreases7,18,19 has remained unknown. Because we found GIV to be upregulated in PAN and GIV is known to enhance Akt signaling in other cell types,28 we anticipated that Akt signaling might be maintained or increased in nephrotic glomeruli. At 7 days after puromycin aminonucleoside (PA) injury, activation of Akt as determined by its phosphorylation at S473 was increased (2.2-fold) (Figure 2, A and B), and no apoptosis was detected (based on caspase 3 cleavage) (Figure 2A) even though nephrin was decreased (Figure 1, A and B). Similarly, when in vitro differentiated mouse podocytes were treated with PA (30 μg/ml), there was very little caspase 3 cleavage (5%) in podocytes at early time points (24 hours) (Figure 2, C and D), indicating that very little apoptosis occurs. However, when treatment was extended to 48 or 72 hours, which mimics prolonged injury in PAN rats, cleavage of caspase 3 increased (28% and 43%, respectively) (Figure 2, C and D).
Figure 2.
Akt/mTORC2 signaling is enhanced and podocyte survival is maintained after PA injury. (A) Phosphorylation of Akt is enhanced (2.2-fold) in glomeruli from PAN rats. Equal aliquots (50 µg) of glomerular lysate from normal or PAN (7-day) rats are immunoblotted for p-Akt (S473), t-Akt, and β-actin. (B) Quantification of data in A (normalized to β-actin). Data are expressed as the fold change in PAN compared with normal controls (**P<0.001; mean±SD n=6). (C) Podocytes survive at early stages of PA injury. At time 0, most of the caspase 3 is in the inactive (procaspase 3) form. At 24 hours, a small amount of caspase 3 cleavage (active caspase) is seen; after longer PA treatment (48 or 72 hours), the amount of cleaved (active) caspase 3 is greatly increased. In vitro differentiated mouse podocytes are treated with 30 μg/ml PA for the indicated times. Equal amounts of whole cell lysate are immunoblotted for caspase 3 and β-actin. (D) Quantification of data in C (normalized to β-actin). Data are expressed as the percentage of total caspase 3 (procaspase 3+active caspase 3) that is active (**P<0.001; mean±SD n=4). (E) Akt2 is the major Akt isoform expressed in glomeruli from rats and differentiated mouse podocytes. Equal amounts of lysate (50 μg) from C2C12 cells (positive control, lane 1), glomeruli from normal (lane 2), or PAN rats (lane 3), and differentiated mouse podocytes (lane 4) are immunoblotted for Akt1, Akt2, Akt3, and β-actin. (F) Akt2 is specifically activated in PAN. Akt2 is immunoprecipitated from glomerular lysate (1 mg) from normal or PAN rats using anti-Akt2 IgG followed by protein A magnetic beads. Bound proteins and lysates (100 μg) are immunoblotted for p-Akt (S473) and β-actin. Control IgG is incubated with a mixture of glomerular lysates from normal and PAN rats. (G) Phosphorylation of SGK1, a substrate of mTORC2 kinase, is enhanced (2.8-fold) in glomeruli from PAN rats. Equal aliquots (50 µg) of glomerular lysate from normal or PAN (7-day) rats are immunoblotted for p-SGK1(S422), t-SGK1, and β-actin. (H) Quantification of data in C (normalized to β-actin). Data are expressed as the fold change in PAN compared with normal controls (**P<0.001; mean±SD n=4). IB, immunoblot; IP, immunoprecipitate.
It was recently reported that Akt2 is the main Akt isoform expressed in mouse and human podocytes and that Akt2 activation protects podocytes during CKD.36 We confirmed37 that Akt2 is the major Akt isoform expressed in rat glomeruli and differentiated mouse podocytes (Figure 2E) and found that phosphorylation of Akt2 at S473 is greatly increased in PAN glomeruli (Figure 2F). Because mTOR complex 2 (mTORC2) is known to phosphorylate Akt at S473 upon growth factor stimulation,38,39 this finding suggested that Akt2 might be activated by mTORC2 in PAN. To find out whether this is the case, we examined activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1), a specific substrate of mTORC2 kinase,40 and found that pSGK1 was enhanced 2.6-fold in PAN glomeruli (Figure 2, G and H). mTOR complex 1 (mTORC1) is also activated by growth factors downstream of PI3K/Akt39 so we checked two substrates of mTORC1, S6 kinase (S6K) and S6 ribosomal protein (S6RP), and found that their activation is increased 2-fold in glomeruli from PAN rats (Supplemental Figure 1). These results indicate that in the absence of nephrin, downstream mTORC2-Akt2 survival signaling and mTORC1 cell growth signaling41 are maintained in podocytes via nephrin-independent pathways.
Interaction of GIV with Activated VEGFR2 Is Increased in PAN Rats
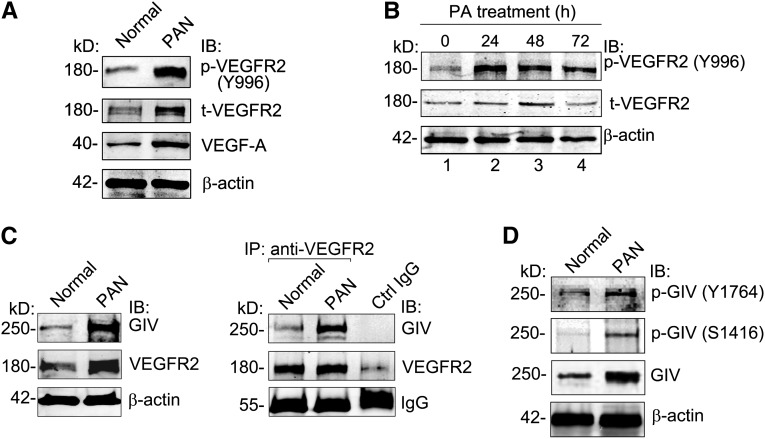
Because GIV is known to bind to activated RTKs, including VEGFR,28–30 and expression of GIV as well as VEGF-A and its receptors is increased in PAN,24 we reasoned that GIV binding to VEGFR2 might be increased in PAN. Interestingly, we found that expression of VEGF-A and total VEGFR2 was increased approximately 2.5-fold in glomeruli from 7-day PAN rats (Figure 3A), and activation of VEGFR2 (p-VEGFR2) (determined by autophosphorylation of Tyr996 on VEGFR2) increased 5.2-fold in both PAN glomeruli (Figure 3A) and PA-treated podocytes (Figure 3B). Moreover, the amount of GIV that coimmunoprecipitated with VEGFR2 from glomerular lysates of PAN rats was increased 3.5-fold (Figure 3C). Thus, VEGF signaling and binding of GIV to VEGFR2 are enhanced in podocytes after PA injury.
Figure 3.
