Abstract
Antibody-mediated rejection (AMR) is a major cause of kidney graft loss, yet assessment of individual risk at diagnosis is impeded by the lack of a reliable prognosis assay. Here, we tested whether the capacity of anti-HLA antibodies to bind complement components allows accurate risk stratification at the time of AMR diagnosis. Among 938 kidney transplant recipients for whom a graft biopsy was performed between 2004 and 2012 at the Lyon University Hospitals, 69 fulfilled the diagnosis criteria for AMR and were enrolled. Sera banked at the time of the biopsy were screened for the presence of donor-specific anti-HLA antibodies (DSAs) and their ability to bind C1q and C3d using flow bead assays. In contrast with C4d graft deposition, the presence of C3d-binding DSA was associated with a higher risk of graft loss (P<0.001). Despite similar trend, the difference did not reach significance with a C1q-binding assay (P=0.06). The prognostic value of a C3d-binding assay was further confirmed in an independent cohort of 39 patients with AMR (P=0.04). Patients with C3d-binding antibodies had worse eGFR and higher DSA mean fluorescence intensity. In a multivariate analysis, only eGFR<30 ml/min per 1.73 m2 (hazard ratio [HR], 3.56; 95% confidence interval [CI], 1.46 to 8.70; P=0.005) and the presence of circulating C3d-binding DSA (HR, 2.80; 95% CI, 1.12 to 6.95; P=0.03) were independent predictors for allograft loss at AMR diagnosis. We conclude that assessment of the C3d-binding capacity of DSA at the time of AMR diagnosis allows for identification of patients at risk for allograft loss.
Keywords: acute rejection, transplantation, transplant outcomes, complement, donor-specific antibodies, antibody-mediated rejection
The prevalence of end-stage renal failure is skyrocketing worldwide. Renal transplantation is currently the best therapeutic option, but the unavailability of organs to meet the ever-increasing demand has resulted in a major organ shortage crisis.1
Prolonging graft life appears to be a straightforward strategy to address this unmet medical need. Advances in transplantation medicine over the last decades has nevertheless only marginally improved long-term graft survival.2 These disappointing results are largely explained by the inadequate control of the humoral arm of recipient immune response, which is now widely recognized as a major cause of late kidney-graft loss.3–8 Integration of experimental and clinical data has led to the emergence of a consensus pathophysiologic sequence, in which the binding of circulating donor-specific antibodies (DSAs) to mismatched HLA molecules expressed by graft microvasculature leads to chronic inflammation and progressive tissue destruction.9,10
The development of sensitive solid-phase assays to detect anti-HLA antibodies has, however, revealed an unsuspected heterogeneity in their pathogenic potential.11–16 A critical issue for patient care is therefore discerning which of the detected antibodies are pathogenic.
On the basis of compelling evidence that complement cascade is an important contributor in antibody-mediated graft destruction,17,18 stratification of the risk of graft loss has essentially relied on assays evaluating the ability of DSA to trigger complement activation. Detection of C4d deposition in renal capillaries was historically the first test to evaluate the capacity of graft-bound antibodies to activate the classic complement pathway.13,19,20 However, this assay showed inconsistent performance in predicting the outcome of antibody-mediated rejection (AMR).21–24 Two tests have been developed to directly assess the capacity of anti-HLA antibodies to bind the complement components that initiate the classic pathway.25–28 The detection of C4d- and C1q-binding anti-HLA antibodies in renal graft recipients correlated with shorter graft survival in small cohorts,29–33 a result just confirmed for C1q-binding assay in a large cohort by Loupy et al.34
While these findings are extremely promising, none of these pioneer studies have evaluated the prognostic value of these tests at the diagnosis of AMR, the actual time when the clinician most needs a risk stratification assay that may guide therapeutic decision making.
In the present study, we evaluated whether the ability of DSAs to bind C3d (a cleavage product of C3 positioned downstream the complement cascade) at the time of AMR allows for accurate risk stratification of kidney-allograft loss.
Results
Characteristics of the Study Population
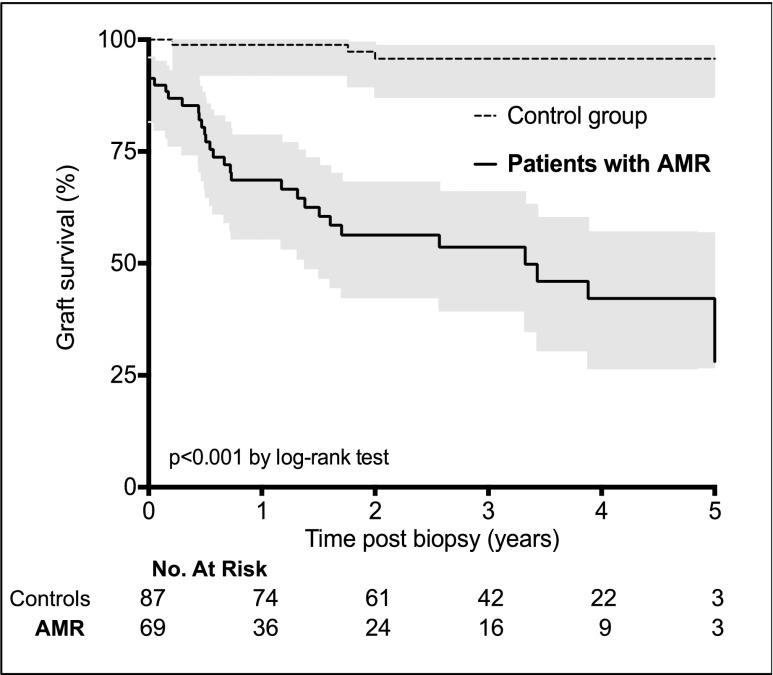
Of 938 kidney transplant recipients followed in our institutions over the study period, 69 (7.3%) fulfilled the diagnostic criteria for AMR35 and were enrolled in the study (see Concise Methods). Table 1 summarizes their characteristics at the time of transplantation and AMR. Kidney allograft survival was compared with that of a control group (i.e., patients who underwent a kidney biopsy and screening for anti-HLA antibodies, and for whom the diagnosis of AMR was excluded) (Supplemental Table 1). Consistent with the literature, kidney allograft survival decreased dramatically after AMR but was highly heterogeneous (68.6%, 53.6%, and 42.2% at 1, 3, and 5 years, respectively) (Figure 1), highlighting the need for tools that allow for accurate risk stratification at diagnosis of AMR.
