Abstract
Patients with Gitelman syndrome (GS), an inherited salt-losing tubulopathy, are usually treated with potassium-sparing diuretics or nonsteroidal anti-inflammatory drugs and oral potassium and magnesium supplementations. However, evidence supporting these treatment options is limited to case series studies. We designed an open-label, randomized, crossover study with blind end point evaluation to compare the efficacy and safety of 6-week treatments with one time daily 75 mg slow-release indomethacin, 150 mg eplerenone, or 20 mg amiloride added to constant potassium and magnesium supplementation in 30 patients with GS (individual participation: 48 weeks). Baseline plasma potassium concentration was 2.8±0.4 mmol/L and increased by 0.38 mmol/L (95% confidence interval [95% CI], 0.23 to 0.53; P<0.001) with indomethacin, 0.15 mmol/L (95% CI, 0.02 to 0.29; P=0.03) with eplerenone, and 0.19 mmol/L (95% CI, 0.05 to 0.33; P<0.01) with amiloride. Fifteen patients became normokalemic: six with indomethacin, three with eplerenone, and six with amiloride. Indomethacin significantly reduced eGFR and plasma renin concentration. Eplerenone and amiloride each increased plasma aldosterone by 3-fold and renin concentration slightly but did not significantly change eGFR. BP did not significantly change. Eight patients discontinued treatment early because of gastrointestinal intolerance to indomethacin (six patients) and hypotension with eplerenone (two patients). In conclusion, each drug increases plasma potassium concentration in patients with GS. Indomethacin was the most effective but can cause gastrointestinal intolerance and decreased eGFR. Amiloride and eplerenone have similar but lower efficacies and increase sodium depletion. The benefit/risk ratio of each drug should be carefully evaluated for each patient.
Keywords: Gitelman syndrome, diuretics, distal tubule
Gitelman syndrome (GS; Mendelian Inheritance in Man 263800) is a rare inherited autosomal recessive salt-losing tubulopathy characterized by hypokalemic metabolic alkalosis, hypomagnesemia, hypocalciuria, and secondary hyperaldosteronism.1 GS has a prevalence of heterozygotes of approximately 1% and an estimated prevalence of homozygotes of approximately 1 in 40,000 in Caucasians.2 It is mainly related to loss-of-function mutations in the SLC12A3 gene encoding for the apically expressed thiazide-sensitive NaCl cotransporter (NCC) of the distal convoluted tubule (DCT).2
The natural history of GS is highly variable in terms of age at clinical diagnosis, biologic phenotype, and severity of clinical manifestations. However, severe hypokalemic rhabdomyolysis or ventricular arrhythmias may occur when acute additional extrarenal potassium and magnesium losses occur (vomiting or diarrhea).3–7
Lifelong oral potassium and magnesium supplementation is the cornerstone of treatment aiming at relieving symptoms and preventing severe hypokalemia-related complications, including cardiac arrhythmias.8 However, oral supplementation may be insufficient to fully correct the electrolyte disorders or may have poor gastrointestinal (GI) tolerability, which can limit dosing.9,10 In the case of persistent symptomatic hypokalemia or intolerance to supplementation, potassium-sparing diuretics, such as amiloride, or mineralocorticoid receptor (MR) antagonists (spironolactone or eplerenone) usually constitute the first therapeutic option.11–14 Another option is low-dose indomethacin, a nonselective inhibitor of cyclooxygenase 1 (COX-1) and COX-2.11,15 However, evidence is limited for all these treatment options, and no randomized controlled trial has so far compared their efficacy, tolerability, or safety in patients with GS.
We designed an open-label, randomized, crossover study to compare the efficacy on plasma potassium concentration and tolerability of slow-release indomethacin, amiloride, and eplerenone in patients with type I GS treated with stable potassium and magnesium supplementation.
Results
Baseline characteristics of 30 patients (17 women) randomized in the study are shown in Table 1; all had typical GS with low BP, hypokalemia, and hypomagnesemia with inappropriate urine potassium and magnesium excretion, low urine calcium excretion, high plasma renin, and normal plasma aldosterone concentrations. Supplemental Figure 1 shows the reasons for exclusion or study drug discontinuation, the number of patients randomized to each treatment sequence, and the number completing the study.
Table 1.