Activated VEGFR2 interacts with GIV in PAN. (A) VEGF-A is increased and phosphorylation of VEGFR2 (p-VEGFR2) is enhanced in glomeruli from PAN rats. Equal aliquots (50 μg) of glomerular lysate from normal or PAN rats are immunoblotted for p-VEGFR2 (Y996), t-VEGFR2, VEGF-A, and β-actin. (B) Activation of VEGFR2 is enhanced in podocytes after PA treatment. In vitro differentiated mouse podocytes are treated with 30 μg/ml PA for the indicated times. Equal amounts of whole cell lysate are immunoblotted for p-VEGFR2 (Y996), t-VEGFR2, and β-actin. (C) Coimmunoprecipitation of GIV with VEGFR2 is greatly increased (3.5×) in PAN glomeruli. For immunoprecipitation, glomerular lysate (1 mg) from normal or PAN rats is incubated with anti-VEGFR2 IgG followed by protein A magnetic beads. Bound proteins and lysates (100 μg) are immunoblotted for GIV, VEGFR2, and β-actin as described in the Concise Methods. Control IgG is incubated with a mixture of glomerular lysates from normal and PAN rats. (D) Phosphorylation of GIV at Y1764 and S1416 is increased in glomeruli from PAN rats. Equal aliquots (50 μg) of glomerular lysate from normal or PAN rats are immunoblotted for p-GIV (Y1764), p-GIV (S1416), GIV, and β-actin. IB, immunoblot; IP, immunoprecipitate.
GIV Is Phosphorylated at Ser1416 and Tyr1764 in PAN
It was recently shown that, upon binding to an RTK, GIV is phosphorylated at Tyr1764 and Tyr1798, and that tyrosine phosphorylated GIV directly binds and activates class 1 PI3Ks, thereby enhancing Akt phosphorylation and actin remodeling during cell migration.30 In addition to enhancing Akt signaling, GIV is also a substrate for Akt, and phosphorylation of GIV at Ser1416 by Akt promotes binding of GIV to actin and actin remodeling.42 Together these phosphorylation events indicate that GIV-dependent signaling is “on.” To check whether PAN injury turns on GIV-dependent signaling, we immunoblotted glomerular lysates with antibodies that specifically recognize pTyr1764 or pSer1416 of GIV. Phosphorylation of GIV at both of these sites was significantly increased in PAN glomeruli (Figure 3D), indicating that GIV is activated and that GIV-dependent signaling is specifically induced upon PAN injury.
GIV Binding to Gαi3 and Activation of Gαi3 Are Increased in PAN
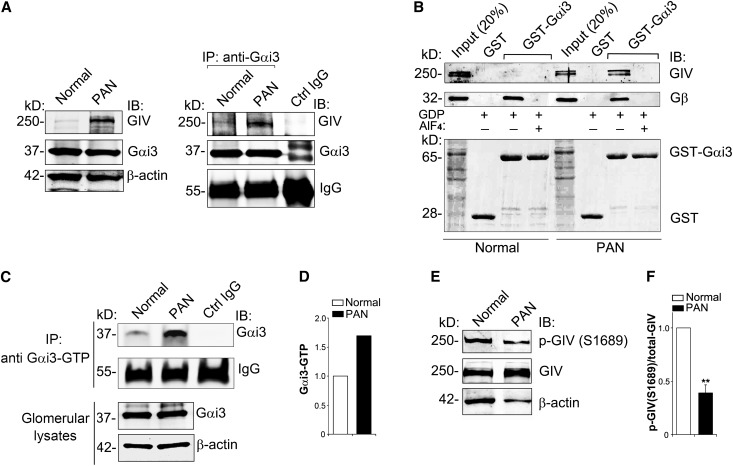
We established earlier that after growth factor stimulation of HeLa cells, GIV binds inactive Gαi3 and serves as a GEF that activates Gαi3, thereby releasing Gβγ43 subunits that directly bind PI3K and activate PI3K/Akt signaling.31 To investigate whether Gαi3 and GIV interact in glomeruli, we immunoprecipitated endogenous Gαi3 and immunoblotted for GIV. The amount of GIV that coimmunoprecipitated with Gαi3 was greatly increased (4.2-fold) in PAN glomeruli, indicating that interaction between Gαi3 and GIV is enhanced (Figure 4A). To determine whether binding is state dependent, we carried out in vitro binding assays with recombinant glutathione S-transferase (GST)-Gαi3 in the presence of GDP alone (inactive state) or in the presence of GDP and AlF4− (active state).44 We found that GIV from PAN glomeruli bound inactive but not active Gαi3, whereas GIV from normal glomeruli did not bind either active or inactive Gαi3 (Figure 4B). Similarly, interaction between inactive GST-Gαi3 and GIV was increased in PA-treated podocytes (data not shown). Next we checked whether PA injury leads to activation of Gαi3 using an antibody that recognizes only active Gαi (Gαi-GTP)45 and found that the ratio of active/total Gαi3 was increased (1.7-fold) in PAN glomeruli (Figure 4, C and D).
Figure 4.
GIV binds and activates Gαi3 upon PA injury. (A) The amount of GIV that coimmunoprecipitates with Gαi3 is greatly increased in PAN rats. Immunoprecipitation is carried out on glomerular lysates with anti-Gαi3 IgG, and bound proteins are immunoblotted for GIV, Gαi3, and β-actin as in Figure 3B. (B) GIV in glomeruli form PAN rats binds inactive (−AlF4−) but not active (+AlF4−) Gαi3, whereas GIV from normal glomeruli does not bind either one. Gβ (positive control) binds inactive Gαi3 from both normal and PAN glomeruli. The upper panel shows GST-Gαi3 incubated with glomerular lysate (1 mg) from normal or PAN rats in the presence of GDP and AlF4− (to activate Gαi3) or GDP alone followed by immunoblotting of bound proteins for GIV and Gβ subunits. The lower panel shows Ponceau S staining. (C) Active Gαi3 (Gαi3-GTP) is increased 1.7-fold in glomeruli from PAN rats. Immunoprecipitation is carried out with an antibody that specifically recognizes only active Gαi3-GTP, and bound proteins are immunoblotted for total Gαi3 and β-actin. (D) Quantification of data in C. Data are expressed as the fold change in Gαi3-GTP level in PAN glomeruli versus normal controls. (E) Phosphorylation of GIV at S1689 is reduced in glomeruli from normal rats. Because the expression of GIV is increased 4-fold in PAN compared with normal glomeruli (Figure 1, A and B), 70 μg of normal glomerular lysate and 20 μg of PAN lysate are loaded (to equalize the total GIV) and immunoblotted for GIV, p-GIV (S1689), and β-actin. (F) Quantification of data in E (normalized to β-actin). Data are expressed as the fold change in PAN compared with normal controls (**P<0.001; mean±SD n=4). IB, immunoblot; IP, immunoprecipitate.
We recently found that the ability of GIV to activate Gαi3 is regulated by phosphorylation at S1689, as GIV-pS1689 fails to bind and activate Gαi3.46 Because interaction between GIV and Gαi3 is increased in PAN, we reasoned that the level of phosphorylation of GIV at S1689 might be altered. When we adjusted the amount of glomerular lysate so that the level of GIV was equal in both normal and PAN glomeruli, phosphorylation of GIV at S1689 was >2-fold higher in normal glomeruli (Figure 4, E and F). These results suggest that binding and activation of GIV by Gαi3 is inhibited by phosphorylation at S1689 in normal glomeruli which prevents its interaction with Gαi3,46 whereas phosphorylation of GIV at S1689 is reduced after PA injury which allows it to bind and activate Gαi3.