Table 1.
Baseline characteristics
| Variable | Patients with AMR (n=69) | Patients with Non–C3d-Binding DSA (n=29) | Patients with C3d-Binding DSA (n=40) | P Valuea |
|---|---|---|---|---|
| Characteristics at the time of transplantation | ||||
| Recipient | ||||
| Men, n (%) | 42 (61) | 16 (61.5) | 26 (65) | 0.40 |
| Age (yr) | 39.2±14.6 | 42.8±15.6 | 36.8±12.4 | 0.07 |
| Retransplantation, n (%) | 24 (35) | 10 (34) | 14 (35) | 0.96 |
| Time since dialysis (mo) | 55.7±64.9 | 65.4±65 | 50.2±65.1 | 0.34 |
| Blood group, n (%) | ||||
| Type A | 38 (55) | 14 (48) | 24 (60) | 0.33 |
| Type B | 6 (9) | 4 (14) | 2 (5) | 0.20 |
| Type O | 24 (35) | 11 (38) | 13 (32.5) | 0.64 |
| Type AB | 1 (1) | 0 (0) | 1 (2.5) | 0.39 |
| Donor | ||||
| Age (yr) | 39.0±17.6 | 40.3±16.1 | 38.1±17.8 | 0.59 |
| Deceased, n (%) | 65 (94) | 28 (97) | 37 (92.5) | 0.47 |
| Transplantation | ||||
| No. of HLA A/B/DR mismatch | 3.7±1.4 | 4.0±1.3 | 3.5±1.4 | 0.22 |
| Combined transplantation, n (%)b | 8 (12) | 3 (10) | 5 (12.5) | 0.78 |
| Cold ischemic time (min) | 948±373 | 977±341 | 902±396 | 0.42 |
| Delayed graft function, n (%) | 14 (20) | 4 (14) | 10 (25) | 0.25 |
| Characteristics of AMR | ||||
| Clinicobiologic characteristics | ||||
| Time post-transplantation (d) | 1453±1600 | 1180±1262 | 1651±1796 | 0.23 |
| Proteinuria (g/d) | 1.6±4.5 | 0.7±1.0 | 2.3±5.8 | 0.12 |
| Creatininemia (μmol/L) | 295±312 | 201±136 | 362±382 | 0.034 |
| eGFRc (ml/min per 1.73 m2) | 33.5±20.4 | 39.2±18.5 | 29.5±20.9 | 0.051 |
| Biopsy for protocol | 11 (16) | 7 (24) | 4 (10) | 0.11 |
| Subclinical AMR, n (%) | 8 (12) | 4 (14) | 4 (10) | 0.63 |
| Histologic characteristics (Banff scoresd ) | ||||
| Microvascular inflammatione | 3.5±1.2 | 3.4±1.0 | 3.5±1.2 | 0.70 |
| Transplant glomerulopathy | 1.1±1.2 | 1.0±1.2 | 1.1±1.2 | 0.76 |
| Interstitial inflammation and tubulitis | 2.6±2.0 | 2.2±1.8 | 2.9±2.1 | 0.16 |
| Interstitial fibrosis and tubular atrophy | 1.6±0.8 | 1.5±0.7 | 1.7±0.8 | 0.34 |
| Arteriosclerosis | 1.0±1.1 | 1.1±1.1 | 0.9±1.0 | 0.48 |
| Endarteritis (vasculitis) | 0.25±0.5 | 0.24±0.5 | 0.25±0.5 | 0.94 |
| Immunologic characteristics | ||||
| Types of DSA, n (%)f | ||||
| Preformed DSA | 12 (17) | 7 (25) | 5 (14) | 0.25 |
| De novo DSA | 43 (62) | 17 (61) | 26 (72) | 0.33 |
| Preformed+de novo DSA | 9 (13) | 4 (14) | 5 (14) | 0.86 |
| No. of DSAs | ||||
| Standard single-antigen assay | 1.8±1.2 | 1.4±0.8 | 2.1±1.4 | 0.019 |
| C3d-binding assay | NA | NA | 1.4±0.8 | – |
| Classes of DSA, n (%) | ||||
| Class I | 8 (12) | 5 | 3 | 0.21 |
| Class II | 47 (68) | 21 | 26 | 0.51 |
| Class I+II | 14 (20) | 3 | 11 | 0.08 |
| MFI of the highest DSA | ||||
| Standard single-antigen assay | 7747±5709 | 3179±2629 | 10898±5152 | <0.001 |
| C3d-binding assay | NA | NA | 11151±7073 | – |
| Treatments | ||||
| Steroids pulses | 60 (87) | 25 (86) | 35 (87.5) | 0.87 |
| Intravenous immunoglobulins | 40 (58) | 14 (48) | 26 (65) | 0.16 |
| Rituximab | 38 (55) | 13 (45) | 25 (62.5) | 0.15 |
| Plasmapheresis | 33 (48) | 11 (38) | 22 (55) | 0.16 |
Unless otherwise noted results are expressed as mean±SD. NA, not adapted.
Comparison between patients with non-C3d-binding DSA and patients with C3d-binding-DSA (chi-squared tests for comparison of proportions and unpaired t test for comparison of continuous variables).
Simultaneous pancreas and kidney transplantations.
Calculated with the Modification of Diet in Renal Disease formula.
Banff scores (0: no significant lesion, 1: mild, 2: moderate, 3: severe).
Sum of the Banff scores for glomerulitis and capillaritis.
Data missing for 5 patients.
