Abstract
A noninvasive means to predict the onset and recurrence of lupus nephritis (LN) before overt renal injury is needed to optimize and individualize treatment. Colony-stimulating factor-1 (CSF-1) is expressed by kidney tubules at the onset of LN, increases with disease progression, and spills into the circulation in lupus-prone mice. We tested the hypothesis that amplified expression of CSF-1 detected in the serum or urine correlates with intrarenal CSF-1 expression and histopathology (increased macrophage accumulation, activity indices) and clinical kidney disease activity and predicts the onset and recurrence of nephritis in patients with systemic lupus erythematosus (SLE). We found increased serum or urine CSF-1 levels in patients with cutaneous, serositis, and musculoskeletal disease; however, the increase in CSF-1 levels was far greater in LN. Moreover, an elevation in serum or urine CSF-1 levels correlated with increasing intrarenal CSF-1 expression and histopathology. By longitudinally tracking patients, we found that elevated serum CSF-1 heralded the initial onset of disease, and a rise in serum or urine CSF-1 predicted recurrences of LN before clinical evidence of glomerular dysfunction and conventional serologic measures, even in patients with other manifestations of SLE. These findings indicate that serial monitoring for a rise in serum or urine CSF-1 levels in patients with SLE reflects kidney histopathology and may predict renal disease activity and the onset and recurrence of LN more accurately than conventional laboratory measures.
Keywords: glomerulonephritis, kidney disease, lupus nephritis, macrophages, rheumatology, renal tubular epithelial cells
Lupus nephritis (LN) is the main cause of morbidity and mortality for patients with systemic lupus erythematosus (SLE).1 Despite current treatment, ESRD is frequent in patients with LN (up to 26%).2 Moreover, relapses or flares of LN are common (27%–66%) and contribute to mortality.3,4 Current methods for diagnosing LN in patients with SLE include proteinuria, active sediment, and a decrease in GFR with confirmation by a renal biopsy.5 Clearly, identification of a biomarker that heralds LN before structural renal damage and the loss of renal function would provide a window to treat early and obviate renal tissue injury.
Colony-stimulating factor-1 (CSF-1) is largely responsible for Mø development, survival, proliferation, and activation.6 We hypothesize that amplified expression of CSF-1 in serum or urine reflects the magnitude of intrarenal CSF-1 and histopathology, correlates with LN clinical disease activity, and heralds the onset and recurrence of LN. This hypothesis is based on the following evidence from our laboratory using lupus-prone MRL-Faslpr mice. Renal tubular epithelial cells (TECs) express CSF-1, and intrarenal CSF-1 expression increases with advancing LN.7–9 The genetic deletion of CSF-1 prevents LN.10 Intrarenal overexpression of CSF-1 via gene transfer,11 as well as systemic overexpression, using CSF-1 transgenic mice incites, hastens, and worsens the severity of LN.8 Indeed, heightened CSF-1 expression within the MRL-Faslpr kidney at the onset of LN spills over into the circulation and leads to monocytosis with renal homing monocytes that are skewed toward an “inflammatory” phenotype.8 Within the kidney, CSF-1 activates and expands Mø,8,12,13 which, in turn, induces destructive apoptosis of TEC.7,14 Because CSF-1 is central to the development and progression of LN in MRL-Faslpr mice, it may prove to be a therapeutic target and predictive biomarker for patients with LN.
We now report that tracking serum and urine CSF-1 levels longitudinally is a noninvasive potential biomarker for monitoring disease activity in patients with LN. We find that a rise in serum CSF-1 may be an earlier harbinger of LN and that an elevation of serum or urine CSF-1 levels may be a more accurate predictor of LN reoccurrences than current standards.
Results
Serum and Urine CSF-1 Levels Are Notably Elevated in Patients with LN
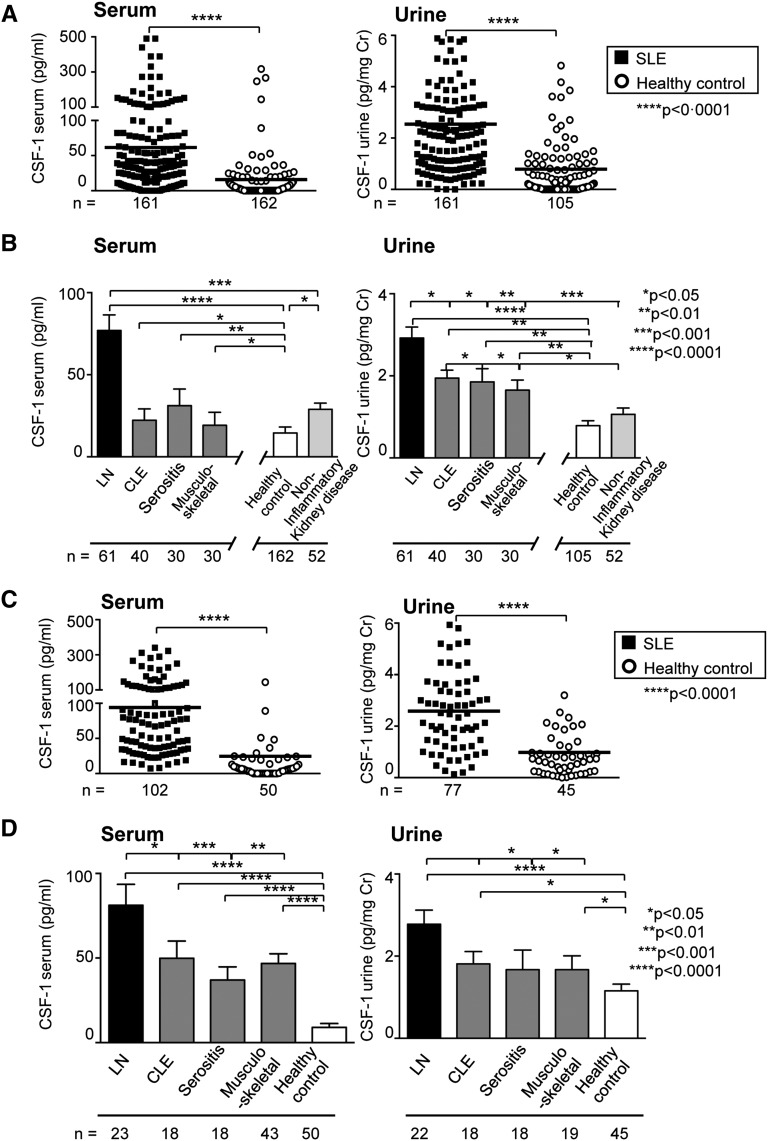
We evaluated CSF-1 levels in two independent cohorts. The cohort from Mainz, Germany, included 161 patients with SLE with diverse disease manifestations and 162 healthy controls, and the cohort from Pavia, Italy, included 102 patients with SLE and 50 healthy controls. To determine whether serum and urine CSF-1 levels increase in patients with SLE, we compared serum and urine CSF-1 in patients with SLE and in healthy controls using these two large cohorts. Patients were included independent of disease duration, clinical activity, and treatment regimen (patient demographic characteristics and clinical profiles are shown in Table 1). We detected increased serum and urine CSF-1 levels in patients with SLE compared with healthy controls in both cohorts (Figure 1), although the range of CSF-1 levels varies between these cohorts. Because SLE is a diverse disease that targets many tissues for destruction, we stratified and compared CSF-1 levels according to the diseased tissue (Figure 1). While serum and urine CSF-1 are elevated in patients with SLE and LN (Table 1) and those with cutaneous lupus erythematosus (CLE), serositis, and musculoskeletal manifestations alone compared with healthy controls and noninflammatory kidney disease (patient demographic characteristics and clinical profiles are shown in Supplemental Table 1), CSF-1 levels are far higher in each cohort in patients with LN (Figure 1).