GIV Mediates Assembly of a VEGFR2/GIV/Gαi3 Molecular Complex
Our findings that VEGFR2 and Akt are strongly activated and that GIV binds activated VEGFR2 as well as Gαi3 in glomeruli from nephrotic rats suggest that GIV provides an important link between VEGF-A stimulation and Akt survival signaling in podocytes. Next we asked whether GIV enhances Akt signaling through formation of a VEGFR2/GIV/Gαi3 complex in podocytes in response to VEGF-A. To determine whether this is the case, cultured podocytes were treated with VEGF-A for 30 minutes, followed by immunoprecipitation with anti-VEGFR2 IgG and immunoblotting for Gαi3, VEGFR2, and GIV. We found that little, if any, Gαi3 coprecipitated with VEGFR2 from lysates of starved cells, but both GIV and Gαi3 coprecipitated with VEGFR2 after VEGF stimulation (Figure 5A). Similar experiments on lysates from control versus PA-treated podocytes showed that the interaction of VEGFR2 with GIV and Gαi3 is also greatly increased after PA treatment (Figure 5B). Thus, both VEGF activation and PA treatment trigger the assembly of VEGFR2/GIV/Gαi3 protein complexes in podocytes.
Figure 5.
GIV binds VEGFR2 and mediates assembly of a VEGFR2/GIV/Gαi3 molecular complex in both VEGF-treated and PA-treated podocytes. (A) GIV and Gαi3 coimmunoprecipitate with VEGFR2 in VEGF-stimulated but not in serum-starved podocytes. Differentiated podocytes are serum starved or stimulated with VEGF (40 ng/ml) for 30 minutes, and cell lysates (500 μg) are incubated with anti-VEGFR2 IgG. Control IgG is incubated with a mixture of lysates from starved and VEGF-stimulated podocytes. Immunoprecipitates and cell lysates (50 μg) are immunoblotted for GIV, VEGFR2, p-Akt (S473), t-Akt, Gαi3, and β-actin. (B) Coimmunoprecipitation of both Gαi3 and GIV with VEGFR2 is greatly enhanced after PA treatment (24 hours) of differentiated podocytes, indicating that their interaction is greatly increased. Differentiated podocytes are incubated (24 hours) with or without PA (30 μg/ml), and cell lysates (500 μg) are incubated with anti-VEGFR2 or control IgG. Immunoprecipitates, and cell lysates (50 μg) are analyzed by immunoblotting with antibodies against GIV, VEGFR2, Gαi3, and β-actin. Control IgG is incubated with a mixture of podocyte lysates from untreated and PA-treated (24-hour) podocytes. IB, immunoblot; IP, immunoprecipitate.
GIV Is Required for Actin Remodeling, Cell Migration, Akt2/mTOR activation, and Cell Survival in Cultured Podocytes
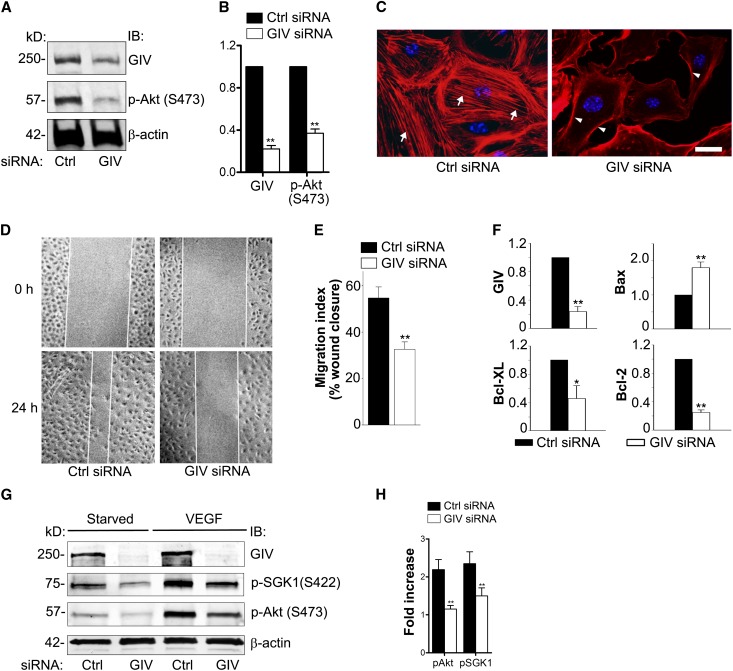
GIV has been shown to act upstream of Akt and to be essential for Akt activation, cell survival, actin remodeling, and cell migration in several cell types (i.e., epithelial cells,29 macrophages,47 and endothelial cells42). Our results in PAN rats suggest that the same is true for podocytes. To directly test whether this is the case, we depleted GIV from differentiated cultured mouse podocytes by the peptide transducing domain-double stranded RNA binding domain (PTD-DRBD) method,48 which is an effective way to introduce small interfering RNAs (siRNAs) into these cells (Figure 6A). In GIV-depleted podocytes, Akt phosphorylation was attenuated (Figure 6, A and B), the actin cytoskeleton reorganized (Figure 6C), and cell migration was impaired (Figure 6, D and E). Moreover, GIV-depleted cells showed increased Bax (1.8×), a proapoptotic marker, and decreased antiapoptotic markers Bcl-2 and Bcl-XL (75% and 54%, respectively) (Figure 6F). GIV depletion also abolished phosphorylation of Akt and SGK1 in response to VEGF-A stimulation (Figure 6, G and H). Thus, GIV plays a key role in enhancing prosurvival Akt signaling and actin remodeling after growth factor stimulation in cultured podocytes.
Figure 6.
GIV enhances Akt phosphorylation, actin remodeling, cell migration, and survival in podocytes. (A) p-Akt is decreased in GIV-depleted podocytes. Differentiated podocytes are transfected with control or GIV siRNA. Forty-eight hours later, cell lysates are immunoblotted for GIV, p-Akt (S473), t-Akt, and β-actin. (B) Quantification of data from 10 experiments expressed as the fold change in GIV siRNA versus Ctrl siRNA-treated podocytes (normalized to β-actin) (**P<0.001; mean±SD). (C) Podocytes treated with control siRNA show stress fibers (arrows), whereas GIV-depleted podocytes show prominent cortical actin (arrowheads). Control or GIV siRNA-treated podocytes are fixed and stained with Phalloidin Alexa Fluor 594 and DAPI. (D) Cell migration assay. Twenty-four hours after wounding, podocytes treated with control siRNA cover most of the wound area, whereas closure is impaired in GIV-depleted cells (GIV siRNA). Podocyte monolayers are scratch-wounded and examined by phase-contrast microscopy after 0 or 24 hours. (E) Quantification of data from eight experiments as in D (**P<0.001; mean±SEM). (F) After siRNA depletion of GIV (75%), proapoptotic Bax is increased (1.8-fold) and antiapoptotic Bcl-2 and Bcl-XL are decreased (to 25% and 46%, respectively) compared with controls (Ctrl siRNA). mRNA levels are determined by quantitative real-time PCR of total RNA (normalized to β-actin mRNA) (*P<0.05; **P<0.001; mean±SEM n=5). (G) VEGF-mediated phosphorylation of Akt and SGK1 is abolished in GIV-depleted podocytes. Podocytes are transfected with control or GIV siRNA by the PTD-DRBD method. Forty-eight hours after transfection, cells are serum starved or stimulated with VEGF (40 ng/ml) for 30 minutes, and cell lysates are immunoblotted for p-Akt (S473), p-SGK1 (S422), and β-actin. (H) Quantification of data from three representative experiments as in G expressed as the fold change in VEGF-mediated stimulation of p-Akt and p-SGK1 (normalized to β-actin) compared with starved controls (**P<0.001; mean±SD). DAPI, 4,6-diamidino-2-phenylindole; IB, immunoblot; Ctrl, control. Bar, 10 μm.