Figure 1.
AMR is associated with worse kidney graft survival. Kaplan–Meier curves for kidney graft survival are shown for patients diagnosed with AMR and for controls (i.e., Control group). Grey shading indicates SEM.
Evaluation of the Ability of DSAs to Activate the Complement Cascade and Association with Allograft Loss
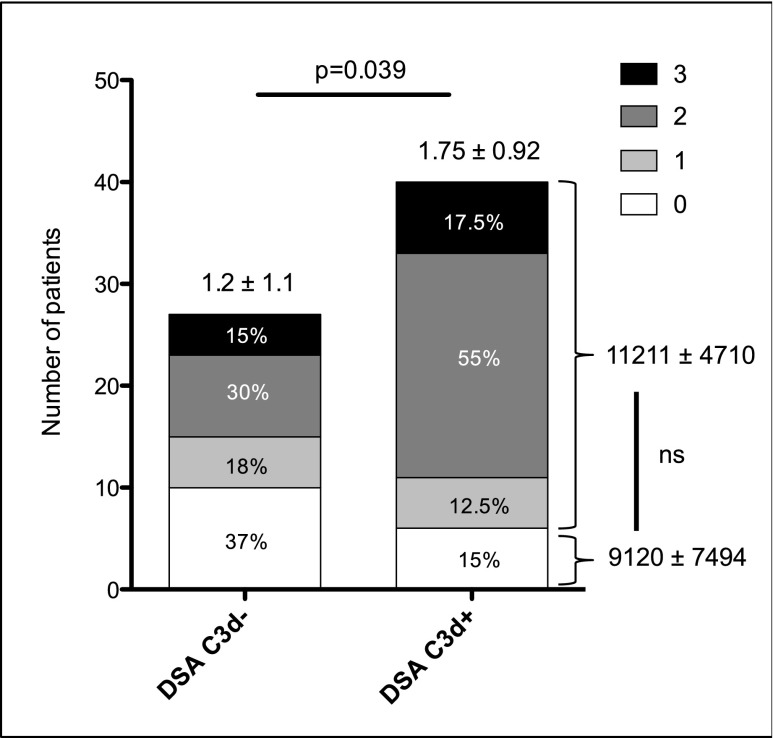
On the basis of abundant literature demonstrating the role of the complement in antibody-mediated graft destruction,17,18 we hypothesized that an assessment of the capacity of antibodies to activate the complement cascade might be useful for predicting AMR outcome. The ability of DSAs to activate the complement cascade was evaluated at the time of rejection by two methods. The gold standard, indirect immunofluorescence technique, was used to detect the presence of C4d deposits in the biopsy specimens. In parallel, serum was tested for the presence of C3d-binding anti-HLA antibodies using a novel single-antigen flow bead assay. Of the 69 patients, 51 (76%) had C4d deposition in renal graft capillaries, and 40 (58%) had circulating C3d-binding DSA. As expected, a positive correlation was observed between the results of the two techniques: Eighty-five percent of patients (n=34) with C3d-positive antibodies had C4d deposits in their biopsy specimens (Figure 2), and C4d deposition scores were higher in patients with C3d-binding antibodies (1.75±0.92 versus 1.2±1.1; P=0.04). Nevertheless, 17 (63%) patients with non–C3d-binding antibodies had detectable C4d deposits in their graft and 6 (15%) patients with C3d-binding antibodies had no C4d deposition (Figure 2). No correlation was observed between the mean fluorescence intensity (MFI) of C3d-binding antibodies and the presence of C4d deposition (Figure 2).
Figure 2.
Presence of C3d-binding DSA correlates with higher C4d scores. Numbers above bars are mean±SD C4d scores. Data are missing for two patients from the DSA C3d-negative group. Numbers on the right side of the figure are the mean±SD MFI of DSAs according to the presence or absence of C4d deposit in the graft (P<0.4). Unpaired t test for comparison.
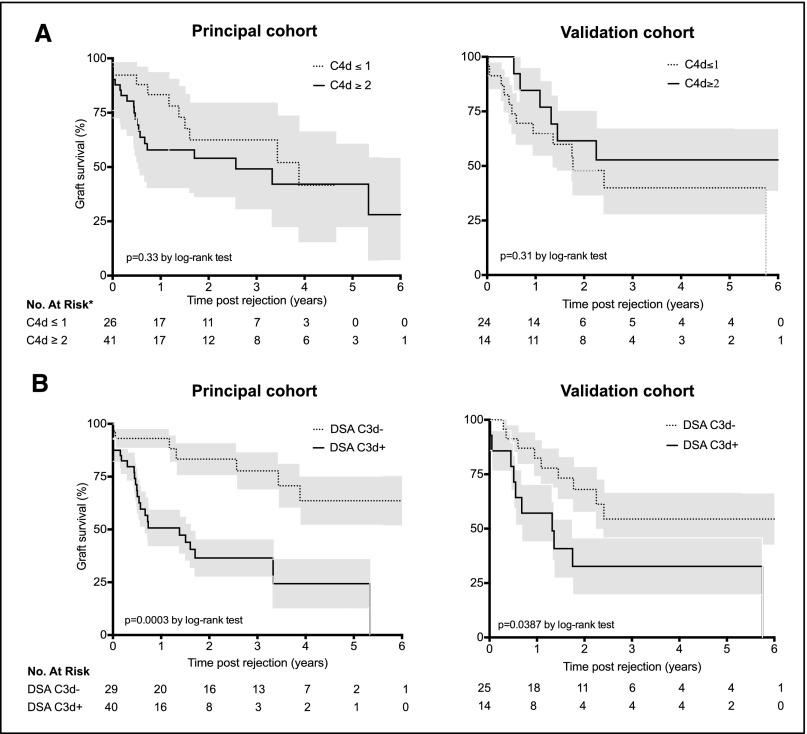
To determine whether these complement activation assays could help in risk stratification, kidney graft survival was analyzed according to, respectively, the biopsy C4d staining or the serum antibody C3d status at time of AMR (Figure 3, left panels). The median follow-up period after rejection was 373 days (range, 1–3010 days). In this cohort of patients, the presence of substantial C4d deposition into the graft (score>1) was not associated with a higher risk of graft loss using Kaplan–Meier estimation (log-rank test, P=0.33) (Figure 3A, upper left panel). In contrast, the presence of circulating C3d-binding DSAs at time of rejection was strongly associated with a higher risk for kidney-graft failure (log-rank test, P<0.001) (Figure 3B, lower left panel). In the C3d-positive group, the estimations of graft survival were 50.7%, 36.5%, and 24.3% at 1, 3, and 5 years, respectively, versus 93.1%, 77.7%, and 63.6% in the C3d-negative group.