Baseline characteristics of 30 randomized patients and the subpopulation of 22 patients who received all study drugs
| Randomized Patients | Patients Who Received All Study Drugs | P Value | |
|---|---|---|---|
| n | 30 | 22 | |
| Age, yr | 39.9±13.5 | 40.6±12.4 | 0.79 |
| Body weight, kg | 66.8±13.2 | 68.6±13.2 | 0.67 |
| Body mass index, kg/m2 | 24.3±4.1 | 24.4±4.1 | 0.86 |
| Systolic BP, mmHg | 113±9 | 113±9 | 0.67 |
| Heart rate, beats/min | 67±10 | 66±10 | 0.92 |
| Plasma potassium, mmol/L | 2.8±0.4 | 2.8±0.5 | >0.99 |
| Plasma magnesium, mmol/L | 0.53±0.08 | 0.52±0.07 | 0.84 |
| Plasma sodium, mmol/L | 140.0±1.2 | 139.9±1.2 | 0.91 |
| Plasma creatinine, µmol/L | 57.7±14.6 | 63.6±17.0 | 0.80 |
| eGFR, ml/min per 1.73 m2 | 124±33 | 123±32 | 0.96 |
| Plasma renin concentration, mU/L | 89 (60 to 155) | 98 (49 to 162) | 0.99 |
| Plasma aldosterone, pg/ml | 44 (28 to 87) | 51 (28 to 87) | 0.88 |
| Urine sodium excretion, mmol/24 h | 186 [148–214] | 177 [148–211] | 0.68 |
| Urine potassium excretion, mmol/24 h | 108 [91–142] | 108 [91–134] | 0.86 |
| Urine magnesium excretion, mmol/24 h | 2.0 [0.9–3.3] | 2.8 [1.0–3.8] | 0.59 |
| Urine calcium excretion, mmol/24 h | 1.10 [0.49–1.85] | 1.21 [0.75–1.85] | 0.51 |
Data are mean±SD, geometric mean (95% CI), or median [interquartile range]. P values were by unpaired t test. Normal values for plasma renin concentration are 20.8 mU/L (95% CI, 17.7 to 24.4) in men and 16.2 mU/L (95% CI, 13.6 to 19.2) in women. Normal values for plasma aldosterone concentration are 45 pg/ml (95% CI, 39 to 52) in men and 34 pg/ml (95% CI, 28 to 40) in women. mU/L, milli-international units per liter.
Tolerability
Indomethacin caused GI intolerance (heartburn, dyspepsia, or gastric irritation) in six patients, resulting in early discontinuation. Eplerenone (150 mg once a day [o.d.].) was discontinued in two other patients for palpitations and dizziness (Supplemental Figure 1). The 20-mg o.d. dose of amiloride was downtitrated to 10 mg o.d. in two patients and 15 mg o.d. in another two patients for palpitations and dizziness. Thus, 22 of 30 patients actually received all three study drugs. Their baseline characteristics were similar to those of the randomized population of 30 patients (Table 1).
Effects of Indomethacin, Eplerenone, and Amiloride on Electrolytic and Hormonal Parameters and GFR
All parameters returned to their respective baseline and pretreatment levels after the 6-week washout periods (Supplemental Table 1). Therefore, only treatment effects are reported for the on-treatment population who actually received all three study drugs (n=22 of 30). Treatment effects analyzed by an ANOVA for a crossover design were significant for the following parameters: plasma potassium, creatinine, aldosterone and renin concentrations, and urine magnesium excretion. Two-by-two comparisons are shown below.