Table 1.
Demographic and clinical characteristics according to study cohort
| Variable | Overall | Biopsy-Proven LN | ||
|---|---|---|---|---|
| Mainz Cohort (n=161) | Pavia Cohort (n=102) | Mainz Cohort (n=50) | Pavia Cohort (n=15) | |
| Race | ||||
| White | 142 | 94 | 42 | 12 |
| Asian | 11 | 3 | 7 | 1 |
| Others | 8 | 5 | 1 | 2 |
| Sex | ||||
| Female | 150 | 95 | 41 | 13 |
| Male | 11 | 7 | 9 | 2 |
| Age (yr) | ||||
| Mean | 48.5±1.9 | 48.9±1.7 | ||
| Range | 22–75 | 18–69 | ||
| Age at SLE diagnosis (yr) | ||||
| Mean | 33.2±2.1 | 31.8±1.8 | 32±1.9 | 34±1.6 |
| Range | 11.1–72.3 | 12.5–71.4 | 11.5–69.1 | 13.1–73.2 |
| Age at diagnosis of LN (yr) | ||||
| Mean | 37.3±2.1 | 38.1±3.3 | ||
| Range | 19.5–69.3 | 17.1–67.2 | ||
| Disease duration at time of LN onset (yr) | ||||
| Mean | 5.0±1.1 | 6.6±131 | ||
| Range | 0–31.1 | 0–24.1 | ||
| Overall duration of SLE (yr) | ||||
| Mean | 13.9±1.6 | 15.7±1.4 | 11.1±1.1 | 12.1±0.9 |
| Range | 0.7–43.5 | 0.9–41.9 | 0.8–32 | 0.5–40.2 |
| Clinical variables at the time of LN diagnosis | ||||
| ANA-positive patients (n) | 157 | 100 | 45 | 15 |
| Anti-dsDNA–positive patients (n) | 126 | 78 | 39 | 6 |
| Creatinine (mg/dl) | 1.1±0.1 | 1.1±0.16 | ||
| Proteinuria (24-hr collection)>0.5 g/d (g/d) | ||||
| Mean | 2.9±0.4 | 1.4±0.4 | ||
| Range | 0.4–17.5 | 0.3–5.6 | ||
| Depressed serum C3 level | ||||
| Patients (n) | 38 | 12 | ||
| Mean | 0.6±0.5 | 0.7±0.1 | ||
| Range | 0.2–1.4 | 0.4–1.1 | ||
| ISN/RPS class (n) | ||||
| Class II | 11 | 2 | ||
| Class III | 10 | 4 | ||
| Class IV | 29 | 9 | ||
| Class V | 4 | 2 | ||
| Medication | ||||
| Prednisolone dose (mg) | 4.3±0.8 | 4.9±0.7 | 8.3±0.6 | 4.5±0.5 |
| Prednisolone (n) | 134 | 81 | 48 | 15 |
| Mycophenolate mofetil (n) | 78 | 36 | 40 | 11 |
| Cyclophosphamide (only as induction therapy) (n) | 31 | 12 | 23 | 10 |
| Azathioprine (n) | 42 | 13 | 4 | 4 |
| Hydroxychloroquine (n) | 92 | 67 | 30 | 12 |
| Angiotensin-blocking agent (n) | 72 | 34 | 42 | 10 |
Means are expressed with SDs.
Figure 1.
Serum and urine CSF-1 are elevated in patients with SLE with LN, CLE, serositis, and musculoskeletal manifestations compared with healthy controls (two cohorts) and noninflammatory kidney disease (only Mainz cohort) but are highest in patients with LN. CSF-1 levels in SLE (A and C) compared with healthy controls and SLE with LN, CLE, serositis, and musculoskeletal manifestations (B and D) compared with healthy controls and noninflammatory kidney disease. CSF-1 was quantified using ELISA assay. Values are means±SEM. Analysis was done using Mann–Whitney U test.
CSF-1 levels in the sera and urine of patients with SLE and LN are sensitive and specific, as shown using receiver-operating characteristic curves (Supplemental Figure 1A). Confidence in our CSF-1 assay was supported by the CSF-1 interassay variability (8.5%) and intra-assay variability (7.2% for serum and 5.1% for urine).15 Moreover, we found little variability in CSF-1 levels using serum and urine specimens that we froze and thawed four times (Supplemental Figure 1B). The difference (mean±SD) of the change of CSF-1 levels was 2.7±0.5 pg/ml in serum and 6.7±0.6 pg/ml in urine. In addition, we found little variability in serum CSF-1 levels (frozen and thawed once) of healthy volunteers and patients with LN in remission at multiple timed intervals (Supplemental Figure 1C). Finally, quantifying CSF-1 levels is both rapid and inexpensive.
These findings indicate that CSF-1 was elevated in patients with LN, although absolute CSF-1 levels differed in the two cohorts. Of note, there was wide diversity within and between the two cohorts. The patients were included irrespective of disease activity (active, flare, remission), severity, duration, and treatment (medications and regimens) (Table 1). These cross-sectional studies showing that CSF-1 levels in the serum and urine are substantially higher in LN than in other manifestations of SLE in each cohort. These rather unfiltered data provided the stimulus to test our hypothesis that CSF-1 levels provide a predictive biomarker of LN.