Figure 3.
C3d-binding assay predicts renal allograft loss after AMR. Kaplan–Meier curves for kidney graft survival after AMR according to C4d graft deposition status (A) or DSA-C3d status (B), in the principal (left column) and the validation (right column) cohorts. *C4d data missing for two patients in the principal cohort and for one patient in the validation cohort. Grey shading indicates SEM.
To validate our findings, we studied an independent cohort of 39 patients diagnosed with an AMR at Bordeaux University Hospital (Supplemental Table 2). As with the principal cohort, C4d deposition in the graft did not correlate with graft survival (log-rank test, P=0.31) (Figure 3C, upper right panel) but patients with C3d-binding DSAs showed significantly worse allograft survival than those with non–C3d-binding DSAs (log-rank test, P<0.05) (Figure 3D, lower right panel).
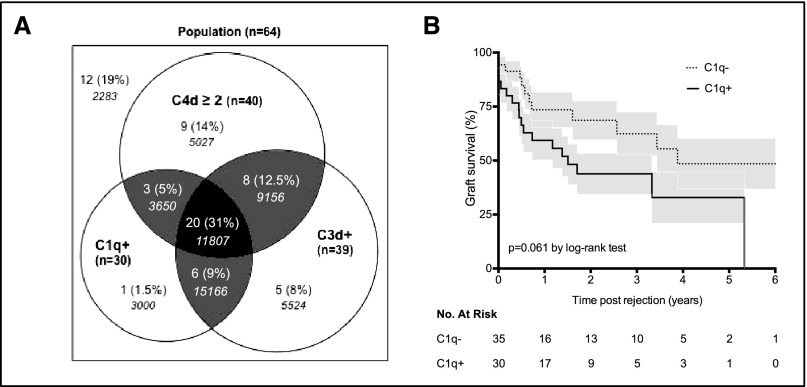
A recent study has established that the onset of C1q-binding DSA during the first year after transplantation is a strong independent risk factor for renal allograft loss.34 To evaluate the prognostic value of this C1q-binding assay at the time of AMR, sera from 65 patients from the principal cohort were tested for the presence of C1q-binding DSA. Figure 4A shows a Venn diagram to summarize the logical relation among the three tests evaluating the ability of donor-specific anti-HLA antibodies to activate the complement (i.e., C4d, C3d, and C1q). Although patients with C1q-binding DSA showed a strong tendency for worse allograft survival, the difference with C1q-negative patients did not reach statistical significance (P=0.06 by log-rank test) (Figure 4B), suggesting that a C3d-binding assay is more powerful in identifying patients at risk for kidney graft failure at diagnosis of AMR (log-rank test comparing allograft survival according to antibody-C3d status for the same 65 patients tested with C1q-binding assay, P<0.001). In calculating the specificity, sensitivity, and the positive and negative predictive values of the three assays (i.e., C4d, C3d and C1q), scores were higher for the C3d-binding assay both for the risk of allograft loss within the first year after AMR and within 3 years after AMR (Table 2).
Figure 4.
Prognostic value of C1q-binding assay at diagnosis of AMR. (A) Venn diagram showing the relation among the three tests evaluating the ability of DSA to activate the complement for 64 patients of the principal cohort (data incomplete for five patients). Numbers in italics indicate MFI of DSA. (B) Kaplan–Meier curves for kidney graft survival after AMR according to DSA-C1q status.
Table 2.
Performance of the three assays to predict allograft loss at 1 and 3 years after AMR
| Variable | C4d Graft Deposition (%) | C1q-Binding Assay (%) | C3d-Binding Assay (%) |
|---|---|---|---|
| Graft loss at 1 year post-rejection | |||
| Sensitivity | 80.0 | 60.0 | 90.0 |
| Specificity | 45.5 | 48.5 | 51.5 |
| Positive predictive value | 47.1 | 41.4 | 52.9 |
| Negative predictive value | 78.9 | 66.7 | 89.5 |
| Graft loss at 3 years post-rejection | |||
| Sensitivity | 69.2 | 61.5 | 84.6 |
| Specificity | 40.0 | 60.0 | 73.3 |
| Positive predictive value | 66.7 | 72.7 | 84.6 |
| Negative predictive value | 42.9 | 47.4 | 73.3 |
Population Characteristics according to C3d Antibody Status
Table 1 shows the characteristics of patients from the principal cohort according to their C3d antibody status (the same information is provided for the patients of the validation cohort in Supplemental Table 2).
Baseline characteristics were similar between the two groups at time of transplantation. Of note, the treatment of AMR consisted of steroid pulses, intravenous immunoglobulins, plasmapheresis, or rituximab and was similar between the two groups. Patients with C3d-binding antibodies had a worse estimated kidney graft function at time of rejection than patients with non–complement-binding antibodies (eGFR, 29.5±0.5 versus 39.2±18.5 ml/min per 1.73 m2, respectively; P=0.05).