The GEF Function of GIV Is Required for Podocyte Survival
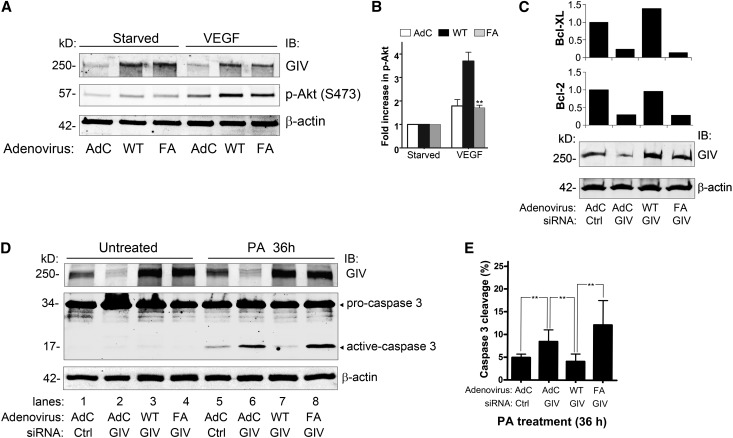
Next we asked whether activation of Gαi3 by GIV is required for podocyte survival. siRNA resistant GIV wild-type (GIV-WT) or a specific mutant, GIV F1685A (GIV-FA), which fails to bind and activate Gαi3, were expressed in podocytes using adenoviral vectors. In cells expressing GIV-WT, Akt phosphorylation (S473) in response to VEGF-A stimulation (Figure 7, A and B) and expression of antiapoptotic markers (Figure 7C) were higher than in those expressing GIV-FA. Notably, expression of Bcl-2 and Bcl-XL in cells expressing GIV-FA was approximately 30% of that in cells expressing GIV-WT, indicating that GIV-WT but not GIV-FA can maintain cell viability. To find out whether GIV affects cell survival after PA injury, we checked caspase 3 cleavage in GIV-depleted podocytes after PA treatment (36 hours) and found that caspase 3 cleavage was increased (1.7-fold) in PA-treated cells (Figure 7, D and E). In addition, expression of GIV-WT rescued cell viability in GIV-depleted cells as shown by decreased caspase 3 cleavage, whereas expression of GIV-FA did not because caspase cleavage was increased 3-fold compared with GIV-WT rescued podocytes (Figure 7D). These results indicate that the GEF function of GIV, and by implication activation of Gαi3, is required for maintaining cell survival and antiapoptotic signaling in podocytes.
Figure 7.
The GEF function of GIV is required for podocyte survival. (A) VEGF-mediated prosurvival Akt signaling is enhanced in podocytes overexpressing GIV-WT (WT) and is inhibited in podocytes overexpressing GIV-FA (FA). Podocytes are transduced with Ad-GIV-WT (WT) or Ad-GIV-FA (FA), or control adenovirus (AdC). Forty-eight hours later, they are switched to 0.4% serum for 24 hours and then stimulated with VEGF (40 ng/ml) for 30 minutes. Equal amounts of cell lysate are immunoblotted for GIV, p-Akt (S473), and β-actin. (B) Quantification of data in A (normalized to β-actin). Data are expressed as the fold change in AdC–, Ad-GIV-WT–, or Ad-GIV-FA–treated podocytes. (C) Depletion of endogenous GIV in cells transduced with control virus (AdC) decreases expression of the antiapoptotic markers Bcl-2 (30%) and Bcl-XL (25%). Overexpression of GIV-WT prevents this reduction in expression of Bcl-2 and Bcl-XL, whereas overexpression of GIV-FA does not. Podocytes are transduced with control (AdC), Ad-GIV-WT, or Ad-GIV-FA, followed (24 hours later) by control or GIV siRNA. After 48 hours, equal amounts of cell lysates are immunoblotted for GIV or β-actin. RNA is isolated and relative mRNA levels of Bcl-2 and Bcl-XL are determined by quantitative real-time PCR. (D) Addition of PA (36 hours) to GIV siRNA-treated podocytes transduced with control virus (lane 6) leads to increased caspase 3 cleavage (1.7-fold) compared with control siRNA-treated cells (lane 5). Overexpression GIV-WT (lane 7) inhibits the PA-mediated increase in caspase 3 cleavage but overexpression of GIV-FA (lane 8) does not. No caspase cleavage is seen in untreated cells (lanes 1–4). Podocytes are transduced with control virus (AdC), Ad-GIV-WT, or Ad-GIV-FA, followed (24 hours later) by control or GIV siRNA. After 48 hours, they are treated with 30 µg/ml PA for 36 hours. Equal amounts of cell lysates are immunoblotted for GIV, caspase 3, and β-actin. (E) Quantification of data in D (normalized to β-actin). Data are expressed as the percentage of active caspase 3/total caspase 3 (procaspase 3+active caspase 3) (**P<0.001; mean±SD n=3). IB, immunoblotting.
Taken together, our results support that at early stages of podocyte injury induced by PA treatment, GIV turns on Gαi3 through its GEF function and provides a critical link between upstream VEGFR2 signaling and downstream Akt2/mTOR survival signaling (Figure 8).
Figure 8.
Working model of GIV’s role in early podocyte injury in PAN nephrosis. (A) In normal podocytes, Akt survival signaling is mediated through the well defined nephrin-dependent PI3K/Akt pathway. (B) At early time points (7 days) after PAN injury, increased GIV expression coincides with decreased nephrin expression and activation of VEGFR2 and Akt. GIV binds to activated VEGFR2 and is tyrosine phosphorylated by VEGFR2. Activated GIV binds and activates Gαi3 (Gαi3-GTP). A VEGFR2/GIV/Gαi3 signaling complex is assembled and prolongs podocyte survival in PAN via activation of PI3K, Akt, mTORC1, and mTORC2. Thus, after PA injury when nephrin levels decrease, GIV takes over the task of enhancing Akt/mTOR survival signaling in response to VEGF.