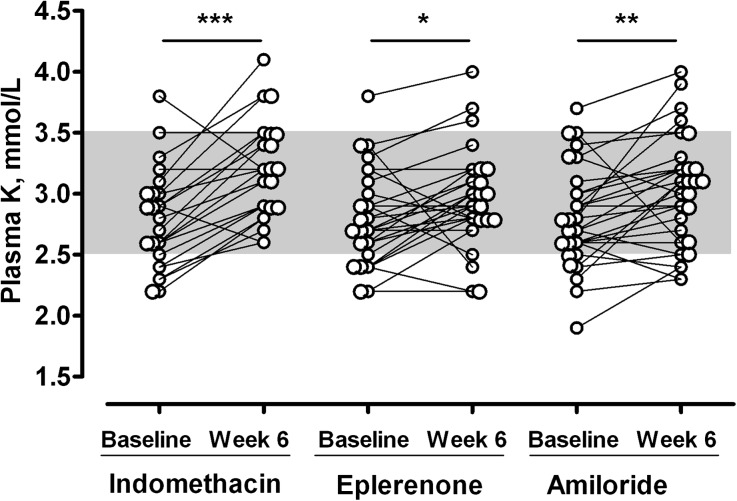
Indomethacin significantly increased plasma potassium by 0.34 mmol/L (95% confidence interval [95% CI], 0.20 to 0.48 mmol/L) compared with the control period (n=22, P<0.001) (Table 2) but did not significantly change plasma magnesium or sodium concentration (Table 2). When using the whole dataset for the indomethacin period (n=24), the net potassium increase from baseline was 0.38 mmol/L (95% CI, 0.23 to 0.53; P<0.001 versus baseline), and 6 of 24 (25%) patients normalized their plasma potassium concentration (≥3.5 mmol/L) (Figure 1). As expected, indomethacin markedly reduced plasma renin concentration (n=22; P<0.001) (Table 2) but did not change plasma aldosterone concentration (n=22; P>0.99) (Table 2). Compared with the control period, eGFR significantly decreased by 10.0 ml/min per 1.73 m2 (95% CI, 4.2 to 15.9 ml/min per 1.73 m2; n=22; P<0.01) (Table 2) after the 6-week treatment with indomethacin. Urine potassium, magnesium, and sodium excretion did not significantly change (Table 2).
Table 2.
Effects of 6-week treatments with 75 mg o.d. slow-release indomethacin, 150 mg o.d. eplerenone, or 20 mg o.d. amiloride in 22 patients with genetically proven GS type I who received all three treatments
| Parameters | Control Period (Week 6) | Indomethacin (Week 6) | Eplerenone (Week 6) | Amiloride (Week 6) | ANOVA (P Value) |
|---|---|---|---|---|---|
| Body weight, kg | 69.3±13.1 | 70.3±13.4a | 68.5±12.6b | 68.7±13.1b | <0.001 |
| Systolic BP, mmHg | 115±9 | 116±11 | 115±11 | 114±10 | 0.24 |
| Heart rate, beats/min | 71.8±10.1 | 73.5±7.6 | 75.8±8.0 | 75.6±9.7 | 0.07 |
| Plasma potassium, mmol/L | 2.8±0.4 | 3.2±0.4c | 3.0±0.5d | 3.0±0.5 | <0.01 |
| Plasma magnesium, mmol/L | 0.54±0.09 | 0.56±0.07 | 0.56±0.13 | 0.58±0.08 | 0.59 |
| Plasma sodium, mmol/L | 140.0±1.6 | 139.1±1.4 | 138.6±1.5 | 138.5±1.6 | 0.17 |
| Plasma creatinine, µmol/L | 61.7±15.1 | 66.6±17.6e | 63.1±15.6 | 64.8±16.4 | 0.03 |
| eGFR, ml/min per 1.73 m2 | 126±28 | 115±25e | 122±24 | 119±28 | 0.05 |
| Plasma renin concentration, mU/L | 90 (61 to 133) | 47 (32 to 69)c | 113 (76 to 167)b | 112 (76 to 166)b | <0.001 |
| Plasma aldosterone, pg/ml | 45 (33 to 62) | 42 (30 to 59) | 131 (94 to 183)b,c | 155 (107 to 124)b,c | <0.001 |
| Urine sodium excretion, mmol/24 h | 187 [137–231] | 187 [127–207] | 186 [143–273] | 193 [136–255] | 0.50 |
| Urine potassium excretion, mmol/24 h | 107 [93–141] | 123 [89–134] | 104 [90–129] | 104 [94–137] | 0.95 |
| Urine magnesium excretion, mmol/24 h | 2.1 [1.0–3.3] | 3.1 [1.4–3.7] | 3.0 [1.5–4.6] | 1.7 [0.8–3.3]a,d | 0.02 |
Data are mean±SD, geometric mean (95% CI), or median [interquartile range]. ANOVA is P value for the treatment effect in ANOVA for a crossover study. Normal values for plasma renin concentration are 20.8 mU/L (95% CI, 17.7 to 24.4) in men and 16.2 mU/L (95% CI, 13.6 to 19.2) in women. Normal values for plasma aldosterone concentration are 45 pg/ml (95% CI, 39 to 52) in men and 34 pg/ml (95% CI, 28 to 40) in women. mU/L, milli-international units per liter.