Serum and Urine CSF-1 Levels Reflect Intrarenal CSF-1 and Extent of Mø-Rich Inflammation in LN
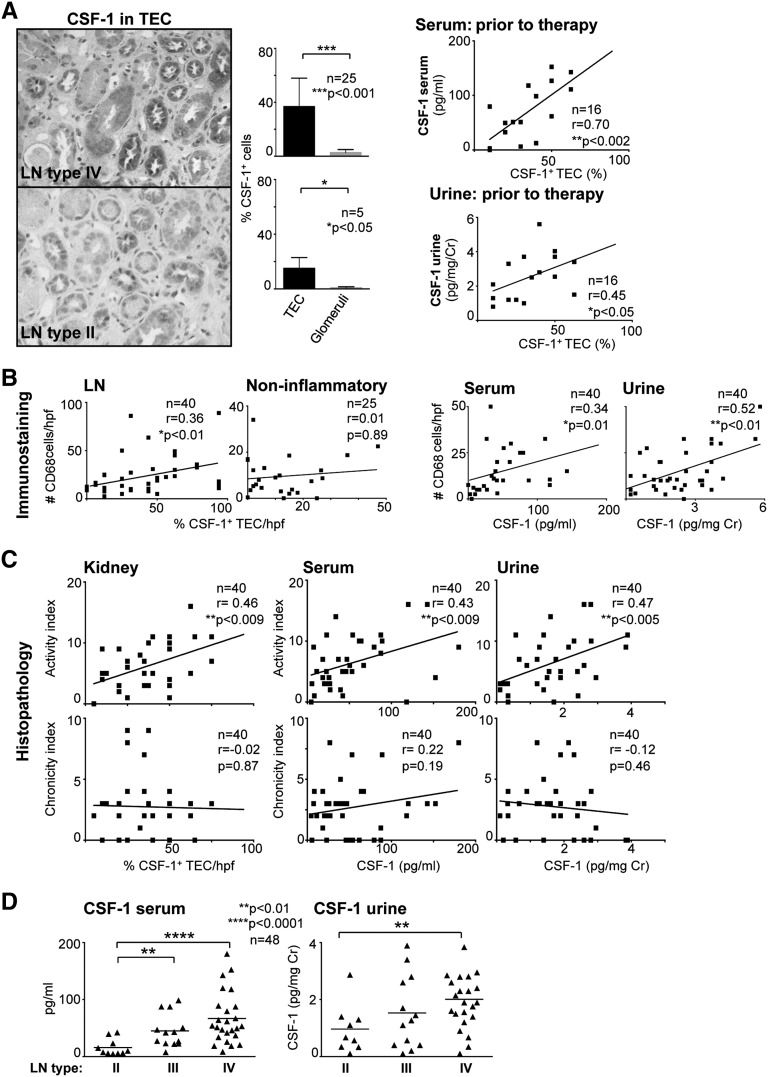
On the basis of the data in Figure 1, we hypothesized that increased intrarenal CSF-1 spills into the serum or urine in patients with LN and thereby reflects the extent of Mø-rich intrarenal inflammation. Using immunostaining, we detected intrarenal CSF-1 expression in patients with LN, primarily within tubules (Figure 2A), which was more extensive in patients with class IV than class II disease. Because therapeutic intervention may skew our data, we restricted our initial CSF-1 analysis to patients with LN before initiation of therapy. We found that the magnitude of intrarenal CSF-1 expression correlated with serum and urine CSF-1 in LN before therapy (Figure 2A). Because CSF-1 promotes Mø-mediated inflammation,7,13 we hypothesized that increased CSF-1 in TEC reflects a rise in intrarenal Mø. We detected an increase in Mø (CD68+) in LN compared with noninflammatory kidney diseases (minimal-change and thin basement membrane disease; data not shown). Moreover, the abundance of intrarenal Mø infiltration was correlated with CSF-1 expression by TEC (Figure 2B) and serum or urine CSF-1 levels (Figure 2B). Thus, elevated serum and urine CSF-1 reflect an increase in intrarenal CSF-1 expression and heightened Mø-mediated renal inflammation.
Figure 2.
Serum and urine CSF-1 levels reflect intrarenal CSF-1 expression and histopathology disease activity. (A) Left panel: Representative photomicrograph (original magnification, ×20) illustrating CSF-1 expression in renal TEC in type IV and type II LN. Graphs of CSF-1 levels in TEC and glomeruli in type IV and type II LN. Right panel: Correlation of serum or urine CSF-1 levels with CSF-1 expression in TEC before therapy. (B) Correlation of intrarenal Mø (CD68+) with CSF-1 in LN and noninflammatory kidney diseases with serum or urine CSF-1 in LN. (C) Correlation of CSF-1 in kidney, serum, and urine with histopathology activity and chronicity indices in LN. (D) Serum and urine CSF-1 levels stratified according to ISN/RPS classification of LN. CSF-1 and CD68 were detected on renal biopsy specimens by immunostaining and serum or urine CSF-1 quantified using ELISA. Values are means±SEM. Analysis was done using Spearman correlation calculation.
CSF-1 in Kidney, Serum, and Urine Levels Reflect Histopathology Activity and Severity, Not Chronicity, of LN
Because CSF-1 levels in the serum and urine reflect intrarenal CSF-1 expression, we hypothesized that the level of CSF-1 in the kidney, serum, and urine reflect renal disease activity in patients with LN, as reflected by histopathology. In fact, the magnitude of CSF-1 expression in the kidney, serum, and urine positively correlated with renal histopathology activity (glomerular proliferation, leukocyte exudation, karyorrhexis/fibrinoid necrosis, cellular crescents, hyaline deposits, and interstitial inflammation) (Figure 2C). This finding is consistent with the hypothesis that amplified CSF-1 expression drives the initiation of LN. In contrast, the magnitude of CSF-1 expression in the kidney, serum, and urine did not correlate with histopathology indices of chronicity (glomerular sclerosis, fibrous crescents, tubular atrophy, and interstitial fibrosis). We suggest that this lack of correlation results from a reduction of CSF-1 owing to destruction of the major source of CSF-1, tubules, during ESRD.8
Because LN is diverse, we determined whether the magnitude of CSF-1 in serum or urine was stratified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2004 classification.16 Serum or urine CSF-1 levels were highest in type IV (diffuse GN), intermediate in type III (focal segmental GN), and lowest in type II (only mesangial alterations) (Figure 2D). As the severity of inflammation increases from type II to Type IV, our findings are consistent with the enhanced expression of CSF-1 in tubules of patients with type IV compared with type II (Figure 2A), as well as the hypothesis that serum and urine CSF-1 levels reflect the magnitude of renal inflammation.
