Abstract
Whether inclusion of the coronary artery calcium score improves cardiovascular risk prediction in individuals with CKD, a population with unique calcium-phosphate homeostasis, is unknown. Among 6553 participants ages 45–84 years without prior cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis, coronary artery calcium score was assessed for cardiovascular risk prediction beyond the Framingham predictors in those with (n=1284) and without CKD and contrasted with carotid intima-media thickness and ankle-brachial index (two other measures of subclinical atherosclerosis). During a median follow-up of 8.4 years, 650 cardiovascular events (coronary heart disease, stroke, heart failure, and peripheral artery disease) occurred (236 events in subjects with CKD). In Cox proportional hazards models adjusted for Framingham predictors, each subclinical measure was independently associated with cardiovascular outcomes, with larger adjusted hazard ratios (HRs; per 1 SD) for coronary artery calcium score than carotid intima-media thickness or ankle-brachial index in subjects without and with CKD (HR, 1.69; 95% confidence interval [95% CI], 1.45 to 1.97 versus HR, 1.12; 95% CI, 1.00 to 1.25 and HR, 1.20; 95% CI, 1.08 to 1.32, respectively). Compared with inclusion of carotid intima-media thickness or ankle-brachial index, inclusion of the coronary artery calcium score led to greater increases in C statistic for predicting cardiovascular disease and net reclassification improvement. Coronary artery calcium score performed best for the prediction of coronary heart disease and heart failure, regardless of CKD status. In conclusion, each measure improved cardiovascular risk prediction in subjects with CKD, with the greatest improvement observed with coronary artery calcium score.
Keywords: cardiovascular disease, coronary calcification, arteriosclerosis, CKD
CKD, defined as reduced kidney function or damage, is an important clinical condition that has a prevalence of approximately 10%–15% in many places of the world and increases the risk of adverse outcomes, including cardiovascular disease (CVD).1 Indeed, approximately one half of individuals with CKD dies because of CVD,2 and thus, the prevention of CVD is crucial in this population. Although appropriate risk classification creates the basis for effective and efficient prevention strategy,3 it is controversial whether conventional risk factors perform well for CVD prediction in persons with CKD.4,5
In this context, coronary artery calcium (CAC) is a promising subclinical measure for CVD prediction, because it has outperformed other subclinical measures for predicting the composite of coronary heart disease (CHD) and stroke in the general population.6,7 However, its performance has not been specifically evaluated in those with CKD, a population with unique calcium-phosphate homeostasis. Indeed, CAC may not correlate well with severity of CHD in this population.8 Also, previous studies did not include CVD subtypes, such as heart failure (HF) and peripheral artery disease (PAD), which are more prevalent and clinically important in persons with CKD compared with those without CKD.9,10
Therefore, the objective of this study was to assess CAC as well as carotid intima-media thickness (IMT) and ankle-brachial index (ABI) in terms of their associations with CVD—CHD, stroke, HF, and PAD—and contributions to prediction improvement among individuals with CKD in a large general population study. These three subclinical measures are recommended in some clinical guidelines for CVD prediction among those with intermediate risk on the basis of conventional predictors.11 A secondary aim was to assess whether resulting findings were specific to those with CKD compared with those without CKD.
Results
Of 6553 study participants, 19.6% (n=1284) had CKD by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation or albumin-to-creatinine ratio. Those with CKD generally had a poorer CVD risk profile (e.g., older age, higher prevalence of hypertension, and diabetes) compared with those without CKD (Table 1). Compared with those without CKD, individuals with CKD were unlikely to be current smokers. There were no substantial racial/ethnic differences between CKD and non-CKD subgroups. CAC and IMT tended to be higher in those with CKD compared with those without CKD. The mean of ABI was similar between CKD and non-CKD groups.
Table 1.