The C3d-binding capacity of antibodies also correlated with a higher number and a higher MFI of DSAs. This difference, which was also observed in the validation cohort, existed both when the highest MFI (10898±5152 versus 3179±2629; P<0.001) (Table 1, Figure 5A) or the sum of MFI (16694±11249 versus 4187±5216; P<0.001) (Figure 5B) were considered. Despite this, some patients in the C3d-negative group had DSAs with high MFI, and conversely some patients in the C3d-positive group had DSAs with low MFI (Figure 5, A and B). This overlap was even more evident when all detected DSAs in the principal cohort were grouped according to their C3d-binding ability and their MFI was plotted (Figure 5C). Of note, when only the 25 sera with the lowest MFI (i.e., <3500) were considered, the difference in allograft survival between the C3d-positive and C3d-negative groups persisted (P<0.004 by log-rank test) (Figure 5D).
Figure 5.
Relation between MFI and C3d-binding capacity of DSA. (A) The MFI of the highest DSA is plotted according to patients’ C3d status. (B) The sum of the MFIs of DSAs is plotted according to patients’ C3d status. Each patient is a dot. (C) The MFIs are plotted for all the DSAs identified according to their ability to bind C3d. Each dot is a C3d-negative DSA and each square is a C3d-positive DSA. Numbers above boxes and scatter plots are mean±SD. Unpaired t test for comparisons. (D) Kidney graft survival after AMR according to the DSA-C3d status for the patients with the lowest DSA-MFI (MFI<3500). Grey shading indicates SEM.
Identification of Independent Predictors for Allograft Loss at Time of AMR
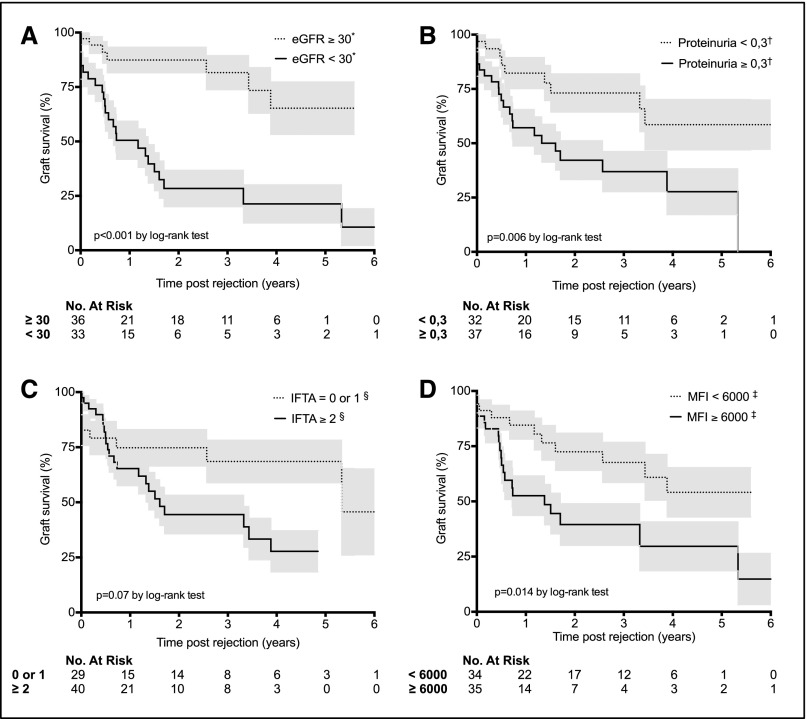
Because the C3d-binding positive and negative groups were statistically different for characteristics that may have influenced graft survival, a Cox regression proportional hazard model was used to test whether C3d antibody status is truly independently associated with graft failure. Exploratory univariate analysis identified five risk factors of graft loss (Table 3, Figure 6): C3d antibody status (hazard ratio [HR], 4.35; 95% confidence interval [95% CI], 1.82 to 10.40; P=0.001); low eGFR (HR, 4.99; 95% CI, 2.12 to 11.70; P<0.001); proteinuria (HR, 2.82; 95% CI, 1.29 to 6.18; P<0.01); interstitial fibrosis and tubular atrophy (HR, 2.05; 95% CI, 0.91 to 4.62; P=0.08); and MFI of the highest DSA (HR, 2.47; 95% CI, 1.17 to 5.23; P=0.02). These five variables were integrated in a multivariate analysis (Table 3), which identified only two independent predictors for allograft loss at diagnosis of AMR: low eGFR (HR, 3.56; 95% CI, 1.46 to 8.70; P<0.01) and C3d-binding DSAs (HR, 2.80; 95% CI, 1.12 to 6.95; P=0.03).
Table 3.