Increased Serum or Urine CSF-1 Longitudinally Track with Elevated Clinical Disease Activity in LN
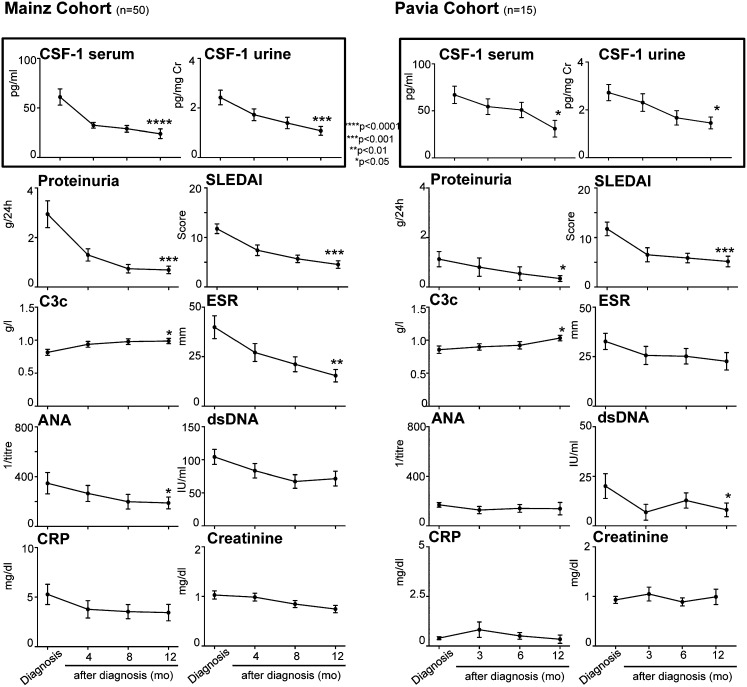
Our cross-sectional findings indicated that serum and urine CSF-1 were elevated in patients with LN and reflect the level of intrarenal infiltration by Møs. These findings prompted us to determine whether CSF-1 is a reliable biomarker reflecting disease activity in LN. Therefore, we longitudinally monitored CSF-1 in the serum and urine of patients with biopsy-proven LN to determine whether a change in CSF-1 levels tracks with clinical disease activity in the two cohorts (Mainz and Pavia) (demographic characteristics and patient profiles are shown in Table 1). In these studies, each patient served as his or her own control. Measurements were taken 4, 8, and 12 months after diagnosis of LN in the Mainz cohort and 3, 6, and 12 months after in the Pavia cohort. Serum and urine CSF-1 levels decreased at 4, 8, and 12 months and at 3, 6, and 12 months after diagnosis in the Mainz and Pavia cohorts, respectively. This finding correlated with improved indices of disease activity (rise in C3c and decrease in antinuclear antibody [ANA], anti–double-stranded DNA [dsDNA] antibodies, and erythrocyte sedimentation rate [ESR] in serum, proteinuria, active sediment, and Systemic Lupus Erythematosus Disease Activity Index [SLEDAI]) (Figure 3). Moreover, we calculated the decline of serum CSF-1 levels as a predictor of the response to therapy and remission (Figure 3). This is of particular interest in daily clinical practice to minimize toxicity, especially in young patients. The decline in serum CSF-1 (≥25%) after initiation of therapy had a positive predictive value (PPV) of 88% and a negative predictive value (NPV) of 58%. The considerably higher PPV compared with NPV is understandable because more patients were in remission than patients who did not reach remission (40/10). Thus, serum or urinary CSF-1 levels tracked with LN clinical disease activity and may potentially predict the response to therapy.
Figure 3.
Longitudinally monitoring CSF-1 in serum positively correlates with disease activity in two cohorts. Serum or urine CSF-1 at diagnosis of biopsy-proven LN and during therapy in comparison to conventional clinical disease activity measures. CSF-1 was quantified using ELISA. Values are means±SEM. Analysis was done using the Kruskal–Wallis test for multiple comparisons. CRP, C-reactive protein.
Serum or Urine CSF-1 Decreases from Diagnosis to Remission and Increases in Advance of Glomerular Dysfunction and Standard Measures during LN Flares
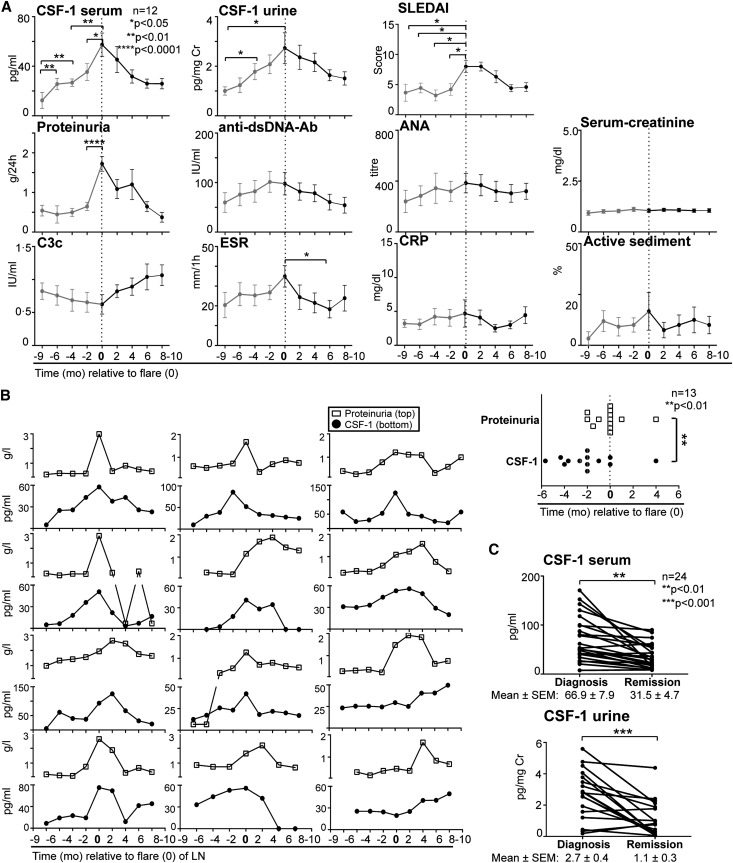
Early diagnosis and therapy are essential to successful treatment of LN flares. We monitored serum and urine CSF-1 levels before, during, and after flares in LN alone without flares in other manifestations of SLE (Figure 4; patient demographic characteristics and clinical profiles are shown in Supplemental Table 2). Serum and urine CSF-1 levels increased well in advance of LN flares (renal flare defined as an increase of urinary protein levels of ≥500 mg/24 hours, in accordance with the American College of Rheumatology criteria and SLEDAI) compared with other conventional clinical measures (Figure 4A, composite). Of the clinical variables, proteinuria was the best indicator of an LN flare in this cohort. To determine whether a rise in CSF-1 is a better predictor of an LN flare than proteinuria in an individual patient, we compared elevated CSF-1 (defined as 50% higher than normal) versus proteinuria at each point before the patient’s renal biopsy. An elevation of serum CSF-1 heralded a LN flare in individual patients earlier (mean, 54 days) than did a rise in proteinuria (Figure 4B, individual). Consistent with these data, serum and urine CSF-1 declined with LN remissions (Figure 4C, individual) and remained stable in patients not in remission (data not shown). Thus, an increase (≥25%) in the CSF-1 level in serum or urine predicted an LN flare before overt glomerular dysfunction and standard clinical measures, at least in a single cohort. Because this analysis was composed of a limited sampling (size, race, ethnicity, and sex), a more extensive prospective multicenter clinical trial is required to validate these findings.