Baseline characteristics according to CKD status
| Characteristics | Overall | CKD | No CKD |
|---|---|---|---|
| N | 6553 | 1284 | 5269 |
| Age, yr | 62 (10) | 69 (9) | 60 (10) |
| Men, % | 48 | 46 | 48 |
| Race, % | |||
| White | 39 | 39 | 39 |
| Black | 27 | 28 | 27 |
| Hispanic | 22 | 21 | 22 |
| Asian | 12 | 12 | 12 |
| Current smoker, % | 13 | 10 | 14 |
| Hypertension, % | 45 | 69 | 39 |
| Antihypertensive, % | 37 | 59 | 31 |
| Systolic BP, mmHg | 127 (21) | 137 (23) | 124 (20) |
| Diastolic BP, mmHg | 72 (10) | 73 (11) | 72 (10) |
| Total cholesterol, mmol/L | 5.0 (0.9) | 5.0 (1.0) | 5.0 (0.9) |
| HDL cholesterol, mmol/L | 1.32 (0.38) | 1.29 (0.37) | 1.32 (0.38) |
| Diabetes, % | 12 | 23 | 10 |
| Fasting glucose, mmol/L | 5.4 (1.7) | 5.9 (2.2) | 5.3 (1.5) |
| eGFRcre, ml/min per 1.73 m2 | 78 (16) | 62 (18) | 82 (13) |
| ACR, mg/g | 5.3 (3.3–10.9) | 22.4 (5.1–70.9) | 4.8 (3.2–8.3) |
| CAC | 0 (0–85) | 31 (0–224) | 0 (0–58) |
| Z score maximum IMT | −0.01 (1.00) | 0.37 (1.11) | −0.10 (0.95) |
| ABI | 1.11 (0.12) | 1.08 (0.14) | 1.12 (0.11) |
CKD defined as eGFRcre<60 ml/min per 1.73 m2 or urinary albumin-to-creatinine ratio≥30 mg/g. Values are mean (SD), percent, or median (interquartile range). eGFRcre, eGFR by the CKD-EPI creatinine equation; ACR, albumin-to-creatinine ratio.
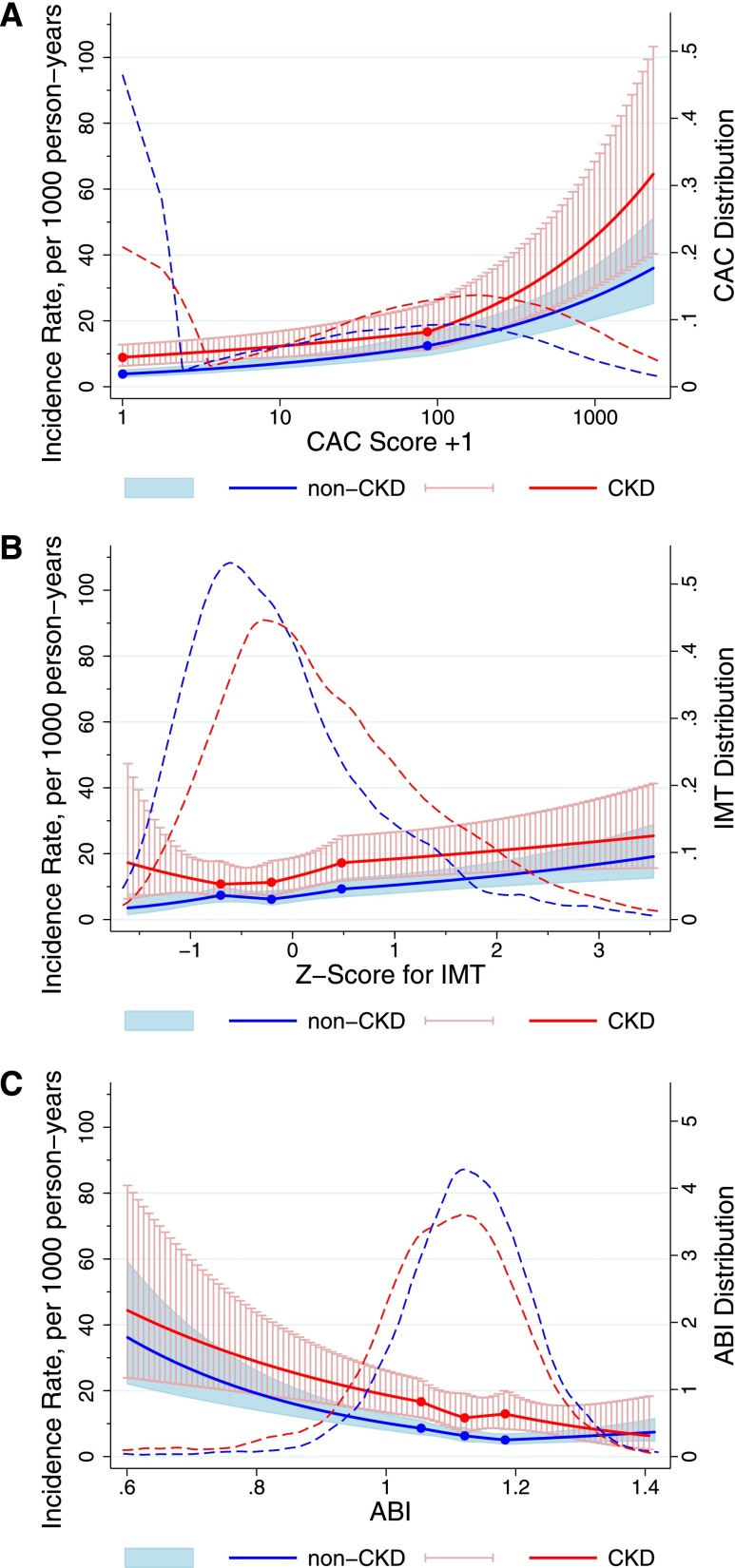
During a median follow-up of 8.4 years, there were 650 incident CVD events, with 236 of these events occurring in participants with CKD. The 650 CVD events included 387 CHD events, 138 strokes, 190 HF events, and 70 events of PAD; 110 participants had two or more constituent events, mostly CHD with HF. The overall incidence rate of CVD was 13.3/1000 person-years. Those with CKD had a higher demographically adjusted incidence rate of CVD than those without CKD across the entire range of values of the subclinical measures, except at high levels of ABI (approximately 1.4) (Figure 1). Although there was generally a graded association between each measure and the risk of CVD, the risk gradient was steeper for CAC and ABI than for IMT, regardless of CKD status. A J-shaped association (higher risk at normal range) was observed for IMT in CKD and ABI in non-CKD groups but not for CAC.
Figure 1.
