Abstract
Patients on dialysis require phosphorus binders to prevent hyperphosphatemia and are iron deficient. We studied ferric citrate as a phosphorus binder and iron source. In this sequential, randomized trial, 441 subjects on dialysis were randomized to ferric citrate or active control in a 52-week active control period followed by a 4-week placebo control period, in which subjects on ferric citrate who completed the active control period were rerandomized to ferric citrate or placebo. The primary analysis compared the mean change in phosphorus between ferric citrate and placebo during the placebo control period. A sequential gatekeeping strategy controlled study-wise type 1 error for serum ferritin, transferrin saturation, and intravenous iron and erythropoietin-stimulating agent usage as prespecified secondary outcomes in the active control period. Ferric citrate controlled phosphorus compared with placebo, with a mean treatment difference of −2.2±0.2 mg/dl (mean±SEM) (P<0.001). Active control period phosphorus was similar between ferric citrate and active control, with comparable safety profiles. Subjects on ferric citrate achieved higher mean iron parameters (ferritin=899±488 ng/ml [mean±SD]; transferrin saturation=39%±17%) versus subjects on active control (ferritin=628±367 ng/ml [mean±SD]; transferrin saturation=30%±12%; P<0.001 for both). Subjects on ferric citrate received less intravenous elemental iron (median=12.95 mg/wk ferric citrate; 26.88 mg/wk active control; P<0.001) and less erythropoietin-stimulating agent (median epoetin-equivalent units per week: 5306 units/wk ferric citrate; 6951 units/wk active control; P=0.04). Hemoglobin levels were statistically higher on ferric citrate. Thus, ferric citrate is an efficacious and safe phosphate binder that increases iron stores and reduces intravenous iron and erythropoietin-stimulating agent use while maintaining hemoglobin.
Keywords: clinical trial, phosphate binders, anemia, dialysis
Phosphorus is ubiquitous in food and excreted by the kidney. Phosphorus control is a universal problem in well nourished patients on dialysis. Hyperphosphatemia is associated with metabolic bone disease, hyperparathyroidism, and increased morbidity and mortality.1,2 Several agents currently available are capable of binding phosphorus in the gastrointestinal (GI) tract and preventing its absorption, but all have limitations. We performed a sequential randomized clinical trial with two randomized periods to determine the safety and efficacy of ferric citrate as a phosphate binder as well as evaluate the capacity of ferric citrate to supplement iron stores and reduce intravenous (iv) iron and erythropoietin-stimulating agent (ESA) use.
Results
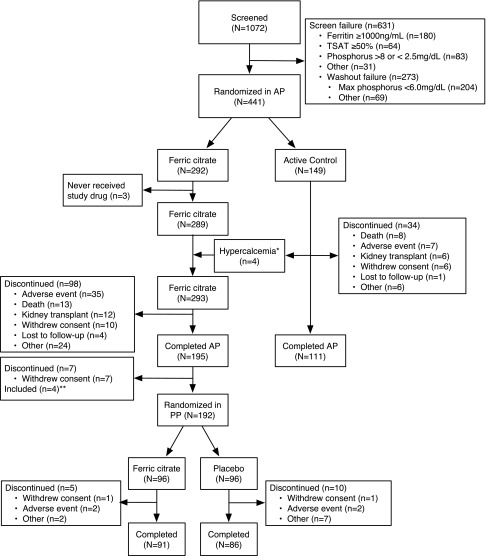
Figure 1 shows the disposition of subjects through the trial. In total, 1072 subjects were screened, and 441 subjects were randomized in the 52-week active control period, with 192 subjects rerandomized in the final 4-week placebo control period. In total, 448 (42%) of 1072 enrolled subjects failed to be randomized because of increased iron stores at screening or inability to develop hyperphosphatemia during washout. Table 1 shows the baseline characteristics of the subjects according to treatment assignment and study period. Subjects entering the 52-week active control period were well balanced between both groups and reflected the United States dialysis population. After randomization, more subjects on ferric citrate discontinued the study drug compared with the active control, notably in the first weeks of the study, most commonly because of GI nonserious adverse events (AEs) (Supplemental Appendix). Tolerability to active control was an entry criterion; 21% of subjects receiving ferric citrate and 15% of subjects receiving active control discontinued use because of an AE (includes AE, death, and kidney transplant in Figure 1).
Figure 1.
Disposition of subjects in the trial. AP, active control period; PP, placebo control period. *Four subjects on calcium acetate stopped calcium acetate and switched to ferric citrate because of persistent hypercalcemia. **Four subjects entered the final 4-week placebo control period who were ineligible, because they did not complete the 52-week active control period on ferric citrate.
Table 1.