P<0.05 versus control period.
P<0.001 versus indomethacin.
P<0.001 versus control period.
P<0.05 versus indomethacin.
P<0.01 versus control period.
Figure 1.
Effect of 6-week treatments with 75 mg o.d. slow-release indomethacin, 150 mg o.d. eplerenone, or 20 mg o.d. amiloride on plasma potassium concentration in patients with genetically proven GS type I. All datasets of valid periods of treatments for indomethacin (n=24), eplerenone (n=28), and amiloride (n=30) are used to connect plasma potassium values under treatment with corresponding pretreatment values. *p< 0.05; **p<0.01; ***p<0.001.
Eplerenone increased plasma potassium by 0.13 mmol/L (95% CI, −0.01 to 0.27 mmol/L) compared with the control period (n=22; P=0.41) (Table 2). When using the whole dataset for the eplerenone period (n=28), the net potassium increase from baseline was 0.15 mmol/L (95% CI, 0.02 to 0.29; P=0.03 versus baseline), and 3 of 28 (11%) patients normalized their plasma potassium concentration (Figure 1). Eplerenone did not change plasma magnesium concentration and slightly decreased plasma sodium concentration (n=22) (Table 2). It markedly increased plasma aldosterone concentration compared with baseline (n=22; P<0.001) (Table 2), but the increase in plasma renin concentration was not significant (n=22) (Table 2). eGFR and urine potassium, magnesium, and sodium excretion did not change significantly (Table 2).
Amiloride increased plasma potassium by 0.18 mmol/L (95% CI, 0.04 to 0.32 mmol/L) compared with the control period (n=22; P=0.09) (Table 2). When using the whole dataset for the amiloride period (n=30), the net potassium increase was 0.19 mmol/L (95% CI, 0.05 to 0.33; P<0.01 versus baseline), and 6 of 30 (20%) patients normalized their plasma potassium concentration (Figure 1). Amiloride increased plasma magnesium concentration and decreased plasma sodium concentration slightly (n=22) (Table 2). As expected, it markedly increased plasma aldosterone concentrations compared with the control period (n=22; P<0.001) (Table 2), but the increase in plasma renin concentration was not significant (Table 2). Compared with the control period, eGFR slightly decreased by 6.4 ml/min per 1.73 m2 (95% CI, −0.54 to 12.20 ml/min per 1.73 m2; P=0.20) (Table 2) after the 6-week treatment with amiloride. Urine potassium and sodium excretion did not change significantly, but magnesium excretion significantly decreased (n=22) (Table 2).
The increase in plasma potassium concentration was significantly greater after the 6-week treatment with indomethacin than eplerenone (increase of 0.21 mmol/L; 95% CI, 0.07 to 0.35 mmol/L; P=0.03; n=22) but not amiloride (P=0.15; n=22) (Figure 1, Table 2).
There was no significant difference between amiloride and eplerenone (P>0.99) (Figure 1, Table 2).
There was no correlation between the changes in plasma potassium concentration in the three possible pairs of treatment responses (Supplemental Figure 2). In patients who received all three treatments (n=22), 16 (73%) patients were responders (increase of at least 0.3 mmol/L in plasma potassium concentration) to at least one of three treatments; 6 of 22 patients (27%) had no response to any of the three drugs.
Among 15 intermediate responders, nonresponders, or patients intolerant to indomethacin, 6 patients became full responders by switching to amiloride (n=3) or eplerenone (n=3). Among 22 intermediate responders or nonresponders to amiloride, 13 patients became full responders by switching to one of the other drugs (indomethacin alone, n=3; eplerenone alone, n=2; both treatments, n=8). Among 19 intermediate responders, nonresponders, or patients intolerant to eplerenone, 10 patients became full responders to one of the other drugs (indomethacin alone, n=3; amiloride alone, n=3; both treatments, n=4).