Adjusted incidence rate of CVD in participants with and without CKD according to CAC, IMT, and ABI and their distributions. The solid lines (red, CKD; blue, non-CKD) show estimated incidence rates of CVD (per 1000 person-years) and 95% CIs (whiskers and shaded area) with spline (knots at thresholds defining quartiles) for (A) CAC, (B) IMT, and (C) ABI. The incidence rate was adjusted to mean age, men, and whites, and the plot was truncated at 0.5th and 99.5th percentiles of each marker. The dashed lines (red, CKD; blue, non-CKD) show the distribution of each subclinical measure on the basis of kernel density estimate.
When further adjusted for other conventional factors, regardless of CKD status, those in the top quartile (Q4) had a significantly higher risk of CVD compared with those in the bottom quartile (Q1 [+Q2 for CAC]) for CAC and ABI but not for IMT (Table 2). The corresponding hazard ratio (HR) was much greater for CAC than for ABI in both CKD and non-CKD groups (HR, 3.02; 95% confidence interval [95% CI], 2.03 to 4.50 versus HR, 1.63; 95% CI, 1.09 to 2.45 and HR, 4.11; 95% CI, 3.11 to 5.44 versus HR, 1.60; 95% CI, 1.21 to 2.13, respectively). The HRs were statistically significant even in Q3 for CAC in both groups. In contrast, no risk gradient was observed across Q1–Q3 for IMT and ABI.
Table 2.
Adjusted HR (95% CI) of CVD according to quartiles of CAC, IMT, and ABI
| Subclinical Measures | CKD | No CKD | ||||||
|---|---|---|---|---|---|---|---|---|
| Q2 | Q3 | Q4 | P for Trend | Q2 | Q3 | Q4 | P Value for Trend | |
| CAC | NAa | 1.73 (1.13 to 2.64) | 3.02 (2.03 to 4.50) | <0.001 | NAa | 1.80 (1.34 to 2.43) | 4.11 (3.11 to 5.44) | <0.001 |
| IMTb | 1.02 (0.59 to 1.75) | 1.12 (0.67 to 1.87) | 1.26 (0.76 to 2.07) | 0.21 | 0.97 (0.69 to 1.36) | 1.00 (0.71 to 1.39) | 1.37 (0.98 to 1.92) | <0.01 |
| ABI | 1.02 (0.65 to 1.60) | 1.01 (0.64 to 1.57) | 1.63 (1.09 to 2.45) | 0.004 | 0.95 (0.71 to 1.28) | 1.05 (0.78 to 1.41) | 1.60 (1.21 to 2.13) | 0.001 |
Adjusted for age, sex, race, systolic BP, antihypertensive medications, total cholesterol, HDL cholesterol, smoking, and diabetes (all continuous predictors were log-transformed). NA, not applicable.
Approximately one half of participants had CAC score=0 and composed the reference group as Q1+Q2.
Z score for overall maximal internal and common carotid IMT.