Discussion
It has recently become evident that both VEGF signaling21–24 and Akt activation36 function in maintaining cell viability after podocyte injury. The molecular pathways that connect growth factor signaling and downstream Akt survival signaling after podocyte injury have remained unknown. Our data clearly point to GIV as the critical linker between upstream VEGFR and downstream PI3K/Akt survival signaling in PAN, a model of MCD. We found that during the early phases of PA injury, VEGFR2 is activated and GIV is upregulated. GIV assembles a VEGFR/GIV/Gαi3 signaling complex that promotes podocyte survival through activation of Gαi3 and enhancement of PI3K/Akt downstream signaling (see Figure 8B). In keeping with previous findings on EGFR,29 our results suggest that tyrosine phosphorylation of GIV by VEGFR2 is the trigger for assembly of the VEGFR2/GIV/Gαi3 complex and subsequent activation of Gαi3. The essential role of GIV in this scenario is illustrated by our findings that in the absence of GIV, Akt signaling is reduced and podocytes undergo apoptosis. The crucial role of GIV’s GEF function is illustrated by the fact that these effects can be reversed by introducing human GIV wild-type protein but not a GIV mutant (GIV-FA) that is incapable of binding and activating Gαi3. Thus, after acute injury as nephrin levels decrease GIV takes over the task of enhancing Akt survival signaling in response to VEGF, and activation of Gαi3 is required for this effect.
We show here that Akt2 is the isoform that is highly expressed in rat glomeruli and is activated in PAN, and that GIV acts upstream of Akt2, mTORC1, and mTORC2. Both mTORC1 and mTORC2 are activated early after PAN injury as indicated by increased phosphorylation of their respective substrates, S6K and SGK1. These results are in keeping with previous studies on podocyte-specific, mTOR-deficient mice, which established that both mTORC1 and mTORC2 are essential for the integrity and proper functioning of the glomerular filtration barrier.41,49
GIV Coordinates Multiple Signaling Pathways
We show here that GIV coordinates growth factor, G protein, and PI3K/Akt/mTOR signaling to promote cell survival and prevent apoptosis in podocytes after PAN injury. GIV is uniquely organized to directly receive incoming receptor signals and to modulate them via G proteins and was previously shown to regulate such diverse cell processes as cell migration,29,47 cancer metastasis,29 and autophagy.50 The C terminus serves as a common platform for binding RTKs, G proteins, and Akt. Interactions of GIV with its binding partners and resultant downstream signaling are controlled by specific phosphorylation events: Phosphorylation of GIV at Tyr1764 and Tyr179830 by RTKs regulates GIV’s ability to activate PI3Ks and activate Akt, and phosphorylation of GIV at S1689 prevents binding of GIV to Gαi.46 We show here that in PAN rats in response to stress, activation of VEGFR2 is increased, phosphorylation of GIV at Tyr1764 is enhanced, and phosphorylation at S1689 is suppressed thus promoting assembly of VEGFR2/GIV/Gαi complexes, facilitating activation of Gαi3, and enhancing downstream PI3K/Akt/mTOR survival signaling.31 The PAN model we used is reversible; thus, the enhanced VEGF stimulation, GIV activation, and Akt signaling stimulated by PA treatment must eventually subside. Because GIV activation is controlled by tyrosine phosphorylation at two specific sites, we anticipate that the GIV/PI3K/Akt signaling pathway might be terminated by specific phosphatases such as Src homology domain 2-containing protein-tyrosine phosphatase-1, which binds and dephosphorylates GIV,51 and by phosphorylation of GIV at S1689 by PKCθ, which inhibits Gαi3-GIV coupling.46
GIV Mediates Actin Remodeling in Podocytes
The normal podocyte has an elaborate actin cytoskeleton with a prominent actin network in the cell body and foot processes that maintains the characteristic foot process and filtration slit organization.1,2,5 It is assumed that in the normal glomerulus, the nephrin/VEGFR2 signaling complex regulates the actin cytoskeleton and maintains foot process architecture.52 The actin network undergoes dramatic reorganization in diseases that are associated with foot process effacement and proteinuria (e.g., PAN).3–5,32,53 Previous work established that GIV binds Akt and actin and is phosphorylated at S1416 by Akt, which mediates actin remodeling during cell migration in other cell types.42 GIV has also been shown to be involved in VEGF-dependent actin reorganization and cell migration during VEGF-mediated angiogenesis in endothelial cells.54 Our finding that GIV is phosphorylated at S1416 by activated Akt in PAN rats, which promotes its binding to and remodeling of actin in response to VEGF/VEGFR2 activation,42 suggests that GIV plays a role in enhancing actin remodeling in podocytes during foot process effacement in PAN.
Relevance of GIV Findings in the PAN Rat Model to Human Glomerular Diseases
Our results demonstrate that VEGF-A and VEGFR2 are upregulated and autophosphorylation of VEGFR2 is increased in glomeruli from PAN rats. This is in keeping with previous studies demonstrating that VEGF-VEGFR2 coupling is prominent in podocytes of biopsies from patients with MCD,55 crescentic nephritis,23 lupus nephritis,23 IgA and membranous nephropathy,23 diabetic nephropathy,22 and HIV-associated nephropahy.56 VEGF-A is abundantly expressed and secreted by podocytes and has been suggested to protect renal glomeruli from injury21,22 and to activate the PI3K/Akt survival pathway and protect glomerular endothelial cells,57 and tubular epithelial cells58 as well as podocytes20 from apoptosis. It seems likely that all of these events are mediated through GIV. Moreover, activation of Akt has been shown to be essential for maintaining podocyte viability in stress induced by nephron reduction36 and oxidized LDL-mediated injury.59 GIV’s ability to amplify Akt signaling is not restricted to a single receptor or class of receptor: It has been shown to serve as a common platform that coordinates signaling downstream of multiple growth factor receptors (EGFR, IGF1R, InsR) as well as some G protein–coupled receptors.28 On the basis of our findings and the properties of GIV, we predict that GIV might similarly act as a linker between other RTKs (e.g., insulinR60) and downstream Akt/mTOR signaling to promote podocyte survival early in the course of other podocytopathies.
Concise Methods
Reagents and Antibodies
All reagents were of analytical grade and were obtained from Sigma-Aldrich or Fisher Biotech; cell culture media were purchased from Invitrogen. PA, type I collagen, and isopropyl β-d-1-thiogalactopyranoside were from Sigma-Aldrich, recombinant mouse VEGF was from R&D Systems, and mouse γ-IFN was from BD Pharmingen. A rabbit polyclonal antibody (pAb), GIV (CT3347), against the C terminus of human GIV (amino acids 1574–1843) was raised and affinity-purified purified as previously described61 and used for staining full-length GIV in kidney sections by immunofluorescence. Rabbit IgG against the extracellular domain of rat nephrin was raised and purified as previously described.62 A mouse mAb that recognizes the ectodomain of rat podocalyxin (5A) was previously described.63 Rabbit pAbs for GIV/Girdin (T-13), pan-Gβ (M-14), Gαi3 (C-10), phospho-VEGFR2/phospho-Flk-1(Y996), p-SGK1 (S422), a mouse mAb for VEGFR2/Flk-1 (A-3), control rabbit IgG, and control mouse IgG were purchased from Santa Cruz Biotechnology. Rabbit pAbs against phospho-Akt (S473), Akt1, Akt2, p-S6K (T389), p-S6RP (S235/236), and caspase 3, and mouse mAbs against Akt3 and total Akt were purchased from Cell Signaling Technology. pAbs were also from Cell Signaling Technology. A mouse mAb for VEGF was from BD Pharmingen and those against vimentin, SGK1, and β-actin were from Sigma-Aldrich. Affinity-purified rabbit pAbs for phospho-GIV (Y1764) and phospho-GIV (S1689) were from Roche and those for phospho-GIV (S1416) were from Immuno-Biologic Laboratories. A mouse mAb specific for activated Gαi subunits was kindly provided by Dr. Graeme Milligan45; IRDye 800CW goat anti-mouse IgG (H+L) and IRDye 680LT goat anti-rabbit IgG (H+L) used for immunoblotting were from Li-Cor Biosciences. For immunofluorescence, 4,6-diamidino-2-phenylindole, Alexa Fluor 594 phalloidin, highly cross-adsorbed Alexa Fluor 594 goat anti-mouse IgG F(ab’)2, and Alexa Fluor 488 goat anti-rabbit IgG F(ab’)2 were purchased from Invitrogen.