Univariate and multivariate analyses of risk factors for death-censored allograft loss
| Variable | Patients (n) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Clinicobiologic factors | |||||
| Recipient sex | |||||
| Female | 27 | 1.00 (Reference) | |||
| Male | 42 | 1.01 (0.49 to 2.08) | 0.98 | a | |
| Recipient age (per 1-yr increment) | 69 | 0.99 (0.96 to 1.01) | 0.30 | a | |
| Retransplantation | |||||
| No | 45 | 1.00 (Reference) | |||
| Yes | 24 | 1.02 (0.49 to 2.12) | 0.95 | a | |
| Donor type | |||||
| Living | 4 | 1.00 (Reference) | |||
| Deceased | 65 | 0.92 (0.22 to 3.91) | 0.91 | a | |
| Donor age (per 1-yr increment) | 69 | 1.00 (0.98 to 1.02) | 0.87 | a | |
| No. of mismatches A/B/DR | |||||
| ≤3 | 20 | 1.00 (Reference) | |||
| >3 | 49 | 0.57 (0.27 to 1.18) | 0.13 | a | |
| Cold ischemia time per 1-min increment | 69 | 1.00 (0.99 to 1.00) | 0.29 | a | |
| Estimated GFR at the time of rejectionb | |||||
| ≥30 ml/min per 1.73 m2 | 36 | 1.00 (Reference) | |||
| <30 ml/min per 1.73 m2 | 33 | 4.99 (2.12 to 11.70) | <0.001 | 3.56 (1.46 to 8.70) | 0.005 |
| Proteinuria at time of rejection | |||||
| <0.3 g/d | 32 | 1.00 (Reference) | |||
| ≥0.3 g/d | 37 | 2.82 (1.29 to 6.18) | 0.009 | — | NS |
| Histologic factorsc | |||||
| Microvascular inflammationd | |||||
| 2 or 3 | 34 | 1.00 (Reference) | |||
| ≥4 | 35 | 1.13 (0.55 to 2.35) | 0.72 | a | |
| C4d graft depositione | |||||
| 0 or 1 | 26 | 1.00 (Reference) | |||
| ≥2 | 41 | 1.45 (0.67 to 3.13) | 0.34 | a | |
| Interstitial inflammation and tubulitis | |||||
| 0 or 1 | 27 | 1.00 (Reference) | |||
| ≥2 | 42 | 0.95 (0.46 to 1.97) | 0.90 | a | |
| Transplant glomerulopathy | |||||
| 0 or 1 | 49 | 1.00 (Reference) | |||
| ≥2 | 20 | 1.24 (0.59 to 2.61) | 0.57 | a | |
| Endarteritis (vasculitis) | |||||
| 0 | 54 | 1.00 (Reference) | |||
| ≥1 | 15 | 0.92 (0.37 to 2.26) | 0.85 | a | |
| Arteriosclerosis | |||||
| 0 or 1 | 52 | 1.00 (Reference) | |||
| ≥2 | 17 | 0.72 (0.31 to 1.67) | 0.44 | a | |
| Interstitial fibrosis and tubular atrophy | |||||
| 0 or 1 | 29 | 1.00 (Reference) | |||
| ≥2 | 40 | 2.05 (0.91 to 4.62) | 0.084 | — | NS |
| Immunologic factors | |||||
| C3d-binding DSA | |||||
| No | 29 | 1.00 (Reference) | |||
| Yes | 40 | 4.35 (1.82 to 10.40) | 0.001 | 2.80 (1.12 to 6.95) | 0.027 |
| MFI of the highest DSA | |||||
| <6000 | 34 | 1.00 (Reference) | |||
| ≥6000 | 35 | 2.47 (1.17 to 5.23) | 0.018 | — | NS |
| No. of DSA | |||||
| 1 | 38 | 1.00 (Reference) | |||
| ≥2 | 31 | 1.67 (0.82 to 3.41) | 0.16 | a | |
The variables that were not tested in multivariate model.
Calculated with the Modification of Diet in Renal Disease formula.
Banff scores (0: no significant lesion, 1: mild, 2: moderate, 3: severe).
Sum of the Banff scores for glomerulitis and capillaritis.
Data missing for 2 patients. Variables at P<0.1 in univariate model were incorporated into the multivariate model.
Figure 6.
Factors associated with worse kidney graft survival at diagnosis of AMR. Kaplan–Meier curves for kidney graft survival after AMR according to (A) GFR estimated by the Modification of Diet in Renal Disease equation at diagnosis (denoted by asterisk and expressed as ml/min per 1.73m2), (B) proteinuria (denoted by dagger and expressed as g/d), (C) interstitial fibrosis and tubular atrophy (IFTA; denoted by section mark and expressed as sum of Banff scores), and (D) highest DSA MFI (denoted by double dagger and expressed as arbitrary units). Grey shading indicates SEM.
Discussion
The recipient’s humoral response against donor HLA molecules is a major cause of kidney graft loss.3–8 Yet AMR has a highly heterogeneous outcome at the individual level,36,37 which makes it crucial to develop reliable prognostic tools to better stratify the risk. Ideally, such a prognostic tool should be able to identify patients at risk of graft loss at the time of the diagnosis of AMR, in order to allow for timely adjustment of the treatment.
We analyzed 69 cases of AMR and identified only two factors that independently predicted allograft failure at the time of diagnosis: (1) a low eGFR and (2) the presence of C3d-binding DSA in the circulation.
The negative prognostic value of a low eGFR could have two explanations. First, the eGFR could reflect the intensity of acute antibody-mediated damages. Alternatively, it could trivially indicate that the prognosis of AMR is worse when it occurs on a graft, which already has established damages. The comparison of graft histology favors the last explanation. Patients with an eGFR<30 ml/min per 1.73 m2 indeed displayed more severe nonspecific chronic lesions (i.e., interstitial fibrosis with tubular atrophy) (Supplemental Table 3) but had similar scores for cellular-mediated (t+i) as well as antibody-mediated (g+ptc) lesions (Supplemental Table 3). Of note, the score for chronic humoral lesions (cg) was also similar between patients with a low and those with a high eGFR, suggesting that the more severe chronic damages observed in the first group were not due to a delay in AMR diagnosis.
Experimental studies have demonstrated that antibodies directed against the graft can cause injuries in the absence of complement38 through antibody-dependent cell cytotoxicity and/or direct activation of endothelial cells.10,39–41 Nevertheless, the combination of complement-dependent and -independent mechanisms is synergistically deleterious for the graft,42 making complement activation a good candidate for risk stratification in AMR.
The binding of C1q to antibodies complexed with antigen activates the serine esterases C1s and C1r, which allow for the cleavage of C4. This, in turn, results in the deposition of C4d in tissue and the assembly of the classic pathway C3 convertase. The latter cleaves C3 into C3a and C3b.17,43 C3a is a potent proinflammatory mediator that causes leukocyte recruitment, while C3b propagates the complement cascade leading to the formation of sublytic membrane attack complexes responsible for the activation of endothelial cells.17,43
C4d staining in renal capillaries historically represented the gold standard technique to detect complement activation.17,19 However, the results of several studies that assessed the performance of this assay in predicting the outcome of AMR have been contradictory. Some studies have reported an association of C4d deposition with an inferior graft survival (for a recent review see Sapir-Pichhadze et al.24). In contrast, other studies have highlighted the limits of this assay in predicting the outcome of AMR.21–23 In agreement with the latter, we concluded from the present work that C4d deposition into the graft at the time of AMR did not allow for efficient discrimination between patients who would lose their graft and those who would not.