Effects of Treatments on BP, Heart Rate, Body Weight, and Quality of Life
The slight changes in systolic/diastolic BP (decrease with the two potassium-sparing diuretics and increase with indomethacin) were as expected and nonsignificant (Table 2). The changes in body weight were as expected (Table 2).
None of the treatments significantly improved or worsened quality of life (Supplemental Table 3).
Discussion
The results of this crossover trial showed that the moderate-to-severe hypokalemia of patients with genetically proven GS was corrected in the short term but only partially by 75 mg/d slow-release indomethacin, 20 mg/d amiloride, or 150 mg/d eplerenone given for 6 weeks combined with a constant oral potassium and magnesium supplementation. At the selected doses, indomethacin was more effective in increasing mean plasma potassium concentration than amiloride or eplerenone, which were of similar efficacy. The effects of the treatments on plasma magnesium concentration were either negligible (amiloride) or absent (indomethacin and eplerenone). As expected, indomethacin and the two diuretics had opposite effects on plasma renin and aldosterone concentrations as well as the slight treatment-induced BP changes. eGFR significantly decreased with indomethacin and less so with amiloride but did not change with eplerenone. Clinical tolerability of the drugs was inversely related to their efficacy, with indomethacin being the least tolerated because of GI intolerance leading to early discontinuation in 6 of 30 (20%) patients, despite coprescription of a proton pump inhibitor.
The crossover nature of the study design with rotation of the three drugs showed that 40% of patients who were intermediate responders, nonresponders, or patients intolerant to indomethacin became full responders by switching to amiloride or eplerenone. Thus, 73% of patients who tolerated the three treatments were responders to at least one of three treatments.
Our primary end point was treatment-induced increase in plasma potassium concentration in patients with GS. Hypokalemia is associated with the intensity of musculoskeletal symptoms and the occurrence of more severe complications, and it has previously been shown to respond to each of three study drugs in preliminary reports.11,15,16 Hypokalemia in patients with GS is caused by secondary consequences of the inherited inactivation of NCC in the proximal DCT, including increased sodium delivery to late DCT and cortical connecting duct and secondary renin-dependent hyperaldosteronism triggered by sodium depletion. This biologic and hormonal phenotype mimics the electrolytic consequences of chronic administration of thiazide diuretics, which specifically block NCC in the kidney.12–14 The rationale behind administering the epithelial sodium channel inhibitor amiloride and the MR antagonist eplerenone is, thus, to neutralize the compensatory mechanisms in the distal nephron that are responsible for hypokalemia in patients with GS.
The dose of 20 mg amiloride has previously been shown to reverse the hydrochlorothiazide-induced hypokalemia and increase plasma magnesium concentration more effectively than 100 mg spironolactone in healthy volunteers.17 Because eplerenone is approximately 75% less potent than spironolactone,18 the selected dose was 150 mg on the basis of the healthy volunteer study mentioned above.17 Eplerenone was uptitrated weekly from 50 to 100 mg o.d. and then, to the maximum dose of 150 mg o.d. administered for 4 weeks. A 6-week administration of either 20 mg o.d. amiloride or 150 mg o.d. eplerenone was equally effective in increasing plasma potassium concentration. However, amiloride and eplerenone normalized plasma potassium concentration in only 20% and 11% of the patients, respectively, when given combined with potassium and magnesium supplementation. Amiloride slightly increased plasma magnesium concentration and significantly reduced urine magnesium excretion, indicating the stimulation of tubular magnesium reabsorption. This observation is in line with a previous report in healthy volunteers.17 It is not known whether a higher dose of amiloride or eplerenone could be more effective in correcting hypokalemia in patients with GS, but this option would be limited by tolerability. Indeed, the use of amiloride or eplerenone is, in part, paradoxical, because both drugs may worsen sodium depletion and hypovolemia in these patients with a salt-losing nephropathy.8 In fact, both drugs induced a marked increase in plasma aldosterone concentrations secondary to (1) diuretic-induced sodium depletion associated with a mild decrease in systolic BP (approximately 2 mmHg) and eGFR (amiloride only), although the rise in plasma renin was nonsignificant, and (2) partial correction of hypokalemia. The progressive titration of amiloride and eplerenone to their highest respective doses may explain the satisfactory clinical and biologic tolerability of the two drugs. Amiloride was downtitrated in only two patients, and eplerenone was discontinued in only two patients for worsened hypovolemia. Whether additional MR activation by high aldosterone concentrations triggered by amiloride may contribute to long-term cardiovascular fibrosis and remodeling and renal fibrosis in patients with a salt-losing tubulopathy is unknown. Indeed, aldosterone has a number of nonclassic, MR-mediated actions, such as tissue remodeling, modulation of vascular tone, and stimulation of inflammation and fibrosis, including the kidney, in the setting of high salt intake.19 Such effects would be neutralized by the use of the MR antagonist eplerenone.