We observed similar results for a composite of CVD when every subclinical measure was modeled as a continuous variable, except that IMT reached significance in this model among those with and without CKD (Table 3). The HRs for every subclinical measure were smaller in those with CKD compared with those without CKD, and the interaction was significant for CAC (P=0.02) and IMT (P<0.001) in univariate models but not multivariable models (P>0.13). When CVD types were analyzed separately, the largest HRs were observed with CAC for CHD and HF in both CKD and non-CKD groups (Table 3). For stroke, none of the subclinical measures showed significant associations among those with CKD, whereas a significant association was only seen for CAC among those without CKD. Regarding PAD, both CAC and ABI showed significant associations regardless of CKD status, with larger adjusted HR for ABI in the CKD group but for CAC in the non-CKD group.
Table 3.
Adjusted HR (95% CI) of CVD for subclinical measures among participants with and without CKD
| Predictors | Subclinical Measure | ||
|---|---|---|---|
| CACa | IMTb | ABI | |
| CVD | |||
| CKD | 1.69 (1.45 to 1.97) | 1.12 (1.00 to 1.25) | 1.20 (1.08 to 1.32) |
| Non-CKD | 1.89 (1.69 to 2.12) | 1.19 (1.08 to 1.31) | 1.27 (1.16 to 1.40) |
| CHD | |||
| CKD | 2.36 (1.88 to 2.98) | 1.18 (1.02 to 1.37) | 1.14 (1.00 to 1.30) |
| Non-CKD | 2.19 (1.87 to 2.55) | 1.31 (1.16 to 1.47) | 1.32 (1.17 to 1.48) |
| Stroke | |||
| CKD | 0.99 (0.74 to 1.32) | 1.04 (0.81 to 1.35) | 1.08 (0.87 to 1.34) |
| Non-CKD | 1.38 (1.09 to 1.75) | 1.01 (0.81 to 1.26) | 1.08 (0.88 to 1.32) |
| HF | |||
| CKD | 1.55 (1.21 to 1.97) | 1.07 (0.88 to 1.30) | 1.01 (0.85 to 1.20) |
| Non-CKD | 1.47 (1.18 to 1.83) | 1.05 (0.86 to 1.27) | 0.99 (0.82 to 1.19) |
| PAD | |||
| CKD | 1.67 (1.11 to 2.53) | 1.22 (0.88 to 1.69) | 2.20 (1.76 to 2.75) |
| Non-CKD | 2.42 (1.62 to 3.61) | 1.44 (1.08 to 1.91) | 1.96 (1.57 to 2.44) |
HR per 1 SD higher (CAC and IMT) or lower (PAD) is shown.
ln(CAC score+1).
Z score for overall maximal internal and common carotid IMT.
CAC showed more evident improvement for CVD prediction in all statistics compared with IMT and ABI, regardless of CKD status (Table 4). In the CKD group, C statistics significantly improved when CAC was added. No significant increment in the C statistic was observed for IMT or ABI. In this population, significantly positive categorical net reclassification improvement (NRI) was only observed for CAC (0.14; 95% CI, 0.03 to 0.24), and it was mainly because of better reclassification among those who developed CVD outcomes during follow-up. Although IMT and ABI showed some improvement, which was determined by the continuous NRI and integrated discrimination improvement (IDI), the estimates were much smaller than those for CAC. The superiority of CAC to IMT and ABI was confirmed in the CKD group, regardless of stage (Supplemental Table 1) and age (Supplemental Table 2) or when restricted to eGFR<45 ml/min per 1.73 m2 and/or kidney damage (Supplemental Table 3). We also observed consistent results in those without CKD (Table 4).
Table 4.