In contrast, we observed that the ability of DSAs to bind the complement component C3d correlated strongly with the risk of graft failure, a result validated in an independent cohort. Because Loupy et al.34 recently reported that the onset of C1q-binding DSAs during the first year after transplantation is a strong independent risk factor for allograft loss and is associated with a higher risk of AMR, we continued evaluating the performance of C1q-binding assay to stratify the risk of graft loss at the time of diagnosis of AMR. Despite a strong tendency, the difference in graft survival between groups that were positive and negative for C1q binding did not reach statistical significance.
The difference in performance for C3d- and C1q-binding assays must be further confirmed in independent studies but can likely be explained by the fact that these two tests analyze different steps of the classic complement pathway. The complement system indeed activates through a triggered-enzyme cascade, in which the activation of a small number of complement proteins at the start is hugely amplified by each successive enzymatic reaction.17,43 C1q is the first component of the classic complement pathway, and it is therefore not surprising that a C1q-binding assay would exhibit a lower sensitivity than a C3d-binding assay. Another plausible explanation could lie in the regulatory mechanisms preventing uncontrolled amplification of the complement cascade. By limiting C3 convertase formation even when substantial amounts of C1q bind to antibodies,43 they could reduce the specificity of a C1q-binding assay. In contrast, the presence of C3d on DSA proves the efficient cleavage of C3 and is therefore more closely related to the pathogenic processes damaging the graft.
Finally, an important point to consider is the relative value of this new C3d-binding assay compared with the mere quantification of DSA titer by the MFI. Our data, as well as the ones reported by Loupy et al.,34 indeed indicate a strong correlation between the MFI of DSAs and their ability to activate the complement. This observation was not unexpected because the binding energy required for C1q activation is achieved only when a single molecule of C1q can bind two or more IgG molecules that are held within 30–40 nm of each other, a situation that requires many molecules of IgG.44 However, C3d-positive DSAs with low MFI do exist and carry higher risk for graft loss (Figure 5D).
Another important utility of the C3d-binding assay may be the identification of patients who would benefit from a treatment with eculizumab. This monoclonal antibody, which is capable of blocking the complement cascade,45,46 cannot be given to every patient diagnosed with AMR because of its costs and adverse effects.
In conclusion, our study shows that the presence of C3d-binding DSAs at the time of AMR is a strong independent predictor of allograft loss. This finding provides a basis for future clinical trials aimed at testing the efficiency of complement inhibitor agents on this subgroup of high-risk patients.
Concise Methods
Study Population
The reports of all kidney allograft biopsies performed between September 1, 2004, and September 1, 2012, at Edouard Herriot Hospital or Jules Courmont Hospital, the two university hospitals in Lyon, France, were screened (2024 biopsies in 938 patients) by means of the pathology department’s computer database (DIAMIC). All kidney transplant recipients who displayed DSAs during the same period were identified through the immunology department’s computer database. Information from the two databases was compared to identify patients (n=77) with microvascular inflammation and concomitant DSAs, thus fulfilling the diagnosis criteria of AMR according to the latest Banff classification.35
A renal pathologist and a nephrologist reviewed all biopsy specimens, and eight patients whose biopsy specimens did not show significant microvascular inflammation were excluded. Serum samples, which had been banked at the time of biopsy, were all retested using the same batch of the same Single Antigen Flow Beads assay (see below).
All patients received an ABO-compatible transplant with negative historical and current complement-dependent cytotoxicity crossmatches. Clinical data were obtained from two independent national registries (Cristal [http://www.sipg.sante.fr/portail/] and Données Informatiques Validées en Transplantation [http://www.divat.fr/]) and crosschecked. The characteristics of the patients and their rejections are summarized in the left column of Table 1.
The end of follow-up was set as September 26th, 2013 (mean follow-up duration for the cohort±SD, 21.6±21.7 months). Allograft survival in this principal cohort was compared with allograft survival in a control cohort of patients for whom the diagnosis of AMR was excluded (see Supplemental Material).
Observations made for the principal cohort were validated with an independent cohort of patients from the kidney transplantation department of the Pellegrin Hospital, University Hospital of Bordeaux, France (see Supplemental Material).
Allograft Pathology
Kidney graft biopsies were performed systematically as part of the routine follow-up procedure at 3 months and 1 year after transplantation, or when rejection was suspected at the other time points.
Renal specimens were fixed in acetic acid-formol-absolute alcohol, and paraffin-embedded sections were stained by routine methods. C4d staining was performed by indirect immunofluorescence on frozen sections using an anti-human C4d complement–rabbit clonal antibody (clone A24-T, produced by DB Biotech, Kosice, Slovak Republic). The renal pathologist (M.R.) and the nephrologist (A.S.) who reviewed the biopsy specimens were blinded to clinical and immunologic information.
Renal allograft lesions were graded according to the Banff classification updated in 2014. Histologic criteria for AMR were defined by a sum of the scores for glomerulitis and peritubular capillaritis ≥2, with or without concurrent positive C4d staining.
Detection and Characterization of Anti-HLA Antibodies
All the analyses were performed in a blinded fashion by a trained immunobiologist. HLA typing of donors and recipients was performed by PCR-SSO reverse (One Lambda, Canoga Park, CA).
Serum samples banked at the time of biopsy were analyzed using Single Antigen Flow Beads assays (LSA class I and class II, Lifecodes, Immucor, Norcross, GA). The MFI was measured on a LABscan IS 200, and all the specificities with a MFI >500 and AD-BCR >5 were considered positive (AD-BCR is MFI adjusted to the quantity of coated antigen per bead).
Serum samples were analyzed in a blinded fashion for the presence of C3d-binding donor-specific anti-HLA antibodies (V.D. at the Etablissement Français du Sang, Lyon, France) with the use of single-antigen flow bead assays according to the manufacturer’s protocol (C3d-binding antibody assay; Lifecodes; see details in Supplemental Material).