We also investigated whether the nonselective COX-1 and COX-2 inhibitor, indomethacin, would increase plasma potassium concentration without worsening sodium and volume depletion in patients with GS. By inhibiting prostaglandin E2 (PGE2) synthesis in the kidney,20 indomethacin corrects hypokalemia in patients with Bartter syndrome, another salt-losing tubulopathy caused by mutations in genes coding for proteins responsible for salt reabsorption in the loop of Henle.21 The dosage regimen (75 mg) was on the basis of the low indomethacin doses (approximately 1 mg/kg) currently used in Bartter syndrome21 and preliminary reports in GS.15 We showed that a 6-week administration of 75 mg o.d. slow-release indomethacin significantly increased plasma potassium concentration from baseline but had no effect on plasma magnesium concentration in patients with GS. This result suggests a role of renal PGE2 in the pathogenesis of GS as shown for loop disorders.21 The net potassium increase after 6 weeks of treatment with indomethacin was greater, although nonsignificantly, than that achieved with amiloride or eplerenone.
In contrast to amiloride and eplerenone, indomethacin decreased plasma renin concentration. Indeed, indomethacin is known to inhibit renin secretion by inhibition of PGE2 synthesis in the juxtaglomerular apparatus.20 Indomethacin blunted the expected physiologic increase in plasma aldosterone concentration in response to the increase in plasma potassium concentration. Moreover, indomethacin has been shown experimentally to inhibit the diuretic effect of thiazides and stimulate sodium reabsorption in the loop of Henle.22 Accordingly, systolic BP increased slightly by approximately 2 mmHg, suggesting that the potassium-sparing effect of indomethacin was associated with a slight correction of hypovolemia. In summary, the effect of indomethacin in patients with GS may be caused by (1) the increase in loop NaCl reabsorption, leading, in turn, to a decrease in NaCl delivery to DCT,23 and (2) the inhibition of renin release by the juxtaglomerular apparatus as observed in rats treated with hydrochlorothiazide.24
Indomethacin normalized plasma potassium concentration in only 25% of the patients who tolerated the drug. A titration of indomethacin dosage by monitoring the effect on plasma renin concentration or urinary excretion rates of prostaglandins may have improved efficacy and/or tolerability of indomethacin. However, whether a higher dose of indomethacin would have been more effective in correcting hypokalemia in these patients is not known,15 but the use of slow-release indomethacin (even at the low dose of 75 mg o.d.) for only 6 weeks had limited tolerability. First, eGFR decreased by 10.0 ml/min per 1.73 m2, despite the mild increase in systolic BP after 6 weeks of treatment. The indomethacin-induced decrease in eGFR was slightly greater than that observed with amiloride, and both decreases were reversible when the drugs were stopped. This result is probably related to the specific effects of COX-2 inhibition on renal hemodynamics, which are specifically enhanced in sodium- and volume-depleted patients with GS.25,26 The risk of a more marked decrease in GFR may be increased with longer-term indomethacin use in the setting of (1) additional extrarenal sodium loss or (2) drug-induced interstitial nephritis.27–30 Second, six patients suffered from GI intolerance related to the indomethacin-induced inhibition of COX-1 at the level of the GI mucosa, leading to early discontinuation, despite coprescription of a proton pump inhibitor. The risk of severe GI adverse events is increased with long-term use of nonsteroidal anti-inflammatory drugs, including indomethacin.31,32 Third, there may also be a theoretical increased risk of cardiovascular events with prolonged exposure to the drug.32 However, this risk is related to the degree of COX-2 inhibition33 and thus, remains probably low at a dose of 75 mg o.d. slow-release indomethacin. In addition, chronic use of a proton pump inhibitor could worsen magnesium depletion by decreasing net intestinal absorption of magnesium.34
The principle limitations of this study were (1) the short duration of exposure to treatments, which may not have been sufficient to fully assess tolerability and sustained efficacy for a chronic disease such as GS; (2) the selected doses of the three drugs, which may have not been maximal to fully assess efficacy; (3) the lack of consideration of other treatment options, such as renin-angiotensin system blockers (however, increasing the doses of each study drug or using a renin-angiotensin system blocker would have been limited by tolerability in these sodium- and volume-depleted patients); and (4) the lack of consideration of sodium chloride supplementation as a more physiologic way to decrease renin and aldosterone secretion. However, extending the duration of each active period or increasing the number of periods would have increased the risk of loss to follow-up of patients, who were already included in this crossover study for 38–42 weeks. Finally, another option would have been to carry out a long-term parallel group study, but this option would have required more patients to include and follow-up, a difficult task in patients with a rare disease, such as GS.