Demographics and baseline characteristics among randomized subjects
| Parameter | Ferric Citrate 4-Wk Placebo Control Period Start (N=96) | Placebo 4-Wk Placebo Control Period Start (N=96) | P Valuea | Ferric Citrate 52-Wk Active Control Period Start (N=292) | Active Control 52-Wk Active Control Period Start (N=149) | P Valuea |
|---|---|---|---|---|---|---|
| Age (yr), median (Q1–Q3) | 54.0 (45.0–62.5) | 56.0 (48.5–62.0) | 0.66 | 56.0 (45.0–63.0) | 54.0 (45.0–63.0) | 0.41 |
| Men, n (%) | 70 (72.9) | 47 (49.0) | 0.001 | 183 (62.7) | 87 (58.4) | 0.38 |
| Race, n (%) | 0.27 | 0.30 | ||||
| Black or African American | 62 (64.6) | 51 (53.1) | 154 (52.7) | 78 (52.4) | ||
| White/Caucasian | 30 (31.3) | 41 (42.7) | 124 (42.5) | 62 (41.6) | ||
| Other/unknown | 4 (4.2) | 4 (4.1) | 14 (4.8) | 9 (6.0) | ||
| Ethnicity, n (%) | 0.38 | 0.86 | ||||
| Hispanic or Latino | 10 (10.4) | 14 (14.6) | 43 (14.8) | 23 (15.4) | ||
| Not Hispanic or Latino | 86 (89.6) | 82 (85.4) | 248 (85.2) | 126 (84.6) | ||
| Cause of ESRD, n (%)b | ||||||
| Diabetic nephropathy | 29 (30.2) | 42 (43.8) | 0.30 | 120 (41.1) | 65 (43.6) | 0.32 |
| Hypertensive nephrosclerosis | 33 (34.4) | 30 (31.3) | 89 (30.5) | 45 (30.2) | ||
| Glomerular disease | 13 (13.5) | 7 (7.3) | 25 (8.6) | 13 (8.7) | ||
| Polycystic kidney disease | 4 (4.2) | 4 (4.2) | 10 (3.4) | 10 (6.7) | ||
| Other | 17 (17.7) | 13 (13.5) | 48 (16.4) | 16 (10.7) | ||
| Prior parathyroidectomy, n (%) | 5 (5.2) | 7 (7.3) | 0.55 | 19 (6.5) | 4 (2.7) | 0.09 |
| Congestive heart failure, n (%) | 33 (34.4) | 32 (33.3) | 0.88 | 93 (31.8) | 50 (33.6) | 0.72 |
| Prior MI/CAD, n (%) | 36 (37.5) | 29 (30.2) | 0.29 | 103 (35.3) | 46 (30.9) | 0.36 |
| Other heart disease, n (%) | 21 (21.9) | 16 (16.7) | 0.36 | 53 (18.2) | 38 (25.5) | 0.07 |
| CVA/TIA, n (%) | 11 (11.5) | 17 (17.7) | 0.22 | 38 (13.0) | 17 (11.4) | 0.63 |
| Previous binder sevelamer, n (%) | NAc | NA | 170 (58.2) | 96 (64.4) | 0.21 | |
| Previous binder calcium acetate, n (%) | NA | NA | 104 (35.6) | 57 (38.3) | 0.59 | |
| Previous binder lanthanum, n (%) | NA | NA | 28 (9.6) | 12 (8.1) | 0.60 | |
| Previous binder other, n (%) | NA | NA | 20 (6.8) | 14 (9.4) | 0.34 | |
| On ESA, n (%) | 58 (60.4) | 63 (65.6) | 0.46 | 238 (81.5) | 123 (82.6) | 0.79 |
| On iv iron, n (%) | 15 (15.6) | 21 (21.9) | 0.27 | 177 (60.6) | 90 (60.4) | 0.97 |
| On vitamin D (or its analogs), n (%) | 82 (85.4) | 78 (81.2) | 0.44 | 223 (76.4) | 126 (84.6) | 0.05 |
| Phosphorus (mg/dl), median (Q1–Q3) | 5.10 (4.30–6.00) | 5.30 (4.50–5.90) | 0.20 | 7.20 (6.30–8.30) | 7.40 (6.20–8.50) | 0.42 |
| Calcium (mg/dl), median (Q1–Q3) | 9.23 (8.63–9.63) | 9.20 (8.75–9.70) | 0.89 | 9.00 (8.40–9.45) | 9.00 (8.50–9.58) | 0.41 |
| Ferritin (mg/dl), median (Q1–Q3) | 858 (520–1096) | 932 (650–1236) | 0.06 | 582 (380–778) | 568 (374–780) | 0.87 |
| TSAT (%), median (Q1–Q3) | 36 (30–47) | 34 (28–46) | 0.54 | 29 (24–37) | 29 (23–35) | 0.73 |
| Hemoglobin (g/dl), median (Q1–Q3) | 11.4 (10.5–12.3) | 10.9 (10.4–11.6) | 0.01 | 11.4 (10.7–12.2) | 11.7 (10.9–12.4) | 0.20 |
| iPTH (pg/ml), median (Q1–Q3) | 354 (197–622) | 343 (206–559) | 0.80 | 515 (331–801) | 480 (278–754) | 0.28 |
In the 52-week active control period, one patient has missing ethnicity. Q1, 25th percentile; Q3, 75th percentile; MI/CAD, myocardial infarction/coronary artery disease; CVA, cerebrovascular accident; TIA, transient ischemic attach; NA, not applicable; iPTH, intact parathyroid hormone.
Chi-squared tests were used to compare treatment percentages, and t tests were used to compare treatment means.
ESRD cause by precedence polycystic kidney disease, glomerular disease, diabetic nephropathy, hypertensive nephrosclerosis, and all others.
All subjects entering the 4-week placebo control period completed the 52-week active control period on ferric citrate.
In the final 4-week placebo control period, baseline mean serum phosphorus was similar in the ferric citrate and placebo groups (Table 2). At the end of the placebo control period, the mean serum phosphorus was lower in the ferric citrate group versus the placebo group, with a mean treatment difference of −2.2±0.2 mg/dl (mean±SEM) (P<0.001). After adjustment for the three baseline factors that exhibited imbalance between the treatment groups at the start of the placebo control period (sex, ferritin, and hemoglobin), the mean treatment difference persisted: −2.2±0.2 mg/dl (mean±SEM). The results were unchanged in sensitivity analyses that excluded the four subjects who entered the placebo control period but were ineligible or excluded the four hypercalcemic subjects noted in Figure 1 (Supplemental Appendix). In the placebo control period, 21 subjects on placebo and 1 subject on ferric citrate reached a serum phosphorus level≥9 mg/dl and were considered treatment failures.
Table 2.
Primary outcome in the 4-week placebo control period
| Outcome | Position in Gatekeeping Procedure | Baseline Mean (SEM) | End of Placebo Control Period Mean (SEM) | End of Placebo Control Period Median (Q1–Q3) | ANCOVA Results (Ferric Citrate Versus Placebo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ferric Citrate | Placebo | Ferric Citrate | Placebo | Ferric Citrate | Placebo | Adjusted Mean Difference | 95% CI | P Value | ||
| Phosphorus (mg/dl) | 1 | 5.12 (0.12) | 5.44 (0.15) | 4.86 (0.13) | 7.21 (0.19) | 4.60 (4.20–5.90) | 7.20 (5.80–8.80) | −2.18 | −2.59 to −1.77 | <0.001 |
A last follow-up observation with carried forward imputation was used to impute missing values for all laboratory outcomes; 91 patients were analyzed for both the ferric citrate and placebo control groups in the 4-week placebo control period. Q1, 25th percentile; Q3, 75th percentile; ANCOVA, analysis of covariance; 95% CI, 95% confidence interval.