Figure 4.
Serum and urine CSF-1 levels increase before proteinuria and flares in LN and declined during remission. (A) Composite patient values. Serum and urine CSF-1 levels in LN compared with glomerular dysfunction and serologic measures before (red), during, and after LN flares. Flare is indicated as 0 time point. CSF-1 was quantified using ELISA. (B) Individual patient values. Left panel: Individual serum CSF-1 levels and proteinuria in patients with LN monitored before flares, at diagnosis, and after flares. Right panel: Comparison of longitudinally tracked elevated CSF-1 (50% greater than normal levels) versus proteinuria (>500 mg/24 hours) for each individual patient at time points before the patient’s flare. Prior to the flare, the rise in CSF-1 levels (58±20 days) preceded an increase in proteinuria (4±13 days). (C) Serum and urine CSF-1 levels in LN at diagnosis (biopsy proven) compared with remission. Values are means±SEM.
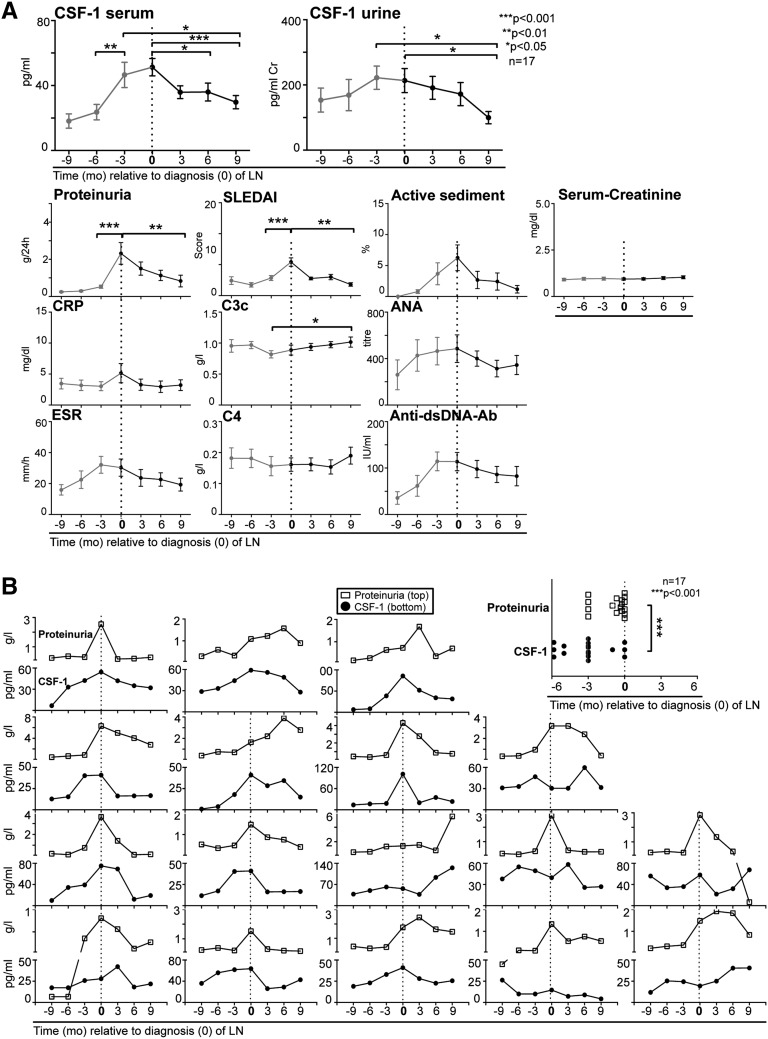
Increased Serum CSF-1 in SLE Heralds Onset of LN in Advance of Glomerular Dysfunction and Standard Clinical Measures
Identification of a biomarker in the serum or urine that predicts initiation of kidney injury would provide an opportunity to obviate irreversible intrarenal disease. Do elevated serum or urine CSF-1 levels indicate imminent LN? To further address this issue, we quantified serum and urine CSF-1 levels in longitudinally tracked patients (patient demographic and clinical characteristics are shown in Supplemental Table 3) with SLE before, at, and after biopsy-proven diagnosis of LN. Serum CSF-1 increased several months in advance of proteinuria and other measures (active sediment and serum creatinine and alterations in serologic variables, including C-reactive protein, C3c, ANA, ESR, C4, anti-dsDNA antibodies, and SLEDAI) in LN (Figure 5A, composite). To determine whether a rise in CSF-1 is an earlier predictor than proteinuria for the onset of LN in each individual patient, we compared the rise in CSF-1 (defined as 50% higher than normal) with proteinuria at each point before the patient’s renal biopsy. An elevation of serum CSF-1 heralded LN flares in individual patients earlier (mean, 66 days) than proteinuria (Figure 5B, individual). Moreover, serum and urine CSF-1 declined after diagnosis upon treatment, and this decline preceded a reduction of proteinuria and serologic measures (C3c, ESR, anti-dsDNA antibodies) (Figure 5, composite). The positive and negative predictors indicating that a rise in serum CSF-1 predicts new-onset LN (Figure 5) and an LN flare (Figure 4) were 83% (PPV) and 63% (NPV). Because these predictive values are based on a limited sampling of largely white female patients, large-scale prospective trials are required to adequately test our hypothesis. Taken together, these findings indicate that a rise in serum CSF-1 is a potential biomarker for predicting the initial onset of LN in advance of glomerular dysfunction and standard clinical measures.