Improvement in CVD prediction by adding each subclinical measure to conventional risk factors
| Predictors | ΔC Statisticsa | Categorical NRI | Continuous NRI | IDI | ||
|---|---|---|---|---|---|---|
| Event | Nonevent | Event | Nonevent | |||
| CKDb | ||||||
| CACc | 0.03 (0.01 to 0.06) | 0.11 (0.01 to 0.21) | 0.03 (−0.01 to 0.06) | 0.29 (0.15 to 0.43) | 0.15 (0.09 to 0.21) | 0.03 (0.02 to 0.04) |
| IMTd | 0.002 (−0.01 to 0.01) | 0.03 (−0.02 to 0.08) | −0.004 (−0.02 to 0.01) | 0.04 (−0.10 to 0.19) | 0.19 (0.14 to 0.25) | 0.003 (0.00 to 0.01) |
| ABI | 0.01 (−0.004 to 0.03) | −0.04 (−0.10 to 0.01) | 0.02 (−0.01 to 0.04) | −0.16 (−0.32 to 0.01) | 0.08 (0.02 to 0.14) | 0.01 (0.003 to 0.02) |
| Non-CKD | ||||||
| CACc | 0.04 (0.02 to 0.06) | 0.19 (0.12 to 0.26) | −0.03 (−0.04 to −0.03) | 0.29 (0.18 to 0.40) | 0.26 (0.23 to 0.28) | 0.03 (0.02 to 0.03) |
| IMTd | 0.01 (−0.001 to 0.01) | −0.02 (−0.06 to 0.02) | 0.002 (−0.004 to 0.01) | −0.03 (−0.15 to 0.08) | 0.14 (0.11 to 0.17) | 0.004 (0.002 to 0.01) |
| ABI | 0.01 (−0.003 to 0.02) | 0.02 (−0.03 to 0.07) | 0.004 (−0.002 to 0.01) | −0.001 (−0.11 to 0.13) | 0.08 (0.05 to 0.11) | 0.01 (0.01 to 0.02) |
HR (95% CI) is shown.
C statistic with only conventional predictors was 0.709 in the CKD group and 0.743 in the non-CKD group.
CKD was defined as eGFR by the CKD-EPI creatinine equation<60 ml/min per 1.73 m2 or urinary albumin-to-creatinine ratio≥30 mg/g.
ln(CAC score+1).
Z score for overall maximal internal and common carotid IMT.
When every CVD type was assessed separately, the best value for each prediction statistic for CHD and HF was consistently observed for a model with CAC, regardless of CKD status (Supplemental Tables 4 and 5). In contrast, little improvement was observed for stroke with any of three measures (Supplemental Table 6), and ABI showed the best improvement in PAD prediction overall (Supplemental Table 7). Finally, we observed similar findings when the CKD-EPI equation with both creatinine and cystatin C was used to define CKD (data not shown).
Discussion
This study compared the additional values of CAC, IMT, and ABI, the most studied subclinical measures,11 for CVD prediction among individuals with and without CKD. Despite the concern about the performance of CAC among those with CKD because of unique calcium-phosphate metabolism,8 the association with global CVD was strongest for CAC in this population, which translated to the most evident improvement in CVD prediction over IMT and ABI as shown in the general population.6,7 When each CVD subtype was analyzed separately, CAC was superior to the other two subtypes for CHD and HF prediction, regardless of CKD status. Little improvement was observed for stroke by any of these measures, whereas ABI best improved PAD prediction overall. Although CAC, IMT, and ABI are recommended for CVD prediction among those with intermediate risk in some clinical guidelines,11 our findings strongly support the use of CAC for better CVD prediction in those with CKD as well as those without CKD.
The evident superiority of CAC to IMT and ABI for the prediction of global CVD consisting of CHD, stroke, HF, and PAD in our study is consistent with previous reports dealing with composite of CHD and stroke.6,7 The outperformance of CAC over IMT and ABI for HF prediction was novel, although this finding may not be surprising with CHD as a leading cause of HF.12 Nevertheless, the burden of HF is expected to grow,13 and thus, our results have important clinical and public health implications. The superiority of CAC was robust across various prediction statistics that have not been previously evaluated in this context, such as continuous NRI and IDI. Furthermore, we used methods for these prediction statistics accounting for censoring.14,15
Despite its strong association with CVD and contribution to improved prediction, there are several caveats about CAC in CVD prediction. Exposure to ionizing radiation of approximately 1 millisievert is a concern.16 This issue is particularly relevant if follow-up evaluation is required to update CVD risk. Cost-effectiveness is another issue to be elucidated. It has been suggested that abnormal CAC score encourages individuals to modify their lifestyle or adhere to a pharmacologic prevention strategy.16 Nevertheless, randomized trials should be conducted to assess whether risk classification with CAC score followed by risk-specific lifestyle or pharmacologic intervention results in reduced CVD incidence. In this context, individuals with CKD may be a good target population for those trials, because the benefit of CAC evaluation may well outweigh its adverse effects given their high CVD risk. Incidental findings during computed tomography are also an important issue under debate.17 Additionally, computed tomography scan is not widely available in low-income countries.18
In this regard, IMT and ABI may have some advantages over CAC (e.g., no radiation, wider availability/accessibility, or lower cost). However, their ability for global CVD prediction was clearly inferior to CAC in both CKD and non-CKD groups. It was somewhat unexpected to see weak associations of IMT with stroke, particularly given an earlier Multi-Ethnic Study of Atherosclerosis (MESA) article reporting a stronger association with stroke for IMT compared with CAC during a median follow-up of 3.9 years.6 However, a recent MESA article with extended follow-up (median=5.8 years) showed no association between IMT and stroke risk.19 This finding may suggest that IMT is useful for short-term prediction of stroke. Nevertheless, our findings are, overall, in line with the fact that the latest guidelines from the American Heart Association and American College of Cardiology do not recommend IMT for predicting atherosclerotic CVD.20 Given that none of three subclinical measures improved stroke prediction in our study, additional investigations are needed for improving stroke prediction.