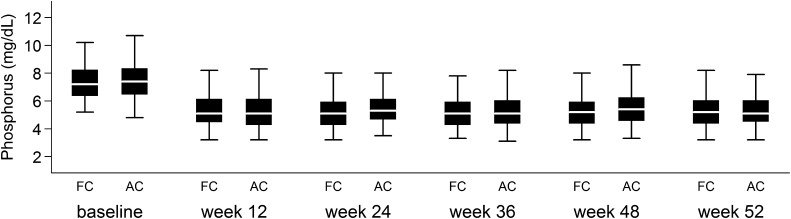
Over 52 weeks, the mean serum phosphorus was not significantly different between the randomized ferric citrate and active control groups (Figure 2). In the active control group, 73 subjects were on sevelamer, 36 subjects were on calcium acetate, and 40 subjects were on both. The median daily dose (pill count) of ferric citrate was 8.0 tablets/d versus 7.7 tablets/d for subjects on active control taking calcium acetate and 9.0 tablets/d for subjects on active control taking sevelamer carbonate (ferric citrate versus sevelamer carbonate, P=0.01; ferric citrate versus calcium acetate, P=0.28; calcium acetate versus sevelamer carbonate, P=0.02). Mean serum phosphorus at the end of 52 weeks was 5.4±1.6 mg/dl (mean±SD) in the ferric citrate group and 5.4±1.7 and 5.3±1.4 mg/dl (mean±SD) for subjects in the active control group taking sevelamer carbonate (P=0.94 compared with ferric citrate) and calcium acetate (P=0.84 compared with ferric citrate), respectively. Values are expressed as mean±SD unless otherwise stated.
Figure 2.
Serum phosphorus levels (milligrams per deciliter) by study time point during the 52-week active control period, with missing values imputed using the last follow-up observation carried forward. Box plots display 5th, 25th, 50th, 75th, and 95th percentiles. Under the repeated measures mixed effects model, the mean difference in serum phosphorus between the ferric citrate and active control groups over weeks 12, 24, 36, 48, and 52 was −0.0127 mg/dl (95% confidence interval, −0.056 to 0.030 mg/dl). AC, active control; FC, ferric citrate.
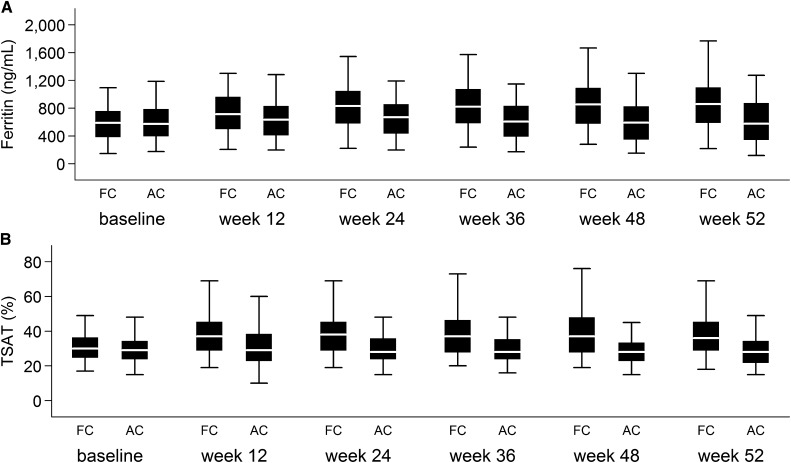
Under the gatekeeping procedure, the trial showed that ferric citrate increased serum ferritin (P<0.001) and transferrin saturation (TSAT; P<0.001) compared with active control and decreased iv iron (P<0.001) and ESA (P=0.04) usage (Table 3). The relative difference in mean TSAT between ferric citrate and active control plateaued at 12 weeks, and the mean ferritin increased at slower rates after week 24 (Figure 3); 19.1% of subjects on ferric citrate and 10.1% of subjects on active control had at least one measurement of serum ferritin>1500 ng/ml. Most of these values were adjudicated to be caused by the administration of iv iron and/or SAEs, and they resolved.3 In the last 6 and 9 months of the active control period, only 43.8% and 47.7% of subjects on ferric citrate received any iv iron compared with 63.0% and 80.1% of subjects on active control (P<0.001), respectively. Over 52 weeks, mean hemoglobin increased in subjects treated with ferric citrate compared with active control (P=0.02) (Table 3). Red blood cell mean cell volume increased with ferric citrate compared with active control (P<0.001). There were no significant differences in serum bicarbonate, serum aluminum, liver function tests, or platelet count between ferric citrate and active control.
Table 3.
Comparisons of main secondary outcomes in the 52-week active control period
| Outcome | Position in Gatekeeping Procedure | Baseline Mean (SEM) | Week 52 Mean (SEM) | Week 52 Median or Cumulative Median (Q1–Q3)a | ANCOVA or Wilcoxon Test Results (Ferric Citrate Versus Active Control) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ferric Citrate | Active Control | Ferric Citrate | Active Control | Ferric Citrate | Active Control | Adjusted Mean Difference | 95% CI | P Value | ||
| Ferritin (ng/ml) | 2 | 593 (18) | 609 (26) | 899 (31) | 628 (31) | 858 (568–1105) | 576 (333–883) | 282 | 197 to 366 | <0.001 |
| TSAT (%) | 3 | 31.3 (0.7) | 30.9 (1.0) | 39.3 (1.1) | 29.7 (1.0) | 36.0 (27.5–47.0) | 28.0 (21.0–34.5) | 9.5 | 6.4 to 12.6 | <0.001 |
| Iv Iron (mg/wk)a | 4 | — | — | — | — | 12.9 (1.0–28.9) | 26.8 (13.4–47.6) | −12.5b | −17.2 to −7.9b | <0.001 |
| ESA dose (units/wk)a | 5 | — | — | — | — | 5303 (2023–9695) | 6954 (2664–12,375) | −1191b | −2632 to 0b | 0.04 |
| Hemoglobin (g/dl) | 11.61 (0.08) | 11.71 (0.11) | 11.42 (0.10) | 11.14 (0.12) | 11.20 (10.50–12.10) | 11.00 (10.25–12.30) | 0.33 | 0.06 to 0.60 | 0.02 | |
| Iron (mg/dl) | 72.6 (1.8) | 69.2 (2.1) | 88.4 (2.7) | 69.6 (2.6) | 82.5 (58.0–102.5) | 65.5 (51.5–81.5) | 17.91 | 10.0 to 25.8 | <0.001 | |
| Phosphorus (mg/dl) | 7.41 (0.10) | 7.56 (0.14) | 5.36 (0.10) | 5.38 (0.13) | 5.20 (4.40–6.10) | 5.10 (4.40–6.20) | 0.01 | −0.30 to 0.32 | 0.95 | |
| Calcium (mg/dl) | 8.90 (0.05) | 8.96 (0.07) | 9.12 (0.05) | 9.27 (0.08) | 9.15 (8.65–9.60) | 9.30 (8.70–9.80) | −0.12 | −0.28 to 0.04 | 0.16 | |
| iPTH (pg/ml) | 621 (27) | 578 (38) | 453 (23) | 432 (29) | 350 (206–611) | 330 (215–519) | 4 | −62 to 70 | 0.90 | |
A last follow-up observation with carried forward imputation was used to impute missing values for all laboratory outcomes. Sample sizes for the 52-week active control period ranged from 247 to 281 patients for the different outcomes in the ferric citrate group and from 132 to 146 patients in the active control group. Q1, 25th percentile; Q3, 75th percentile; ANCOVA, analysis of covariance; 95% CI, 95% confidence interval; iPTH, intact parathyroid hormone.