In conclusion, this study is the first and largest randomized controlled study performed in patients with GS providing evidence of moderate efficacy of three treatments currently used on the basis of limited case series. Although the three drugs have different mechanisms of action, they all increase plasma potassium concentration on the short term, which however, is normalized in a minority (20%–25%) of patients with GS.
Because we did not specifically address the long-term benefits and risks with the three treatments used in our study, these treatments still remain the last resort in patients with GS who remain with severe or symptomatic hydroelectrolytic abnormalities, despite potassium, magnesium, and possibly, salt supplementation at optimally titrated and tolerated doses. The decision should also take into consideration the clinical setting (e.g., the need for nonsteroidal anti-inflammatory drugs for chondrocalcinosis), the specific contraindications of each drug, and the patient's informed choice. BP and plasma potassium, magnesium, and creatinine concentrations need to be monitored to accurately assess efficacy and tolerability. Patients should be informed of the possibility of renal failure or hyperkalemia occurring with long-term treatment during episodes of dehydration or with concomitant use of potentially nephrotoxic and/or hyperkalemic drugs. Additional studies are needed to address the long-term efficacy and tolerability of these treatment options.
Concise Methods
Detailed methods are given in Supplemental Material.
Participants
Eligible patients were men and women (18–60 years old) diagnosed with genetically proven GS at five tertiary French hospitals. All patients gave written informed consent before participating in the study. The protocol (ClinicalTrials.gov Identifier: NCT01146197) was approved by the Comité de Protection des Personnes, Paris-Île de France III, France and adhered to the Declaration of Helsinki.
Design of the Study
This study was a seven-period, three-treatment, open-label, randomized, crossover study with blind end point evaluation. The initial baseline assessments were performed after a 2- to 6-week washout period to stop any COX inhibitor (2 weeks) or potassium-sparing diuretic (6 weeks) effects. Patients were then randomly assigned to sequentially receive slow-release indomethacin (75 mg for 6 weeks) combined with a proton pump inhibitor (omeprazole at 20 mg/d) or eplerenone (uptitrated weekly from 50 to 100 mg o.d. and then, to the maximum dose of 150 mg o.d. administered for 4 weeks) or amiloride (uptitrated weekly from 10 to 15 mg o.d. and then, to the maximum dose of 20 mg o.d. administered for 4 weeks) in addition to oral potassium and magnesium supplementation.
Randomization
The randomization sequence was generated by a computer without stratification using randomized blocks of small size and permutation of treatments within each block. Investigators, patients, and research staff were blinded to the randomization list.
Study Evaluations
Biochemical, hormonal, hemodynamic, and safety assessments were performed after each 6-week washout and active treatment periods. Blood was sampled at approximately 9:00 a.m. in fasting conditions after the patient rested for 1 hour in a semirecumbent position. Seated BP and heart rate were measured at home with a validated electronic device (OMRON M6; Omron Co., Kyoto, Japan) as described previously.35 In addition, we assessed quality of life using the Short Form Health Survey self-administered questionnaire adapted for the French population36 at randomization and the end of each active treatment period.