As anticipated, ABI was generally a stronger predictor of PAD than CAC and IMT. Thus, if the goal is to best predict PAD, ABI should be a first-line subclinical measure. It is yet to be determined whether we need a prediction tool for each CVD subtype or only global CVD.21–23 There are certain advantages for global CVD prediction in terms of comprehensiveness, simplicity, and probably cost. However, risk factors for each individual CVD subtype are not identical. For example, the contribution of diabetes and smoking is larger for PAD than CHD.13 Thus, the prevention strategy can be different across subtypes of CVD. Indeed, some potent drugs reducing CVD risk, such as statin, have shown varying effects on CVD subtypes.24 This aspect is particularly important from the international point of view, because the ratio of CHD and stroke incidence substantially varies across regions.25 Thus, the selection of subclinical measures should be tailored depending on the demographic and clinical circumstances. Nevertheless, CHD is a worldwide issue,26 and the burden of HF is growing worldwide.13 Therefore, CAC would likely lead to better CVD prediction in a broad range of regions and populations.
There are several limitations of our study. A relatively small number of participants had CKD. Even so, it is still one of the largest CKD datasets ever tested simultaneously for CAC, IMT, and ABI in terms of CVD prediction. Indeed, there were sufficient numbers of CVD events among those with CKD to show the significant improvement in risk prediction by CAC. Most participants with CKD were at mild or moderate stages, and thus, generalization of our data needs to be carefully done to apply to those with more advanced CKD (e.g., patients on dialysis). Nevertheless, in the United States, the vast majority of people with CKD is at the mild to moderate stages,27 and thus, our findings have public health implications. We had only a single-time point assessment of subclinical measures, which is susceptible to random errors and short-term variability and might result in some misclassification, expected to bias the results toward the null.
In conclusion, CAC, IMT, and ABI were independently associated with global CVD, including CHD, stroke, HF, and PAD, and improved cardiovascular prediction in those with CKD and without CKD. The improvement for global CVD prediction was most evident for CAC in both CKD and non-CKD groups on the basis of its strong associations with CHD and HF. Our results suggest that CAC is useful to better classify CVD risk in individuals with CKD as well as those without CKD.
Concise Methods
Study Participants
The MESA consists of 6814 participants without known history of CVD who were ages 45–84 years at the initial examination conducted during 2000–2002. Subjects were sampled from six communities in the United States: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern New York City, NY; and St. Paul, MN. MESA was designed to enroll four racial/ethnic groups: white, black, Hispanic, and Chinese.7 Baseline information on demographics, lifestyle, medical history, and medication use was obtained by trained interviewers. Blood samples were obtained at rest after a 12-hour fast.7
Of 6814 participants, 9 participants were discovered to have had prevalent CVD at baseline, and 32 participants did not have follow-up data. We also excluded participants who had missing values of kidney measures (n=33), conventional predictors (n=45), or one or more of three subclinical measures of interest (n=142), leaving a final study population of 6553 participants. Those with missing data were more likely to have a somewhat poorer CVD risk profile compared with those without missing data (mean age, 64 versus 62 years; current smokers, 16% versus 13%; diabetes, 17% versus 12%; hypertension, 52% versus 45%). The study was approved by the Institutional Review Boards of all constituent institutions, and all participants provided informed consent.