Induction of PAN Nephrosis
Male rats (approximately 150 g; Charles River Laboratories) were injected once intraperitoneally with PA (15 mg/100 g body wt) as previously described.34 Animals were euthanized on day 7 after injection. All animal experiments were done according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and the experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of the University of California, San Diego.
Culture and Transfection of Mouse Podocytes
Conditionally immortalized mouse podocytes (a gift from Dr. Peter Mundel)64 were cultured at 33°C in RPMI 1640 (Invitrogen) containing 10% FBS (Hyclone), 100 U/ml of penicillin–streptomycin, and 10 U/ml of mouse γ-IFN. To induce differentiation, podocytes were replated and cultured at 37°C in plates coated with 10 mg/ml type I collagen in the absence of γ-IFN. After 14-day differentiation, siRNA depletion was performed by using the PTD-DRBD mediated delivery method.48 PTD-DRBD was purified from Escherichia coli and mixed with siRNA. Silencer negative control siRNA was purchased from Ambion. siRNA for targeting mouse GIV (sense: 5′-GCAACAAGCUUACCUCAAUTT-3′ and anti-sense: 5′-AUUGAGGUAAGCUUGUUGCTT-3′) was designed and synthesized by Allele Biotechnology & Pharmaceuticals Inc. Cells were harvested 48 hours after transfection and used for immunoblotting, immunoprecipitation, or immunofluorescence.
Preparation of Lysates and Immunoblotting
Rat glomeruli were isolated from kidney cortices of male Sprague-Dawley rats by graded sieving as previously described.63 Isolated glomeruli or cultured podocytes were lysed in RIPA buffer (100 mM sodium phosphate, 150 mM NaCl, 2 mM EDTA, 2 mM dithiothreitol [DTT], 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, phosphatase inhibitor cocktail III [Sigma-Aldrich], and protease inhibitor cocktail [Roche Diagnostics], 0.5 mM sodium orthovanadate, 10 mM NaF, 10 mM sodium polyphosphate). The protein concentration of glomerular or podocyte lysates was measured with a BCA Protein Assay Kit (Pierce Biotechnology Inc.) according to the manufacturer’s instructions. Glomeruli or cultured podocyte lysates were mixed with an equal volume of 5× Laemmli SDS sample buffer and boiled for 10 minutes. Proteins were separated on 8% or 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore) as described.34 Membranes were blocked in blocking buffer (5% BSA, 0.1% Tween-20 in PBS) and incubated with primary antibodies (4°C overnight) and then with secondary antibodies (1 hour at room temperature). Bands were scanned and quantified by two-color detection with the Odyssey Infrared Imaging system (Li-Cor Biosciences). Primary antibody dilutions were as follows: 1:500 for GIV, VEGFR2, p-VEGFR2 (Y996), nephrin, p-Akt (S473), p-SGK (S422), SGK, p-S6K (T389), p-S6RP (s235/236), Gαi3, Gβ, caspase 3, VEGF-A, and p-GIV (S1689); 1:250 for p-GIV (S1416) and p-GIV (Y1764); and 1:1000 for t-Akt and β-actin. Secondary antibody dilutions were 1:15,000 for IRDye 680 goat anti-rabbit and 1:15,000 for IRDye 800 goat anti-mouse IgG.
Immunofluorescence Microscopy
For imaging of semithin sections of glomeruli, rat kidney samples were immersion fixed in 4% paraformaldehyde for 4 hours at 4°C, cryoprotected, and frozen in liquid nitrogen.65 Semithin cryosections (0.5 μm) were cut with a Leica Ultracut UCT microtome equipped with an FCS cryoattachment at −100°C. Sections were blocked for 1 hour in PBS containing 5% normal goat serum, 2% fish skin gelatin, and 0.1% acetylated BSA, incubated with primary antibodies 2 hours at room temperature (GIV-CT3347 pAb, 1:100; vimentin mAb, 1:1000; and podocalyxin mAb 5A, 1:1000),66 followed by detection with Alexa 594 goat anti-mouse and Alexa 488 goat anti-rabbit IgG in blocking buffer for 1 hour. Cultured mouse podocytes were fixed at room temperature with 3% paraformaldehyde for 30 minutes, permeabilized (0.1% Triton X-100) for 10 minutes, and incubated for 1 hour each with primary and secondary antibodies as previously described.29 Primary antibody dilutions were as follows: 1:10 for GIV-CT antibody and 1:100 for Podocalyxin (5A). Secondary antibody dilutions were 1:1000 for Alexa 594 goat anti-mouse and Alexa 488 goat anti-rabbit IgG, 1:1000 for Alexa Fluor 594 phalloidin, and 1:3000 for 4,6-diamidino-2-phenylindole (Invitrogen). Samples were examined with a Zeiss Axiophot microscope (Carl Zeiss Inc., Thornwood, NY), and images were acquired using Volocity software (Improvision) and processed using ImageJ (NIH) and Photoshop software (Adobe Systems).
Protein Purification
GST or GST-Gαi3 fusion constructs were expressed in E. coli strain BL21 (DE3) (Invitrogen) as previously described67 and induced overnight at 25°C with 1 mM isopropyl β-d-1-thiogalactopyranoside (Sigma-Aldrich). Pelleted bacteria from 1 liter of culture media were resuspended in 10 ml of GST lysis buffer (25 mM Tris-HCl, pH 7.5, 20 mM NaCl, 1 mM EDTA, 20% glycerol, 1% Triton X-100, 2× protease inhibitor cocktail [Roche Diagnostics]). After sonication, lysates were centrifuged at 4°C (12,000×g for 20 minutes). Solubilized proteins were affinity-purified on glutathione-Sepharose 4B beads (GE Healthcare). Proteins were eluted, dialyzed overnight against PBS, and stored at −80°C.