Week 52 levels are summarized for all outcomes during the 52-week active control period with the exception of iv iron and ESA dose, which are summarized over the entire follow-up period.
Hodges–Leman estimate of location shift and 95% confidence limit.
Figure 3.
Iron parameters by study time point during the 52-week active control period. (A) Serum ferritin and (B) serum TSAT, with missing values imputed using the last follow-up observation carried forward. Box plots display 5th, 25th, 50th, 75th, and 95th percentiles. AC, active control; FC, ferric citrate.
Through 1 month after drug discontinuation in the 52-week active control period, 4.5% (13 of 292 subjects; 0.054 per patient-year) of subjects in the ferric citrate group and 5.4% (8 of 149 subjects; 0.057 per patient-year) of subjects in the active control group died (Supplemental Appendix). No deaths were attributed to the study drug. The sum of serious AEs and non-serious AEs was similar between the two groups (ferric citrate=90.3%; active control=89.3%). However, 39.1% of subjects receiving ferric citrate and 49.0% of subjects on active control experienced an SAE. The percentages of subjects with GI SAEs in the ferric citrate and active control groups were 6.9% and 12.8%, respectively. The corresponding percentages of subjects in the ferric citrate and active control groups with infection SAEs were 12.5% and 18.1% and cardiac SAEs were 7.3% and 12.1%, respectively (Supplemental Appendix). The proportions of patients with recorded AEs in the noted categories were increased slightly by adding patients who had their first recorded AEs in those categories after discontinuation of the study drug (Supplemental Appendix). Subjects who stopped the study drug were followed in the study, if able, with all study procedures. Available evidence does not support the hypothetical concern that the lower overall rates of SAEs and SAEs in GI, cardiac, and infection categories were caused by censoring of subjects who stopped study drug (Supplemental Appendix).
Discussion
Most patients on dialysis require phosphate binding and the repletion of iron stores. Our study, on the basis of a prospectively designated sequential gatekeeping strategy, achieved all of the five predesignated outcomes: ferric citrate is an efficacious phosphate binder, raises iron stores, which was evidenced by significantly increasing serum ferritin and TSAT, and decreases iv iron and ESA usage. No other approved phosphate binder increases iron stores and decreases iv iron and ESA usage. The gatekeeping strategy assured that the overall type 1 error for all five of the designated outcomes did not exceed 5% by evaluating the statistical significance of each outcome only if all previous outcomes in the sequence were statistically significant at the 5% level.
Oral ferric citrate had been studied as a phosphate binder in several preliminary studies.4–11 Earlier studies suggested that ferric iron binds phosphorus in a dose-dependent fashion. In the 4-week placebo control period, ferric citrate was shown to effectively reduce serum phosphorus compared with placebo, with a compelling P value (<0.001). All previous phosphate binders were deemed to be efficacious in comparisons with placebo. The trial design that we selected shows the efficacy of ferric citrate in lowering serum phosphorus compared with placebo in subjects who had been maintained on ferric citrate for 52 weeks in the active control period. This trial also shows that ferric citrate provides similar control of phosphorus compared with active control over 52 weeks. Our sequential trial design, with the placebo period following the year-long active control period, more closely replicates efficacy in a real patient care setting, in which patients only achieve phosphorus control if they are maintained on the binder over a longer time than could be achieved in a short placebo trial. Also, ferric citrate had a lower average pill burden compared with sevelamer carbonate, with comparable phosphorus control. The few currently available phosphate binders have limitations, including aluminum toxicity,12 diarrhea,13–17 hypercalcemia,14 and patient tolerability issues.14–18 There were more discontinuations for all causes, including renal transplantation, in the ferric citrate versus active control groups (33% ferric citrate versus 23% active control). This result was largely because of more GI non-serious AEs, such as diarrhea and bloating, early in the study in subjects receiving ferric citrate (Supplemental Appendix). Of note, a study entry criterion was tolerability to calcium acetate or sevelamer carbonate, and therefore, patients who had GI intolerance to these active control drugs were excluded from the study. Enrolling subjects with known intolerance to these drugs could have led to their immediate withdrawal if randomized to active control in many cases. Studies have reported discontinuations, including intolerance and all other causes (including renal transplantation), of up to 39% with sevelamer15 and up to 71% with lanthanum.16,19 Given that patients on dialysis take a relatively high number of these pills a day, it may be that a high discontinuation rate is a phosphate binder class effect. Patients on dialysis would benefit from another binder choice independent of the other attributes of ferric citrate.