Figure 5.
Elevated serum and urine CSF-1 are a prognostic indicator of LN. (A) Composite patient values. Longitudinal tracked serum and urine CSF-1 levels in patients with SLE before (red) and at diagnosis of LN (time of renal biopsy, indicated as 0 time point) and at follow-up visits compared with conventional clinical indices of LN. Most patients had active LN without other lupus manifestation symptoms. Arthralgia, malar rash, and mild cutaneous manifestations were seen in <25% of the evaluated patients with LN. CSF-1 was quantified using ELISA. (B) Individual patient values. Left panel: Display of CSF-1 and proteinuria in each individual longitudinally tracked patient before, at, and after renal biopsy. Right panel: Comparison of elevated CSF-1 (defined as 50% higher than baseline) versus proteinuria (defined as urinary protein levels of ≥500 mg/24 hours) at each point before the patient’s renal biopsy. Before new-onset LN, the rise in CSF-1 levels (93±15 days) preceded an increase in proteinuria (27±9 days). Values are means±SEM. CRP, C-reactive protein.
Discussion
On the basis of data from lupus-prone mice, we hypothesized that amplified CSF-1 expression accurately predicted LN and correlated closely with disease activity. In a cross-sectional study using two cohorts, we show that serum and urine CSF-1 levels were increased in patients with SLE and CLE, serositis, and musculoskeletal manifestations. Within each cohort, CSF-1 levels were substantially greater in patients with LN. Using longitudinally tracked patients with SLE, we found that a rise in serum CSF-1 heralded the onset of LN and that a rise in serum or urine CSF-1 predicted the recurrence of LN before glomerular dysfunction and conventional serologic measures. This pattern is evident even in patients with other manifestations of SLE. Moreover, serum and urine CSF-1 levels increase with progressing clinical disease activity and correlate with established renal histopathology variables. Taken together, these findings suggest that serial monitoring serum or urine CSF-1 levels in SLE patients is a potential biomarker for predicting the onset, recurrence, and disease activity of LN that is more accurate than conventional laboratory measures.
Prior studies identifying biomarkers for LN analyzed an individual molecule or combinations of molecules (as reviewed in references 17–19 among cross-sectional studies), mainly in the urine.20,21 In a cross-sectional single-cohort study using a modest sampling of patients with LN, we previously reported that serum or urine CSF-1 was elevated in patients with LN compared with those who had other noninflammatory kidney diseases and healthy controls; in that study we also found that amplified CSF-1 levels correlated with more severe clinical disease activity.8 Another study indicated that flares were more likely in patients with persistently increased regulated upon activation normal T cell expressed and presumably secreted or M-CSF (also known as CSF-1) levels in urine after induction therapy using group survival curve analysis.22,23 By comparison, our current longitudinally analysis indicates that a rise in serial monitored serum CSF-1 levels in individual patients without documented LN heralds the onset of LN, while both serum and urine predict recurrences of biopsy-proven LN more accurately than do standard measures. Is CSF-1 a more accurate and sensitive predictive biomarker for LN than other longitudinally monitored molecules? Urinary, but not serum, neutrophil gelatinase-associated lipocalin predicted renal flares in patients with LN and is reportedly a more sensitive forecaster of flares in patients with a history of LN than are dsDNA antibody titers, but its predictive value was not superior to that of C3c and C4 in patients with LN.21,24 By comparison, our longitudinal analysis of individual patients suggests that elevated serum and urine CSF-1 levels may predict LN recurrences, and elevated serum CSF-1 may herald the onset of LN in advance of glomerular dysfunction and multiple clinical LN measures, including dsDNA. Thus, our current study indicates that monitoring serum or urine CSF-1 offers a potential individualized approach to the management of patients with LN.
Although the current classification (ISN/RPS) of LN is largely based on glomerular,16 rather than tubulointerstitial, pathology, the magnitude of tubulointerstitial damage determines the fate of the kidney in LN and other renal diseases.25,26 In fact, extensive Mø-rich interstitial infiltrates,27,28 inflammation, tubular atrophy, and interstitial fibrosis are accurate morphologic predictors of a poor renal prognosis.25,29,30 Thus, tracking molecules that mediate tubulointerstitial inflammation and injury are candidates for predicting renal disease outcomes in patients with SLE. We now show that increased serum or urine CSF-1 levels reflect the magnitude of CSF-1 expression in the tubules and Mø-rich inflammation in the interstitium and predict the onset, recurrence, and disease activity of LN. This finding agrees with our prior experimental data indicating that CSF-1 expression is upregulated in TEC during early and sustained renal inflammation, fosters the expansion of activated Mø in the renal interstitium, and, in turn, induces TEC apoptosis.8 Our current findings suggest that Mø-mediated interstitial inflammation precedes and perhaps triggers glomerular disease in LN. This view is consistent with our experimental data in which transient kidney injury in lupus-prone hosts induces CSF-1; this, in turn, leads to Mø-mediated tubulointerstitial, followed by a rise in autoantibodies and immune complex–mediated GN.31 Because we now show that a rise in serum and urine CSF-1 heralds overt renal tubulointerstitial injury, tracking serum or urine CSF-1 potentially identifies a time-related opportunity to treat SLE before the onset of irreversible kidney damage.
The CSF-1–dependent Mø phenotype linked to lupus, rather than CSF-1 alone, is central to triggering LN. Our prior studies indicate that whereas increased CSF-1 drives Mø-mediated renal destructive inflammation in lupus-prone mice, CSF-1 fosters Mø-mediated renal repair in normal hosts.31 This apparent dichotomy results from the time-related balance of M1 Mø destroyers, shifting to M2 Mø, healers in normal kidneys, as opposed to the continuing dominance of M1 Mø in lupus-prone hosts. Thus, we suggest that CSF-1–driven aberrant Mø linked to lupus is the likely culprit that triggers LN in patients.