Immunoprecipitation and In Vitro Binding Assays
Lysates for immunoprecipitation or in vitro binding assays were prepared by resuspension of glomeruli or podocytes in Triton X-100 lysis buffer (25 mM HEPES, 125 mM K-acetate, 5 mM Mg-acetate, 0.4% Triton X-100, phosphatase inhibitor cocktail III [Sigma-Aldrich], and protease inhibitor cocktail [Roche Diagnostics], 400 μM Na orthovanadate, pH 7.2) and passed through a 28-gauge needle at 4°C and cleared (14,000×g for 10 minutes) before use in subsequent experiments. For immunoprecipitation, rat glomerular lysates (1 mg total protein) or mouse podocyte lysates (500 µg total protein) were incubated 3 hours or overnight at 4°C with 2 µl anti-VEGFR2 or anti-Gαi3 or rabbit IgG (Santa Cruz Biotechnology) in Triton X-100 lysis buffer. Protein A magnetic beads (EMD Millipore) were added and incubated at 4°C for an additional 1 hour. Beads were washed (×4) with 1 ml of PBS-T (4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 0.1% Tween 20, 10 mM MgCl2, 5 mM EDTA, and 2 mM DTT), and the bound immune complexes were eluted by boiling in 2× Laemmli SDS sample buffer. For in vitro protein binding assays, purified GST-Gαi3 fusion proteins or GST alone (10 μg) were immobilized on glutathione-Sepharose beads and incubated in binding buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 0.4% NP-40, 10 mM MgCl2, 5 mM EDTA, 2 mM DTT, protease inhibitor cocktail [Roche Diagnostics]) containing either 30 μM GDP or 30 μM GDP, 30 μM AlCl3, and 10 mM NaF for 90 minutes at room temperature. We added 500 μg of glomerular or mouse podocyte lysate in Triton X-100 lysis buffer to each tube and binding reactions were carried out overnight at 4°C with constant rotation. Beads were washed four times with 1 ml PBS-T wash buffer supplemented with GDP or GDP, AlCl3, and NaF, and boiled in 2× Laemmli SDS sample buffer.34
Cell Migration Assays
Differentiated wild-type and GIV-depleted podocytes were plated on type I collagen–coated coverslips in six-well plates until 100% confluent. Each coverslip was then scratched with a sterile 200-μl pipette tip, washed with PBS, and placed into fresh medium. Images were captured by phase-contrast microscopy under a ×10 objective at 0 and 24 hours after wounding and analyzed using ImageJ software to quantify cell migration (expressed as the percentage of wound area covered) as previously described.47,67
Semiquantitative RT-PCR
Total RNA was extracted with the RNeasy Protect Mini Kit (Qiagen) from rat glomeruli according to the manufacturer’s instructions. cDNA was prepared using a SuperScript III RT kit (Invitrogen). The sequence-specific primers for GIV with a size of 204 bp were forward primer 5′-TAT GCC ACT TTA CCT CGT GCA A-3′ and reverse primer 5′-CCT AGA CCT GCT TTT TGA ATT TCT-3′. The sequence-specific primers for glyceraldehyde-3phosphate dehydrogenase with a size of 555 bp were forward primer 5′-AAT GCA TCC TGC ACC ACC AAC TGC-3′ and reverse primer 5′-GGA GGC CAT GTA GGC CAT GAG GTC-3′. The PCR conditions were as follows: 5 minutes at 94°C for the initial denaturation, followed by 1 minute at 94°C, 1 minute at 50°C, and 1 minute at 72°C for amplification, and a final extension at 72°C for 5 minutes. The PCR products were separated on a 1.5% agarose gel, and an image of the gel stained with ethidium bromide was captured using the Quantity One (Bio-Rad).
Adenoviral Vectors
Hemagglutinin-tagged full-length human GIV (wild-type or F1685A) resistant to siRNA against murine GIV was cloned into pShuttle-cytomegalovirus vector.68 The resultant plasmid was linearized with PacI restriction endonuclease and transformed into electrocompetent AdEasier E. coli (Agilent Technologies) using a Gene Pulser Electroporator (Bio-Rad). Correctly recombined clones were chosen based on sequencing and restriction endonuclease analyses, linearized with PacI and transfected into Cre8 cells (a derivative of HEK293T cells, a gift from Dr. Ora Weisz, University of Pittsburgh). Serial amplification of the adenovirus and subsequent purification of the adenoviral particles on cesium chloride gradients was carried out as previously described.68 Purified viral particles were dialyzed against PBS, aliquoted, and stored at −80°C. For expression of WT-GIV and GIV-F1685A, cultured cells were incubated with the respective viruses at 50 multiplicity of infection for 5 hours. The medium was replaced and cultures were maintained for an additional 3–5 days before being used for protein or functional assays.
Quantitative Real-Time PCR
Quantitative real-time PCR was carried out on an ABI Prism 7300 Sequence Detection System (Applied Biosystems) using SYBR GreenER qPCR SuperMix for ABI PRISM (Invitrogen) according to the manufacturer’s instructions. The previously verified primers for mouse genes were as follows: Bcl-2 forward primer 5′-AGGAGCAGGTGCCTACAAGA-3′ and reverse primer 5′-GCATTTTCCCACCACTGTCT-3′,69 Bcl-xL forward primer 5′-GCTGGGACACTTTTGTGGAT-3′ and reverse primer 5′-TGTCTGGTCACTTCCGACTG-3′,70 β-actin forward primer 5′-AGATGTGGATCAGCAAGCAG-3′70 and reverse primer 5′-GCGCAAGTTAGGTTTTGTCA-3′,70 and Bax forward primer 5′-TGCAGAGGATGATTGCTGAC-3′ and reverse primer 5′-GATCAGCTCGGGCACTTTAG-3′.69 Specific primers for mouse GIV were designed by online software as follows: forward primer 5′-GTGATCTCTACTGCTGAAGG-3′ and reverse primer 5′-TGTTGCTCCCTAGACCTGCT-3′. PCR reactions were carried out at 94°C for 5 minutes, followed by 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, with a final extension at 72°C for 5 minutes. All reactions were run in triplicate. After data collection, the relative mRNA expression level of a specific gene in the total RNA was calculated and normalized using mouse β-actin as an internal control.
Statistical Analyses
Data were expressed as the mean±SD or mean±SEM. Statistical significance was evaluated using the t test. P<0.05 and P<0.001 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Mehul Shah for his valuable assistance with construction and purification of the adenoviruses.
This research was supported by a postdoctoral fellowship from the American Heart Association (to H.W.) and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R37-DK17724 to M.G.F.). P.G. was supported by the Burroughs Wellcome Fund.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090985/-/DCSupplemental.