Assessment of Subclinical Measures at Baseline
The Agatston score was recorded as the average of two scans obtained by multidetector or electron beam computed tomography28,29 on the basis of the phantom-adjusted sum of calcium measures from the left and right coronary arteries.30 CAC measurement was centrally conducted at the MESA Computed Tomography Reading Center (Harbor-University of California Los Angeles Research and Education Institute, Los Angeles, CA).29 Both interobserver and intraobserver agreements were excellent (κ≥0.90).31
B-mode ultrasonography of the right and left near and far walls of the internal carotid and common carotid arteries was performed by trained technicians using the Logiq 700 Ultrasound Device (General Electric Medical Systems, Waukesha, WI).6 The MESA Ultrasound Reading Center (Department of Radiology, Tufts–New England Medical Center, Boston, MA) measured maximal IMT of the internal and common carotid sites as the mean of the maximum IMT of the near and far walls of the right and left sides. As previously conducted,6 we used a composite Z score for overall maximal IMT (the sum of the values at the two carotid IMT sites after standardization [subtraction of the mean and division by the SD of each measure] divided by the SD of the sum). If only one of two measures was available, a Z score for the single site was used. The rescan and the reread coefficients of variation were 7.1% and 3.5%, respectively.7
Regarding ABI, systolic BP was measured at the bilateral brachial, dorsalis pedis, and posterior tibial arteries in the supine position using a handheld Doppler instrument with a 5-MHz probe.32 To avoid a potential bias from subclavian stenosis, the higher value was used for the brachial artery pressure. For ankle artery pressure, the highest pressure from dorsalis pedis or posterior tibial was used for that leg. ABI was calculated as the ratio of brachial-to-ankle artery pressure for each leg, and the smaller value was recorded.33 The intrareader and inter-reader coefficients of variation were 5.1% and 3.3%, respectively.7
Definition of CKD
CKD was primarily defined as reduced eGFR (<60 ml/min per 1.73 m2) on the basis of the CKD-EPI creatinine equation34,35 or the presence of a spot urine albumin-to-creatinine ratio (≥30 mg/g).36 We repeated the analysis using eGFR on the basis of the CKD-EPI equation with both creatinine and cystatin C.37 Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method. Cystatin C was measured by a BNII Nephelometer on plasma specimens (N Latex Cystatin C; Dade Behring, Inc., Deerfield, IL). Urinary albumin and creatinine were measured using the Array 360 CE Protein Analyzer (Beckman Instruments, Inc., Drea, CA) and the Vitros 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY), respectively.
Covariates
The covariates used for the reference model in this study included all of the predictors used in the Framingham risk score for global CVD prediction.22 BP was measured three times with participants in the sitting position after 5 minutes of rest, and the average of the last two readings was recorded. The use of antihypertensive medication was determined by self-report. Diabetes mellitus was defined as a fasting glucose≥7.0 mmol/L, self-reported history of diabetes, or use of glucose-lowering medications. Serum glucose was measured by rate reflectance spectrophotometry. Use of antihypertensive and glucose-lowering medications was on the basis of review of medication containers. Total cholesterol and HDL cholesterol were determined using the cholesterol oxidase method. Smoking status was dichotomized as current versus former/never.
Cardiovascular Outcomes
Our primary outcome was a composite CVD including the first incidence of CHD, stroke, HF, and PAD, which was implemented in the Framingham global CVD prediction tool.22 Each CVD subtype was also analyzed separately. MESA conducts continuous, comprehensive surveillance for all CVD-related hospitalizations and deaths, and experts (including cardiologists, physician epidemiologists, and neurologists) adjudicate all CVD events.6,7 Incident CHD included definite or probable myocardial infarction, resuscitated cardiac arrest, definite or possible coronary death, definite angina pectoris (symptom with objective evidence of ischemia [coronary angiography or electrocardiogram]), or probable angina followed by coronary revascularization.38 Incident stroke was defined as new onset of neurologic symptoms lasting 24 hours or leading to death with a clinically relevant lesion on brain imaging not caused by a nonvascular cause.39 Incident HF included definite or probable congestive HF with symptoms/signs (e.g., dyspnea or edema) supported by a physician diagnosis, HF treatment, pulmonary congestion, or cardiac dysfunction. Incident PAD included definite or probable cases of lower extremity atherosclerosis/thrombosis or abdominal aneurysm on the basis of a physician diagnosis, imaging (e.g., ultrasound), exercise test, ABI<0.8, or vascular procedures.