The 52-week active control period was a randomized, standalone clinical trial, and it showed that ferric citrate administration over 52 weeks increased iron stores, which was shown by increased serum ferritin, serum iron, and transferrin saturation, despite significantly reducing administration of iv iron and ESA usage. Patients on dialysis lose blood, are commonly iron deficient, and require sufficient iron stores for ESA responsiveness.20–23 Historically, oral supplementation with ferrous salts in the absence of food failed to meet the erythropoietic demands of absolute and functional iron deficiencies in patients with ESRD, largely because of GI intolerance that limited dosing to approximately 200 mg elemental iron per day.23–25 This inefficiency of oral iron supplementation led to the widespread use of iv iron in patients on dialysis, with the Dialysis Outcomes and Practice Patterns Study Practice Monitor reporting over 70% of patients on dialysis receiving iv iron at any given time in 2011.26 Ferric citrate delivered much larger doses of elemental iron with food (up to 2520 mg/d) compared with previous oral preparations, and hence, it reduced or eliminated the need for iv iron. Although other factors may have allowed our subjects to tolerate much higher daily doses of oral iron compared with subjects of previous trials with other oral iron preparations (2520 mg/d versus approximately 200 mg/d), the delivery of the ferric iron preparation with food rather than ferrous iron in the fasting state plausibly accounts for the difference in tolerability.
At the discretion of the treating physicians, iv iron was allowed in all groups as long as the serum ferritin was ≤1000 ng/ml or TSAT was ≤30%. Although subjects receiving ferric citrate achieved higher iron stores than those receiving active control, they received significantly less iv iron, with the majority no longer requiring iv iron in the last 6 months of the 52-week active control period. This result is presumably secondary to the GI absorption of iron from ferric citrate. It is unlikely that the increases in serum ferritin were related to inflammation, because TSAT also increased, and subjects on ferric citrate had evidence of less inflammation, such as fewer infection-associated SAEs, than subjects on active control.27 We did not compare the ferric citrate group with a control group with no iv iron administration and hence, cannot comment on its performance as a standalone iron therapy.
Ferric citrate in this study reduced ESA usage, whereas slightly higher hemoglobin values were achieved compared with active control. Studies, including the Dialysis Patients’ Response to IV Iron with Elevated Ferritin Study, suggest that giving iv iron to subjects with serum ferritin up to 1200 ng/ml and TSAT up to 50% will increase hemoglobin levels and ultimately, decrease ESA usage.28,29 Current standard of care, as opposed to many current guidelines, includes protocols to continue iv iron, irrespective of hemoglobin levels, until these iron goals are exceeded. Indeed, our study showed that subjects on ferric citrate have higher iron stores and decreased iv iron and ESA use. A pharmacoeconomic analysis on the basis of these results found that the decrease in ESA and iv iron usage seen with ferric citrate would save $2101 per patient per year.30 In addition, one could postulate that decreased iv iron and ESA usage could result in decreased nursing time to administer iv medications, which could be redirected to other aspects of patient care, and decreased risk of infections because of fewer iv injections.31 The above noted annual per patient cost savings projected for using ferric citrate as a phosphate binder is welcome, because currently, patients with ESRD represent 1.4% of Medicare patients but use 7.2% of Medicare spending.32
The potential for iron overload by oral absorption is extremely low, except in cases of hemochromatosis. Unlike iv iron currently administered in dialysis units, oral iron absorption is tightly regulated in the GI tract. Soluble iron is transported into the duodenal enterocyte, but ferric iron (Fe3+) must first be reduced to ferrous iron (Fe2+) before transport. This process and subsequent iron passage into the plasma are tightly regulated by iron regulatory proteins and hepcidin, respectively. As result of this regulation, iron absorption is restricted when iron stores are sufficient and increased when iron stores are deficient.33 Our results showing the plateau of TSAT over 52 weeks and the decrease in the rate of rise of ferritin among subjects on ferric citrate suggest that the GI iron absorption from ferric citrate was regulated. In the absence of hemochromatosis, iron overload is not defined by arbitrary cutpoints in serum ferritin or TSAT or increased iron staining in end organs but rather, end organ dysfunction. Because there were fewer SAEs in organ systems usually associated with iron overload in ferric citrate subjects, our data do not support iron overload, despite higher serum ferritin levels and TSAT. Currently, the guidelines from different organizations, the protocols used by major dialysis companies, and the available literature have varying recommendations for serum ferritin and TSAT upper limits. On the basis of any physician’s assessment of the ideal iron stores for a given patient, the use of ferric citrate could be adjusted in a manner analogous to the adjustments made in the use of calcium acetate when hypercalcemia occurs. Because all dialysis units currently monitor iron parameters routinely, ferric citrate use will require no new directives, and physicians could modulate its use as needed to achieve a target iron store status.
Supporting the safety of ferric citrate are the similar rates of death, all SAEs, and non-SAEs over 52 weeks compared with active control. Indeed, subjects on ferric citrate experienced fewer SAEs (39.1% versus 49%) compared with subjects on active control. Subjects who stopped study drug were followed in the study, if able, with all study procedures, and the lower overall rates of SAEs and SAEs in GI, cardiac, and infection categories were not caused by censoring of subjects who stopped the study drug.
By necessity, randomization was limited to those patients on dialysis for whom participation was deemed safe. This limits the generalizability of our results to patients who share the characteristics of the subjects in this trial. However, of note, the most common reasons that patients were excluded from entry into our study were baseline ferritin>1000 ng/ml and/or TSAT>50% or inability to achieve a phosphorus>6.0 mg/dl in washout. They were not excluded by clinical characteristics, such as cardiac status, cause of ESRD, or other clinical features that represent the general health of the ESRD population. Another limitation of this study was that, although the study drug bottles were not labeled, the discoloration of the stool by oral iron necessitated an open-label design, which can introduce investigator or subject bias. This design is unlikely to affect the actual measurement of serum phosphorus, the primary outcome, or the iron parameters but could influence the reporting of AEs. Our study does not address the long-term safety of this compound.
This study showed that ferric citrate is an efficacious and safe oral phosphate binder. In addition, iron absorbed from ferric citrate increases iron stores and reduces iv iron and ESA use while sustaining hemoglobin.