References
- 1.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Garg P, Holzman LB: Podocytes: Gaining a foothold. Exp Cell Res 318: 955–963, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser J, Sever S: Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 64: 357–366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Vasudevan KM, Garraway LA: AKT signaling in physiology and disease. Curr Top Microbiol Immunol 347: 105–133, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K: Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73: 697–704, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K: Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73: 926–932, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F: Cloning of rat nephrin: Expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, Kerjaschki D, Holthöfer H: Nephrin in experimental glomerular disease. Kidney Int 58: 1461–1468, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Hosoyamada M, Yan K, Nishibori Y, Takiue Y, Kudo A, Kawakami H, Shibasaki T, Endou H: Nephrin and podocin expression around the onset of puromycin aminonucleoside nephrosis. J Pharmacol Sci 97: 234–241, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: The urinary podocyte as a marker for the differential diagnosis of idiopathic focal glomerulosclerosis and minimal-change nephrotic syndrome. Am J Nephrol 20: 175–179, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Wen D, You L, Zhang Q, Zhang L, Gu Y, Hao CM, Chen J: Upregulation of nestin protects podocytes from apoptosis induced by puromycin aminonucleoside. Am J Nephrol 34: 423–434, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Müller-Deile J, Worthmann K, Saleem M, Tossidou I, Haller H, Schiffer M: The balance of autocrine VEGF-A and VEGF-C determines podocyte survival. Am J Physiol Renal Physiol 297: F1656–F1667, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Schrijvers BF, Flyvbjerg A, De Vriese AS: The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Tufro A, Veron D: VEGF and podocytes in diabetic nephropathy. Semin Nephrol 32: 385–393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohenstein B, Colin M, Foellmer C, Amann KU, Brekken RA, Daniel C, Hugo CP: Autocrine VEGF-VEGF-R loop on podocytes during glomerulonephritis in humans. Nephrol Dial Transplant 25: 3170–3180, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kanellis J, Levidiotis V, Khong T, Cox AJ, Stacker SA, Gilbert RE, Cooper ME, Power DA: A study of VEGF and its receptors in two rat models of proteinuria. Nephron, Physiol 96: 26–36, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Bailey E, Bottomley MJ, Westwell S, Pringle JH, Furness PN, Feehally J, Brenchley PE, Harper SJ: Vascular endothelial growth factor mRNA expression in minimal change, membranous, and diabetic nephropathy demonstrated by non-isotopic in situ hybridisation. J Clin Pathol 52: 735–738, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertuccio CA: Relevance of VEGF and nephrin expression in glomerular diseases. J Signal Transduct 2011: 718609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A: Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Ghosh P, Garcia-Marcos M, Farquhar MG: GIV/Girdin is a rheostat that fine-tunes growth factor signals during tumor progression. Cell Adhes Migr 5: 237–248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh P, Beas AO, Bornheimer SJ, Garcia-Marcos M, Forry EP, Johannson C, Ear J, Jung BH, Cabrera B, Carethers JM, Farquhar MG: A Galphai-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Biol Cell 21: 2338–2354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C, Ear J, Pavlova Y, Mittal Y, Kufareva I, Ghassemian M, Abagyan R, Garcia-Marcos M, Ghosh P: Tyrosine phosphorylation of the Gα-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal 4: ra64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Hayre M, Degese MS, Gutkind JS: Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol 27: 126–135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Kerjaschki D, Sharkey DJ, Farquhar MG: Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98: 1591–1596, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG: Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20: 1491–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drenckhahn D, Franke RP: Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988 [PubMed] [Google Scholar]
- 36.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F: AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M, Bechtel W, Zschiedrich S, Pfeifer D, Laloë D, Arrondel C, Gonçalves S, Krüger M, Harvey SJ, Busch H, Dengjel J, Huber TB: Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 83: 1052–1064, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM: Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Laplante M, Sabatini DM: mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Martínez JM, Alessi DR: mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416: 375–385, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Grahammer F, Wanner N, Huber TB: mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 29[Suppl 1]: i9–i18, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M: Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell 9: 389–402, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Marcos M, Ghosh P, Farquhar MG: GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci U S A 106: 3178–3183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman DE, Lee E, Mixon MB, Linder ME, Berghuis AM, Gilman AG, Sprang SR: Crystallization and preliminary crystallographic studies of Gi alpha 1 and mutants of Gi alpha 1 in the GTP and GDP-bound states. J Mol Biol 238: 630–634, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Lane JR, Henderson D, Powney B, Wise A, Rees S, Daniels D, Plumpton C, Kinghorn I, Milligan G: Antibodies that identify only the active conformation of G(i) family G protein alpha subunits. FASEB J 22: 1924–1932, 2008 [DOI] [PubMed] [Google Scholar]
- 46.López-Sánchez I, Garcia-Marcos M, Mittal Y, Aznar N, Farquhar MG, Ghosh P: Protein kinase C-theta (PKCθ) phosphorylates and inhibits the guanine exchange factor, GIV/Girdin. Proc Natl Acad Sci U S A 110: 5510–5515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh P, Garcia-Marcos M, Bornheimer SJ, Farquhar MG: Activation of Galphai3 triggers cell migration via regulation of GIV. J Cell Biol 182: 381–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, Dowdy SF: Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol, 27: 567–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB: Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Marcos M, Ear J, Farquhar MG, Ghosh P: A GDI (AGS3) and a GEF (GIV) regulate autophagy by balancing G protein activity and growth factor signals. Mol Biol Cell 22: 673–686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittal Y, Pavlova Y, Garcia-Marcos M, Ghosh P: Src homology domain 2-containing protein-tyrosine phosphatase-1 (SHP-1) binds and dephosphorylates G(alpha)-interacting, vesicle-associated protein (GIV)/Girdin and attenuates the GIV-phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway. J Biol Chem 286: 32404–32415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A: Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem 286: 39933–39944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, Costantini F, Murohara T, Takahashi M: Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol 10: 329–337, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Ostalska-Nowicka D, Malinska A, Zabel M, Witkiewicz W, Nowicki M: Nephrotic syndrome unfavorable course correlates with downregulation of podocyte vascular endothelial growth factor receptor (VEGFR)-2. Folia Histochem Cytobiol 49: 472–478, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Korgaonkar SN, Feng X, Ross MD, Lu TC, D’Agati V, Iyengar R, Klotman PE, He JC: HIV-1 upregulates VEGF in podocytes. J Am Soc Nephrol 19: 877–883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang QX, Nakhaei-Nejad M, Haddad G, Wang X, Loutzenhiser R, Murray AG: Glomerular endothelial PI3 kinase-α couples to VEGFR2, but is not required for eNOS activation. Am J Physiol Renal Physiol 301: F1242–F1250, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Villegas G, Lange-Sperandio B, Tufro A: Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G: Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol 16: 1936–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ: Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le-Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG: Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem 280: 22012–22020, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Lehtonen S, Lehtonen E, Kudlicka K, Holthöfer H, Farquhar MG: Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol 165: 923–936, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG: The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 12: 1589–1598, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 65.McCaffery JM, Farquhar MG: Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol 257: 259–279, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 137: 929–944, 1990 [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Marcos M, Ghosh P, Ear J, Farquhar MG: A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration. J Biol Chem 285: 12765–12777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He TC: A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2: 1236–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Zhou Y, Kong X, Zhao P, Yang H, Chen L, Miao J, Zhang X, Yang J, Ding J, Guan Y: Peroxisome proliferator-activated receptor-α is renoprotective in doxorubicin-induced glomerular injury. Kidney Int 79: 1302–1311, 2011 [DOI] [PubMed] [Google Scholar]
- 70.Iwasawa M, Miyazaki T, Nagase Y, Akiyama T, Kadono Y, Nakamura M, Oshima Y, Yasui T, Matsumoto T, Nakamura T, Kato S, Hennighausen L, Nakamura K, Tanaka S: The antiapoptotic protein Bcl-xL negatively regulates the bone-resorbing activity of osteoclasts in mice. J Clin Invest 119: 3149–3159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.