Statistical Analyses
We conducted all analyses in those with CKD and without CKD separately. We first evaluated the continuous association between each subclinical measure and the incidence rates of CVD using a Poisson regression model with linear spline terms (taking knots at the three thresholds determining quartiles of each measure) and adjustment for age, sex, and race. The knot at the threshold of two lowest quartiles was omitted for CAC, because approximately one half of participants had a CAC score of zero at baseline. The CAC score was log-transformed after adding 1 unit to the original score.6
We quantified independent associations between each subclinical measure and incident CVD using Cox proportional hazards models with adjustment for the Framingham CVD predictors. HRs were computed by quartiles (those with CAC score=0 composed the two lowest quartiles) and for a 1-SD increment in each measure. A P value for trend across quartiles was obtained from Cox models, with a median value of each measure for each quartile assigned to relevant individuals. We did not observe significant interactions of sex and race with conventional predictors in both CKD and non-CKD groups (P>0.08) and thus, incorporated these variables in prediction models together with the conventional risk factors. All continuous Framingham CVD predictors were log-transformed as originally proposed.22
From Cox proportional hazards models including conventional predictors with and without a subclinical measure, we evaluated the C statistic difference, categorical and continuous NRIs, and IDI using methods accounting for censoring.14,15 For categorical NRI on the basis of existing clinical thresholds for CHD risk (i.e., 10% and 20% in 10 years)3 and with CHD accounting for >50% of all CVD in our data, we considered 5-year CVD risk of <10%, 10%–19%, and ≥20% as low, intermediate, and high risk, respectively. Good calibration was confirmed for all models using a modified Hosmer–Lemeshow statistic (all chi-squared<18 with 9 degrees of freedom).22,40 All analyses were conducted in Stata 12.1 (StataCorp., College Station, TX), and a P value<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis (MESA) for their valuable contributions.
This research was supported by National Heart, Lung, and Blood Institute Contracts N01-HC-95159–N01-HC-95169 and National Center for Research Resource Grants UL1-RR-024156 and UL1-RR-025005.
A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014020173/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, Hague N, New J, Farmer CK: Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 72: 92–99, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217–224, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL: Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 168: 1333–1339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM: Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 308: 788–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharples EJ, Pereira D, Summers S, Cunningham J, Rubens M, Goldsmith D, Yaqoob MM: Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. Am J Kidney Dis 43: 313–319, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr., Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, American College of Cardiology Foundation/American Heart Asscoiation Tasl Force on Practice Guidelines : 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 122: 2748–2764, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW: 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119: e391–e479, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 125: e2–e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB: Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med 23: 2109–2123, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr., Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ, Retnakaran R: Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: Systematic review and meta-analysis. BMJ 346: f1654, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Earls JP: The pros and cons of searching for extracardiac findings at cardiac CT: Studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology 261: 342–346, 2011 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization : Global Forum to Improve Developing Country Access to Medical Devices, Geneva, Switzerland, World Health Organization, 2010 [Google Scholar]
- 19.Jain A, McClelland RL, Polak JF, Shea S, Burke GL, Bild DE, Watson KE, Budoff MJ, Liu K, Post WS, Folsom AR, Lima JAC, Bluemke DA: Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: The multi-ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging 4: 8–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW: 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published online ahead of print November 12, 2013]. Circulation [Google Scholar]
- 21.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB: Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke 25: 40–43, 1994 [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB: General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117: 743–753, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R, Cholesterol Treatment Trialists’ (CTT) Collaboration : Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T: Cardiovascular disease and risk factors in Asia: A selected review. Circulation 118: 2702–2709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL: Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 367: 1747–1757, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Szklo M, Ding J, Tsai MY, Cushman M, Polak JF, Lima J, Barr RG, Sharrett AR: Individual pathogens, pathogen burden and markers of subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Med (Hagerstown) 10: 747–751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr., Sidney S, Bild DE, Williams OD, Detrano RC: Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 234: 35–43, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 31.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA: Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 113: 30–37, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S: The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 56: 1506–1512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charles LE, Fekedulegn D, Burchfiel CM, Fujishiro K, Landsbergis P, Diez Roux AV, Macdonald L, Foy CG, Andrew ME, Stukovsky KH, Baron S: Associations of work hours with carotid intima-media thickness and ankle-brachial index: The Multi-Ethnic Study of Atherosclerosis (MESA). Occup Environ Med 69: 713–720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS, Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito H, Pacold IV, Durazo-Arvizu R, Liu K, Shilipak MG, Goff DC, Jr., Tracy RP, Kramer H: The effect of including cystatin C or creatinine in a cardiovascular risk model for asymptomatic individuals: The multi-ethnic study of atherosclerosis. Am J Epidemiol 174: 949–957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawasaki R, Xie J, Cheung N, Lamoureux E, Klein R, Klein BEK, Cotch MF, Sharrett AR, Shea S, Wong TY, MESA : Retinal microvascular signs and risk of stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Stroke 43: 3245–3251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S: A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 16: 965–980, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.