Concise Methods
This phase 3, sequential, randomized, open-label trial was conducted at 60 sites in the United States and Israel. Members (J.B.L., M.S., M.J.K., and J.P.D.) of the Collaborative Study Group (CSG) wrote the protocol, and the independent CSG statistician (T.G.) had full access to the clinical trial database and performed or supervised the analyses of the designee for this manuscript. The rationale and study design of this trial have been published previously.9
Recruitment began in December of 2010, and the study was completed in November of 2012. The Institutional Review Board at the Clinical Coordinating Center (CCC) and each site approved this trial. All subjects gave written informed consent before any investigational procedures, and the trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. This trial is registered with ClinicalTrials.gov (NCT01191255). All laboratory analyses were performed at the central laboratory, with the exception of serum bicarbonate, which was measured locally. All authors contributed to the final manuscript. This trial was conducted under a Special Protocol Assessment agreement with the US Food and Drug Administration (FDA).
Subject Population
Eligible subjects were adult patients with ESRD on three times per week hemodialysis or peritoneal dialysis for at least 3 months before the screening visit who were prescribed 3–18 doses of commercially available phosphate binder and had serum ferritin<1000 ng/ml, serum TSAT<50%, and serum phosphorus≥2.5 and ≤8.0 mg/dl at the screening visit. Major exclusion criteria included parathyroidectomy within 6 months before the screening visit, an absolute requirement for oral iron or vitamin C therapy, or intolerance to calcium acetate and sevelamer.
Study Design
This trial had three periods. A 2-week washout period was followed by a 52-week randomized, open-label, active control period to determine the safety of ferric citrate as well as its capacity to supplement iron stores and reduce iv iron and ESA usage. This period was followed by a 4-week, randomized, open-label, placebo control period to determine the efficacy of ferric citrate to control phosphorus compared with placebo.
Eligible subjects entering the washout period had all phosphate binders stopped and were randomized only if the serum phosphorus was ≥6.0 mg/dl after a maximum of 2 weeks. Subjects who maintained eligibility were randomized in a 2:1 ratio to ferric citrate or active control. Ferric citrate was supplied as 1-g tablets containing 210 mg ferric iron. Dose adjustments of ferric citrate were determined by a protocol-supplied titration schedule (Supplemental Appendix).4,8 The study provided active control study drugs (calcium acetate: 667-mg capsules; sevelamer carbonate: 800-mg tablets) titrated according to the FDA-approved package inserts14,15 that could be used alone or combined. Central laboratory phosphorus levels guided dosing in both treatment groups and were performed monthly after the first 12 weeks of the trial. Subjects were instructed to take study drug with or within 1 hour of meals or snacks. Compliance was assessed by pill counts. Subjects were considered treatment failures if they were ≥80% compliant with 12 doses/d of either ferric citrate or active control and had two consecutive visits with a serum phosphorus>8.0 mg/dl. These subjects discontinued the study drug but completed all study visits. Subjects assigned to calcium acetate with adjusted serum calcium>10.5 mg/dl unresponsive to conservative management were also considered treatment failures. Per the protocol, these subjects were switched to ferric citrate and allowed to enter the final 4-week placebo control period.
Those subjects who were on ferric citrate after 52 weeks were rerandomized to either continue on ferric citrate or receive placebo for the 4-week placebo control period. Serum phosphorus levels were checked weekly, and any subject who developed a phosphorus level≥9.0 mg/dl was considered a treatment failure.
AEs were recorded from the screening visit to the final visit. A CSG medical monitoring committee (M.J.K. and J.P.D.) reviewed and adjudicated all SAEs within 24 hours of reporting to the CCC.
Concomitant Therapies
Vitamin D, its analogs, cinacalcet, fasting calcium supplements, variations in dialysate calcium concentration, and ESAs were permitted during the study at the discretion of the treating physician; iv iron therapy was prohibited if the subject had serum ferritin>1000 ng/ml or TSAT>30%, and iv iron was permitted, at the discretion of the site, if ferritin was ≤1000 ng/ml and TSAT was≤30%.
Randomization
Randomization was implemented using random permuted blocks, with stratification for clinical site by an interactive web randomization system.
Outcomes
Change in serum phosphorus during the final 4-week placebo control period was the primary outcome for determining the efficacy of ferric citrate to control serum phosphorus. Changes over 52 weeks in ferritin and TSAT were designated as main secondary outcomes to characterize the effect of ferric citrate on iron stores, and cumulative doses of iv iron and ESA were designated as the main secondary outcomes to determine the effect of ferric citrate on iv iron and ESA usage. A sequential gatekeeping strategy,34 using two-sided α=0.05 at each step, was used to protect the study-wise α-level for the treatment comparisons of the primary outcome and the four prespecified main secondary outcomes in the following sequence: (1) change in serum phosphorus during the final 4-week placebo control period, changes in (2) ferritin and (3) TSAT from baseline to week 52 in the 52-week active control period, and finally, the cumulative use of (4) iv iron and (5) ESA over the 52-week active control period. All other hypothesis tests were performed at the two-sided α=0.05 level without adjustment for multiple comparisons.
Statistical Analyses
Approximately 350 subjects were planned to be randomized in a 2:1 ratio to either ferric citrate or active control. Approximately 163 subjects from the ferric citrate group would then enter the final 4-week placebo control period assuming a 30% dropout rate in the 52-week active control period. The final sample size of 192 subjects for the placebo control period provided at least 95% power at a two-sided significance level of 5% to detect a mean difference between ferric citrate and placebo of 1.2 mg/dl on the basis of the assumption of a common SD for the two groups of 2 mg/dl.
Continuous data were summarized using means with SDs or medians and 10th to 90th percentile intervals as appropriate. Frequencies and percentages were used to summarize categorical data. The primary analysis was performed using analysis of covariance (ANCOVA) to compare the mean change in serum phosphorus from baseline (week 52) with week 56 of the final 4-week placebo control period between the ferric citrate and placebo groups, controlling for baseline phosphorus. In a sensitivity analysis, the primary ANCOVA was repeated after adjustment for the three additional baseline factors (sex, ferritin, and hemoglobin) exhibiting imbalances between treatment groups. Separate ANCOVA models were applied during the 52-week active control period to compare the mean changes in ferritin, TSAT, hemoglobin, phosphorus, and other laboratory parameters from baseline with each follow-up assessment (Supplemental Appendix) between the ferric citrate and active control groups, controlling for the baseline value of the outcome. Wilcoxon rank-sum tests were used to compare the cumulative average iv iron use and ESA dose between the treatment groups. Wilcoxon rank-sum tests were also used to confirm the ANCOVA results for serum ferritin, TSAT, and intact parathyroid hormone, because these outcomes exhibited moderate departures from normality.