Although a molecule that is an accurate biomarker does not necessarily have to participate in the pathogenesis of disease, CSF-1 is central to initiating the pathogenesis of LN in mice. Because the ultimate goal of a therapeutic is to spare tissue destruction, the most promising therapeutic targets are initiators of tissue injury. As circulating CSF-1, largely generated by the kidney at the onset of inflammation, initiates a cascade of events culminating in kidney damage,8 neutralizing of CSF-1 and blocking the CSF-1 receptor or signaling cascade may preserve renal structure and function. Thus, the CSF-1 pathway is a potential therapeutic target for LN. Similarly, the most valuable biomarkers are those that most accurately identify the inception or reactivation of a disease process before the advent of clinically detectable disease and immutable tissue injury. Even if human translational studies show that neutralizing the CSF-1 pathway is not as powerful in obviating LN in patients as our experimental studies suggest, this does not diminish the importance of CSF-1 as a potential accurate and sensitive predictive biomarker for LN.
In conclusion, fueled by our longitudinal retrospective data largely generated in white women, future trials are clearly needed for adequately powered and thorough tests of our hypothesis. Thus, we intend to plan large-scale multicenter longitudinal, prospective trials to assess the value of serial CSF-1 levels in the serum and urine as a tool for individualized care of patients with SLE. It is essential that these trials include patients in other racial/ethnic groups, in particular those with a higher prevalence of SLE (African Americans, Asians, and Hispanics); include both sexes; and examine the effect in stratified groups of patients with SLE (e.g., based on LN subclassification, type of treatment, and age). Taken together, our findings suggest that quantifying CSF-1 in the serum or urine is a potentially reliable and inexpensive biomarker for managing the individualized treatment of patients with SLE.
Concise Methods
Serum, Urine, and Renal Biopsy Specimens
To diagnose LN (comporting with the ISN/RP 2004 classification)16 and assess histopathology activity and chronicity indices,32 human kidney sections from renal biopsy specimens were provided by the Department of Pathology, Johannes-Gutenberg University, Mainz and Friedrich-Alexander University Erlangen-Nuernberg, Germany. Renal pathologists, without access to the patient’s clinical data, evaluated these biopsy specimens. We analyzed urine and serum samples that were collected from two cohorts: Mainz, Germany, and Pavia, Italy. Unless otherwise stated, the data reported are from Mainz. Specimens were taken from patients who fulfilled at least four of the American College of Rheumatology criteria for the classification of SLE, or noninflammatory kidney disease (minimal-change disease, amyloidosis, nephrosclerosis) after informed consent. Volunteers (age range, 18–70 years) were screened for health by exclusion of any prior kidney diseases, diabetes, hypertension, and autoimmune diseases. Serum and urine was collected at a single visit. Freshly voided urine and drawn blood samples were collected, centrifuged, divided into aliquots, and stored at −30°C before analysis. The use of these specimens was reviewed and approved by the Standing Committee for Clinical Studies of the Johannes-Gutenberg University and the University and IRCCS Policlinico S. Matteo Foundation, Pavia, Italy, in adherence to the Declaration of Helsinki. All samples were analyzed retrospectively in Mainz.
Disease Activity
Disease activity was evaluated by standard clinical serologic activity measures (C3c, C4, ANA, anti-dsDNA antibodies, creatinine, C-reactive protein, ESR [after 1 hour/2 hours]) and urine measures (proteinuria [24-hour collection] and active sediment). Activity indices were assessed in the same serum and urine samples as the CSF-1 measurements at each time point. Clinical remission for patients with LN was defined as normal GFR and proteinuria <500 mg/24 hours according to the 2012 consensus recommendations from the European League Against Rheumatism/European Renal Association-European Dialysis and Transplant Association.5 The criteria for renal flares was increasing proteinuria or serum creatinine, declining C3/C4 levels, or rising anti-dsDNA titers. The criteria for new-onset LN included newly developed proteinuria or increasing serum creatinine, declining C3/C4 levels or rising anti-dsDNA titers in patients with established SLE.5 The following standard values of serologic activity markers were determined: C3 (0.9–1.8 g/L) and C4 (0.1–0.4 g/L) by enzyme immunoassay, ANA (1:80–1:5120) by immunofluorescence, dsDNA (30–200 IU/ml) by ELISA, C-reactive protein (<5 mg/dl) by nephelometry or turbidimetry in an automated analyzer, creatinine (0.5–0.8 mg/dl) by isotope dilution mass spectrometry, proteinuria (<150 mg/24 hours) by immunoturbidimetric assay, and active sediment(<5%) by microscopy.
CSF-1 ELISA
We measured human CSF-1 levels in serum and urine using a human CSF-1 ELISA kit according to the manufacturer’s instructions. Samples were thawed and spun down and the supernatant fraction used for the ELISA. Undiluted serum and urine samples were analyzed. All measurements were made in duplicate. The laboratory personnel were blinded to clinical data. The ELISA antibodies and reagents were purchased from R&D Systems (McKinley Place, MN). The minimum detectable level of CSF-1 is <9 pg/ml.
Immunostaining
We evaluated serial sections (4 μm) of human kidney biopsy specimens. Antigens were retrieved by immersion in citrate buffer followed by blocking of endogenous peroxidase activity and nonspecific binding of avidin and biotin as previously described.8 We incubated kidney sections with a primary antibody, goat anti-human CSF-1 antibody (N-16; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human CD3 antibody (LabVision, Kalamazoo, MI), and mouse anti-human CD68 (C-20; Santa Cruz Biotechnology) and detected the primary antibody by incubation with biotinylated rabbit anti-goat antibody, goat anti-rabbit antibody, and goat anti-mouse antibody, respectively, followed by development with 3-3-diaminobenzidine (Vector Laboratories; Burlingame, CA). We verified staining specificity by replacing the primary antibody with goat IgG (for anti-CSF-1 antibody), rabbit IgG (for anti-CD3 antibody, anti–caspase-3 antibody), and mouse IgG (for anti-CD68 antibody) (eBioscience, San Diego, CA). CSF-1 specificity was verified by preabsorbing the anti–CSF-1 antibody with peptide (Santa Cruz Biotechnology). We determined the percentage positive TECs or positive infiltrating cells in 10 randomly selected high-power fields.