Sixty-seven samples of the 69 were further analyzed for the presence of C1q-binding donor-specific anti-HLA antibodies in a blinded fashion (J.L.T. at the Immunology Department of Pellegrin Hospital, Bordeaux, France) with the use of single-antigen flow bead assays according to the manufacturer’s protocol (C1q-binding antibody assay: C1qScreen, One Lambda; see details in Supplemental Material). There was no serum left for two patients, and for two serum samples, results of the test were not interpretable because of high serum background.
Statistical Analyses
Categorical variables were expressed as percentages and compared with the chi-square test. Continuous variables were expressed as mean±SD and compared using the t test. Graft survival was calculated from the date of diagnosis until the beginning of hemodialysis. Survival curves were constructed with the Kaplan–Meier method and compared with the log-rank test.
The Cox proportional hazards regression model was used in both univariate and multivariate models. All significant variables in the univariate analysis with a level set at P<0.1 were incorporated into multivariate models. Complementary analyses with a level set at P<0.02 or P<0.01 to minimize the number of covariates in the multivariate regression model were performed, and they led to similar conclusions (not shown).
All tests were two sided, and P values <0.05 were considered to represent statistically significant differences. Statistical analyses were done using SPSS software, version 20.0 (IBM, Armonk, NY).
Disclosures
Lifecodes (Immucor) donated reagents but was not involved in the study design, collection and analysis of data, writing the report, or the decision to submit the paper for publication.
Supplementary Material
Acknowledgments
We thank Chantal Buisson, Audrey Eynaud, Géraldine Georget, Frederique Dijoud, and Céline Dagot for their contribution to data collection. We also thank Dr. Jean-Emmanuel Serre for technical assistance. E.M., L.B., and O.T. are members of the CENTAURE Transplantation Research Network.
There was no funding source for this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101144/-/DCSupplemental.
References
- 1.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am J Transplant 12[Suppl 1]: 1–156, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Terasaki PI: A personal perspective: 100-year history of the humoral theory of transplantation. Transplantation 93: 751–756, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Kuypers DR: Diagnosis and prevention of chronic kidney allograft loss. Lancet 378: 1428–1437, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M: Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am J Transplant 14: 255–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nankivell BJ, Alexander SI, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Thaunat O: Humoral immunity in chronic allograft rejection: Puzzle pieces come together. Transpl Immunol 26: 101–106, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D: Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant 8: 324–331, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D, Duong van Huyen JP, Bruneval P, Legendre C, Nochy D: Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9: 2561–2570, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, Ratner LE, Cohen DJ, Radhakrishnan J: Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol 23: 2061–2071, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stegall MD, Chedid MF, Cornell LD: The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol 8: 670–678, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Valenzuela NM, Reed EF: Antibodies in transplantation: The effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Biol 1034: 41–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feucht HE, Felber E, Gokel MJ, Hillebrand G, Nattermann U, Brockmeyer C, Held E, Riethmüller G, Land W, Albert E: Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol 86: 464–470, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB: Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 10: 2208–2214, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF: Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH: Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Sapir-Pichhadze R, Curran SP, John R, Tricco AC, Uleryk E, Laupacis A, Tinckam K, Sis B, Beyene J, Logan AG, Kim SJ: A systematic review of the role of C4d in the diagnosis of acute antibody-mediated rejection [Published online ahead of print May 14, 2014]. Kidney Int 10.1038/ki.2014.166 [DOI] [PubMed] [Google Scholar]
- 25.Bartel G, Wahrmann M, Exner M, Regele H, Schillinger M, Hörl WH, Böhmig GA: Determinants of the complement-fixing ability of recipient presensitization against HLA antigens. Transplantation 83: 727–733, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Smith JD, Hamour IM, Banner NR, Rose ML: C4d fixing, luminex binding antibodies—a new tool for prediction of graft failure after heart transplantation. Am J Transplant 7: 2809–2815, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Sequeira F, Tyan DB: Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol 72: 849–858, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Lachmann N, Todorova K, Schulze H, Schönemann C: Systematic comparison of four cell- and Luminex-based methods for assessment of complement-activating HLA antibodies. Transplantation 95: 694–700, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB: C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 91: 342–347, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Bartel G, Wahrmann M, Schwaiger E, Kikić Ž, Winzer C, Hörl WH, Mühlbacher F, Hoke M, Zlabinger GJ, Regele H, Böhmig GA: Solid phase detection of C4d-fixing HLA antibodies to predict rejection in high immunological risk kidney transplant recipients. Transpl Int 26: 121–130, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, Briley KP, Haisch CE, Bolin P, Parker K, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation 95: 1113–1119, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Lawrence C, Willicombe M, Brookes PA, Santos-Nunez E, Bajaj R, Cook T, Roufosse C, Taube D, Warrens AN: Preformed complement-activating low-level donor-specific antibody predicts early antibody-mediated rejection in renal allografts. Transplantation 95: 341–346, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB: Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant 16: 12–17, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana J-P, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff meeting report writing committee : Banff 2011 Meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen J-P, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana J-P, Hill GS, Glotz D, Legendre C, Jouven X: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB: A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant 12: 313–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colvin RB, Smith RN: Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Thaunat O, Louedec L, Dai J, Bellier F, Groyer E, Delignat S, Gaston AT, Caligiuri G, Joly E, Plissonnier D, Michel JB, Nicoletti A: Direct and indirect effects of alloantibodies link neointimal and medial remodeling in graft arteriosclerosis. Arterioscler Thromb Vasc Biol 26: 2359–2365, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela NM, McNamara JT, Reed EF: Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant 19: 33–40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM, 3rd: Antibody and complement in transplant vasculopathy. Circ Res 100: 191–203, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Roumenina LT, Zuber J, Frémeaux-Bacchi V: Physiological and therapeutic complement regulators in kidney transplantation. Curr Opin Organ Transplant 18: 421–429, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW: Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM: Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Stegall MD, Moore N, Taner T, Li H, Dean PG: Down-regulating humoral immune responses: implications for organ transplantation. Transplantation 97: 247–257, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.