After first defining outcome measurements after study drug discontinuation as missing, all missing values were imputed for each end point using separate last follow-up value carried forward algorithms within the sequential 52-week active control period and the final 4-week placebo control period. The analyses of laboratory parameters using last follow-up value carried forward were confirmed using corresponding ANCOVAs on the basis of longitudinal mixed effect models with unstructured covariance matrices to account for repeated measurements. The longitudinal mixed model for serum phosphorus during the 52-week active control period was used to obtain a 95% confidence interval for the mean difference between the ferric citrate and active control groups over the visits on weeks 12, 24, 36, 48, and 52 to characterize the maximum difference compatible with the data. Additional mixed effect analyses using two-slope linear splines with post hoc selection of knot points were used to compare the relative rates of increase in TSAT and ferritin during the early and later portions of follow-up, with separate mean slopes estimated for TSAT before and after 12 weeks and ferritin before and after 24 weeks. Frequencies and proportions of subjects reaching AEs are tabulated for the safety population, which included subjects who received at least one dose of study medication. Numbers of subjects with AEs were tabulated until the time of discontinuation of study drug for non-serious AEs and until 30 days after discontinuation of study drug for SAEs.
Statistical analyses were performed using SAS, versions 9.3 and 9.4 (Cary, NC).
Disclosures
All authors declare that they have received research grants from, acted as consultants for, and received travel support from Keryx Biopharmaceuticals, Inc.
Supplementary Material
Acknowledgments
We thank Edmund J. Lewis, the Principal Investigator of the Collaborative Study Group (CSG), for his ongoing advice and support. We also thank Robert Niecestro for his invaluable support in the design and conduct of the trial and his expertise in regulatory affairs.
The trial was funded by Keryx Biopharmaceuticals, Inc. Additionally, this study was supported, in part, by a grant from the The Patient Protection and Affordable Care Act of 2010. The Internal Revenue Service issued the funding under the Qualifying Therapeutic Discovery Project administered under section 48D of the Internal Revenue Code.
The following members of the CSG participated in the Phosphate Binding and Iron Delivery with Ferric Citrate in ESRD (PERFECTED-CSG-15) Trial as Coordinators and Investigators: J.B.L. and M.S. (Clinical Coordinating Center); and M.J.K. and J.P.D. (Medical Monitors). CSG site investigators and coordinators included I. Cohen and N. Lizzul (Mayo Clinic Arizona); R. Cohen and E. Camp (Southwest Kidney Institute); A. Felsenfeld, S. Graham, E. Daza, and K. Knibloe (Veterans Administration); C. Sun and L. Estrada (Apex Research Riverside); W. Chiang, R. Darwish, and S. Amini (Whittier Internal Medicine); D. Jalal, D. Spiegel, and B. Farmer (University of Colorado); I. Chang and H. Beeson (Western Nephrology); K. Kapatkin and T. Laneve (PAB Clinical Research); P. Fitzpatrick and J. Wright (Mayo Clinic Jacksonville); A. Rabiei and S. Asghari (ASA Clinical Research); M. Seek and D. Usrey (Discovery Medical Research Group); S Zeig and V. Gervais (Pines Clinical Research); M. Smith and M. Collins (Nephrology Associates); Z. Sharon and D. Darwin (Atlanta Nephrology); M. Sinsakul, D. Jones-O'Brien, and K. Lockwood (Rush University); S. Arfeen and S. Martin (Nephrology Specialists, PC); E. Reisin and S. Barry (Louisiana State University); D. Weiner, L. Chan, and A. Well (Tufts Medical Center); B. Athreya and A. Burkhart (Pioneer Valley Nephrology); B. Greco and J. Whitbeck (Western New England Renal & Transplant Associates); K. Nossuli and V. Sharma (Washington Nephrology Associates); K. Umanath and M. Zidan (Henry Ford Hospital); F. Al-Saghir and J. Powell (Michigan Kidney Consultants); A. Hiremath and D. Udell (Nephrology and Hypertension Clinic); A. Pfleuger and D. Hamiel (Mayo Clinic Rochester); A. Goel and A. Hurst (VA Medical Center); J. Manley and T. Mueller (Mountain Kidney & Hypertension Associates); P. Chuang and D. Griswell (Metrolina Nephrology Associates); J. Middleton and D. Schumm (Duke University); R. Moore and F. Abbot (Trial Management Associates); I. Bowline and V. Mauck (Piedmont Dialysis); W. Shapiro and R. Liang (Brookdale Physician's Dialysis); F. Whittier and D. Dziegelesk (Clinical Research); S. Kant and H. Duncan (University of Cincinnati); R. Heyka and R. Naude (Cleveland Clinic); U. Bhatt and C. Stratton (The Ohio State University); C. Sholer and D. Dion (Chris Sholer, MD, PC); S. Goral and R. Neubauer (University of Pennsylvania); R. Burgos-Calderon and P.F. Fontanez (University of Puerto Rico); C. Galphin and C. Yancy-Spurgeon (Southeast Renal Research Institute); D. Linfert, A. Fortner, J. Giese, and S. Meier (Nephrology Associates); G. Schulman and J. Zirchenbach (Vanderbilt University); P. Van Buren, J. Inrig, T. Tyler, and T. Lightfoot (University of Texas Southwestern Medical Center); A. Basford, S. Fadem, and N. Dickson (Kidney Specialists of North Houston); A. Frome and B. Armentrout, (East Central Dialysis Center); J. Olivero and F. Ricks (Med Center Dialysis); J. Abraham, K. Raphael, and J. Zitterkoph (University of Utah); K. Bolton and N. Mchedlishviii (University of Virginia Health System); A. Assefi and R. Dadmarz (Nephrology Associates of Northern Virginia); R. Cheriyan and M. Obeid (Virginia Nephrology Group); O. Ayodeji and L. Jones-Brandon (Peninsula Kidney Associates); G. Feldman and M. Nicholas (VA Medical Center); D. Negoi and D. de Waal (Fletcher Allen Health Care); S. Blumenthal and C. Veenendaal (University of Wisconsin) Y. Yagil and D. Pinhas (The Barzilai Medical Center); D. Schwartz and N. Platner (Sourasky Medical Center).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014020212/-/DCSupplemental.