Statistical Analyses
Statistical analyses were based on the 161 patients and 162 controls from Mainz and 102 patients and 50 controls from Pavia. The data representing the mean±SEM were prepared using GraphPad PRISM, version 6.0. We used the nonparametric Mann–Whitney U test for comparison between two groups and the Kruskal–Wallis test for comparisons between three or more groups. For correlation analysis, we used the Spearman correlation coefficient. No correction for multiple testing was done, and we report P values for all tests. Because this is an explorative study, we have chosen not to use the term “statistically significant” but rather to discuss differences that cannot be explained by random variation alone. The area under the curve calculation of nonparametric receiver-operating characteristics was used for CSF-1 sensitivity and specificity. We calculated the intra-assay variability and interassay variability as defined elsewhere.15 We calculated the PPV and NPV using the serum CSF-1 levels that were tracked in individual patients in Figures 4 and 5. For each patient, the laboratory results nearest to the predefined time points 60 and 120 days before flare were used for this calculation. The difference in percentages between these values was then calculated and compared with the defined cutoff value (an increase of 25%). No other values between the defined time points were used for this calculation.
Disclosures
V.R.K. has an equity interest in Biogen Idec, a company with research and development interests in lupus. V.R.K.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Supplementary Material
Acknowledgments
We thank Professor Juergen Kriegsmann for providing kidney biopsy tissues and Bettina Maar and Sabine Schmitt for technical support. This work was supported by the Alliance for Lupus Research (V.R.K.) and the MAIFOR research program of the Johannes-Gutenberg University Mainz (J.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121356/-/DCSupplemental.
References
- 1.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO, Chwalinska-Sadowska H, de Ramón E, Fernández-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR, European Working Party on Systemic Lupus Erythematosus : Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82: 299–308, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Fiehn C, Hajjar Y, Mueller K, Waldherr R, Ho AD, Andrassy K: Improved clinical outcome of lupus nephritis during the past decade: Importance of early diagnosis and treatment. Ann Rheum Dis 62: 435–439, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok CC, Ying KY, Tang S, Leung CY, Lee KW, Ng WL, Wong RW, Lau CS: Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum 50: 2559–2568, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Sprangers B, Monahan M, Appel GB: Diagnosis and treatment of lupus nephritis flares—an update. Nat Rev Nephrol 8: 709–717, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dörner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CG, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT, European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association : Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71: 1771–1782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pixley FJ, Stanley ER: CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 14: 628–638, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR: CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR: Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol 20: 2581–2592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yui MA, Brissette WH, Brennan DC, Wuthrich RP, Rubin-Kelley VE: Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol 139: 255–261, 1991 [PMC free article] [PubMed] [Google Scholar]
- 10.Lenda DM, Stanley ER, Kelley VR: Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol 173: 4744–4754, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Moore KJ, Naito T, Martin C, Kelley VR: Enhanced response of macrophages to CSF-1 in autoimmune mice: A gene transfer strategy. J Immunol 157: 433–440, 1996 [PubMed] [Google Scholar]
- 12.Menke J, Iwata Y, Rabacal WA, Basu R, Stanley ER, Kelley VR: Distinct roles of CSF-1 isoforms in lupus nephritis. J Am Soc Nephrol 22: 1821–1833, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenda DM, Kikawada E, Stanley ER, Kelley VR: Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol 170: 3254–3262, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR: Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest 103: 73–80, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramljak S, Musholt PB, Schipper C, Flacke F, Sieber J, Borchert M, Forst T, Pfutzner A: The precision study: Examining the inter- and intra-assay variability of replicate measurements of BGStar, iBGStar and 12 other blood glucose monitors. Exp Opin Med Diagnostics 7: 511–516, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Brunner HI, Bennett MR, Mina R, Suzuki M, Petri M, Kiani AN, Pendl J, Witte D, Ying J, Rovin BH, Devarajan P: Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum 64: 2687–2697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Thomas J, Blanco I, Putterman C: Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol 40: 138–150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mok CC: Biomarkers for lupus nephritis: A critical appraisal. J Biomed Biotechnol 2010: 638413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, Hojaili B, Mackay M, Aranow C, Stohl W, Rovin BH, Michaelson JS, Putterman C: Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res Ther 11: R143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein T, Pitashny M, Levine B, Schwartz N, Schwartzman J, Weinstein E, Pego-Reigosa JM, Lu TY, Isenberg D, Rahman A, Putterman C: Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford) 49: 960–971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S, Li J, Wang L, Liu T, Liu H, Cheng G, Liu D, Deng Y, Gou R, Wan Y, Jia J, Chen C: Urinary levels of RANTES and M-CSF are predictors of lupus nephritis flare. Inflam Res 56: 304–310, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Yang PT, Xiao WG, Zhao LJ, Lu J, He LM, Kasai H, Ito M: Increase in the level of macrophage colony-stimulating factor in patients with systemic lupus erythematosus. Ann Rheum Dis 67: 429–430, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Hinze CH, Suzuki M, Klein-Gitelman M, Passo MH, Olson J, Singer NG, Haines KA, Onel K, O’Neil K, Silverman ED, Tucker L, Ying J, Devarajan P, Brunner HI: Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum 60: 2772–2781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Wu LH, Tan Y, Li LH, Wang CL, Wang WK, Qu Z, Chen MH, Gao JJ, Li ZY, Zheng X, Ao J, Zhu SN, Wang SX, Zhao MH, Zou WZ, Liu G: Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int 77: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hunter MG, Hurwitz S, Bellamy CO, Duffield JS: Quantitative morphometry of lupus nephritis: The significance of collagen, tubular space, and inflammatory infiltrate. Kidney Int 67: 94–102, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, Bariéty J: Predictive power of the second renal biopsy in lupus nephritis: Significance of macrophages. Kidney Int 59: 304–316, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY: Local macrophage proliferation in human glomerulonephritis. Kidney Int 54: 143–151, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Hill GS, Delahousse M, Nochy D, Mandet C, Bariéty J: Proteinuria and tubulointerstitial lesions in lupus nephritis. Kidney Int 60: 1893–1903, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Nagaraja HN, Nadasdy T, Song H, McKinley A, Prosek J, Kamadana S, Rovin BH: A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int 81: 401–406, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata Y, Boström EA, Menke J, Rabacal WA, Morel L, Wada T, Kelley VR: Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J Immunol 188: 4568–4580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin HA, 3rd, Boumpas DT, Vaughan EM, Balow JE: Predicting renal outcomes in severe lupus nephritis: Contributions of clinical and histologic data. Kidney Int 45: 544–550, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.