References
- 1.Delmez JA, Slatopolsky E: Hyperphosphatemia: Its consequences and treatment in patients with chronic renal disease. Am J Kidney Dis 19: 303–317, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umanath K, Blumenthal SS, Koury M, Sika M, Greco BA, Jalal DI, Reisin E, Manley J, Zeig S, Negoi DG, Hiremath AN, Lewis JB, Dwyer JP, for the Collaborative Study Group: Ferric citrate as a phosphate binder reduces IV iron and erythropoiesis stimulating agent (ESA) use. Presented at the American Society of Nephrology Kidney Week, Atlanta, GA, November 7, 2013 [Google Scholar]
- 4.Dwyer JP, Sika M, Schulman G, Chang IJ, Anger M, Smith M, Kaplan M, Zeig S, Koury MJ, Blumenthal SS, Lewis JB, Collaborative Study Group : Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: A short-term randomized trial. Am J Kidney Dis 61: 759–766, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Hsu CH, Patel SR, Young EW: New phosphate binding agents: Ferric compounds. J Am Soc Nephrol 10: 1274–1280, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Sika M, Koury M, Smith M, Greene T, Sinsakul MV, Korbet S, Lewis JB, for the Collaborative Study Group: Evaluation of ferric citrate as a phosphate binder in dialysis patients requiring high doses of phosphate binders. Presented at the American Society of Nephrology Renal Week, San Diego, CA, October 29, 2009 [Google Scholar]
- 7.Sika M, Sinsakul MV, Niecestro RM, Chiang S: Prolonged use of ferric citrate (FC) as a phosphate binder reduces IV iron use in patients with ESRD. Presented at the American Society of Nephrology Renal Week, Denver, CO, November 20, 2010 [Google Scholar]
- 8.Sinsakul M, Sika M, Koury M, Shapiro W, Greene T, Dwyer J, Smith M, Korbet S, Lewis J, Collaborative Study Group : The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract 121: c25–c29, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Umanath K, Sika M, Niecestro R, Connelly C, Schulman G, Koury MJ, Lewis JB, Dwyer JP, Collaborative Study Group : Rationale and study design of a three-period, 58-week trial of ferric citrate as a phosphate binder in patients with ESRD on dialysis. Hemodial Int 17: 67–74, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH: An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: Ferric citrate. Nephrol Dial Trasplant 17: 265–270, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y: Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: Results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36: 478–487, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Molony DA, Murthy B: Accumulation of metals and minerals from phosphate binders. Blood Purif 23[Suppl 1]: 2–11, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Finn WF, SPD 405-307 Lanthanum Study Group : Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: Safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol 65: 191–202, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Fresenius Medical Care North America : PhosLo (Package Insert), Waltham, MA, Fresenius Medical Care North America, 2007 [Google Scholar]
- 15.Genzyme Corporation : Renvela (Package Insert). Cambridge, MA, Genzyme Corporation, 2010 [Google Scholar]
- 16.Shire US Inc. : Fosrenol (Package Insert). Wayne, PA, Shire US Inc., 2012 [Google Scholar]
- 17.Moriniere P, Vinatier I, Westeel PF, Cohemsolal M, Belbrik S, Abdulmassih Z, Hocine C, Marie A, Leflon P, Roche D, Fournier A: Magnesium hydroxide as a complementary aluminium-free phosphate binder to moderate doses of oral calcium in uraemic patients on chronic haemodialysis: Lack of deleterious effect on bone mineralisation. Nephrol Dial Trasplant 3: 651–656, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Sevelamer reduces the efficacy of many other drugs. Prescrire Int 18: 164–165, 2009 [PubMed] [Google Scholar]
- 19.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Skikne BS, Ahluwalia N, Fergusson B, Chonko A, Cook JD: Effects of erythropoietin therapy on iron absorption in chronic renal failure. J Lab Clin Med 135: 452–458, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Sargent JA, Acchiardo SR: Iron requirements in hemodialysis. Blood Purif 22: 112–123, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Streja E, Miller JE, Nissenson AR: Intravenous iron versus erythropoiesis-stimulating agents: Friends or foes in treating chronic kidney disease anemia? Adv Chronic Kidney Dis 16: 143–151, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Besarab A, Coyne DW: Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol 6: 699–710, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Fudin R, Jaichenko J, Shostak A, Bennett M, Gotloib L: Correction of uremic iron deficiency anemia in hemodialyzed patients: A prospective study. Nephron 79: 299–305, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Macdougall IC, Tucker B, Thompson J, Tomson CR, Baker LR, Raine AE: A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 50: 1694–1699, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Fuller DS, Pisoni RL, Bieber BA, Gillespie BW, Robinson BM: The DOPPS practice monitor for US dialysis care: Trends through December 2011. Am J Kidney Dis 61: 342–346, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Rambod M, Kovesdy CP, Kalantar-Zadeh K: Combined high serum ferritin and low iron saturation in hemodialysis patients: The role of inflammation. Clin J Am Soc Nephrol 3: 1691–1701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, Kopelman RC, Dahl NV, Coyne DW: Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol 19: 372–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR, DRIVE Study Group : Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Rodby R, Umanath K, Hsieh A, Niecestro R, Lewis J, Dwyer J: Phosphorus binding with ferric citrate reduces erythropoiesis-stimulating agent (ESA) and IV iron usage and cost in patients with ESRD. Presented at the National Kidney Foundation Spring Clinical Meetings, Las Vegas, NV, April 23, 2014 [Google Scholar]
- 31.Bansal A, Sandhu G, Gupta I, Kalahalli S, Nayak R, Zouain E, Chitale RA, Meisels I, Chan G: Effect of aggressively driven intravenous iron therapy on infectious complications in end-stage renal disease patients on maintenance hemodialysis [published online ahead of print July 23, 2012]. Am J Ther [DOI] [PubMed] [Google Scholar]
- 32.USRDS : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 33.Fleming RE, Ponka P: Iron overload in human disease. N Engl J Med 366: 348–359, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Dmitrienko A, Offen WW, Westfall PH: Gatekeeping strategies for clinical trials that do not require all primary effects to be significant. Stat Med 22: 2387–2400, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.