Laboratory Methods
Biochemical and hormone measurements were performed blind to the randomization sequence in a centralized laboratory. Plasma and urinary electrolytes and creatinine were measured as described previously.37 eGFR was calculated by the Modification of Diet in Renal Disease formula.38 Plasma renin and aldosterone concentrations were measured as described previously.39
Statistical Analyses
The primary objective of the study was to determine whether slow-release indomethacin would significantly increase the plasma potassium concentration compared with the control period, which was defined as the first 6-week period after the 2- to 6-week washout period. Using a within-patient SD of 0.3 mmol/L, we calculated that 30 patients were needed to detect a 0.3-mmol/L difference in plasma potassium concentration between the indomethacin and control periods with 80% power and 5% α-error for a two-tailed test.
Because eight patients had to stop one of the study drugs for intolerance, leading to a missing period for each of these patients (see Results), data were analyzed by an ANOVA for a crossover design on the on-treatment population who actually received all three study drugs (n=22 of 30).40 The model included treatment, sequence, and period as fixed effects and subject nested within sequence as the random effect. When the F test was significant (P<0.05) and there was no period, sequence, or carryover effect, paired comparisons were made between treatments by the Holm method.41
We also performed an analysis restricted to main criteria, i.e., plasma potassium concentration, using paired t tests comparing 6-week on-treatment versus pretreatment values on all datasets of valid periods of treatments.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Prof. P. Houillier at the Georges Pompidou Hospital for helping with patient recruitment. We thank the patients for participating in the study. We thank the nursing staff, Jeanne Meunier, and Valérie Paquet of the Clinical Investigation Center of Georges Pompidou Hospital, the nursing staff and Debohra Postil of the Clinical Investigation Center of Limoges, and the nursing staff, Pierre Jean Saulnier, and Elodie Rogeon of the Clinical Investigation Center of Poitiers. We thank Chantal Andrieux from the Clinical Research Unit for helping us with regulatory processes.
This work was supported by French Ministry of Health and Assistance Publique des Hôpitaux de Paris Grant PHRCAOM08193. A.B. was the recipient of a “Contratd’Interface” Grant from Institut National de la Santé et de la Recherche Médicale.
The funding sources had no involvement in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030293/-/DCSupplemental.
References
- 1.Knoers NV, Levtchenko EN: Gitelman syndrome. Orphanet J Rare Dis 3: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Malafronte C, Borsa N, Tedeschi S, Syrèn ML, Stucchi S, Bianchetti MG, Achilli F, Bettinelli A: Cardiac arrhythmias due to severe hypokalemia in a patient with classic Bartter disease. Pediatr Nephrol 19: 1413–1415, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Qayyum M, Farooq F: Quadriparesis in an adult—Gitelman syndrome. J Pak Med Assoc 61: 182–184, 2011 [PubMed] [Google Scholar]
- 5.Scognamiglio R, Negut C, Calò LA: Aborted sudden cardiac death in two patients with Bartter’s/Gitelman’s syndromes. Clin Nephrol 67: 193–197, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Slyper AH: Growth, growth hormone testing and response to growth hormone treatment in Gitelman syndrome. J Pediatr Endocrinol Metab 20: 257–259, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Pachulski RT, Lopez F, Sharaf R: Gitelman’s not-so-benign syndrome. N Engl J Med 353: 850–851, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Park PG, Jr, Karet Frankl FE: Gitelman syndrome. BMJ 344: e3590–851, 2012 [DOI] [PubMed] [Google Scholar]
- 9.McMahon FG, Ryan JR, Akdamar K, Ertan A: Upper gastrointestinal lesions after potassium chloride supplements: A controlled clinical trial. Lancet 2: 1059–1061, 1982 [DOI] [PubMed] [Google Scholar]
- 10.Peters M, Jeck N, Reinalter S, Leonhardt A, Tönshoff B, Klaus G G, Konrad M, Seyberth HW: Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med 112: 183–190, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Monnens L, Bindels R, Grünfeld JP: Gitelman syndrome comes of age. Nephrol Dial Transplant 13: 1617–1619, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Yoshida M, Nakayama M, Tsutaya S, Ogawa K, Maeda H, Miyata M, Oiso Y: Eplerenone improved hypokalemia in a patient with Gitelman’s syndrome. Intern Med 51: 83–86, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Morton A: Eplerenone in the treatment of Gitelman’s syndrome. Intern Med J 38: 377, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Morton A, Panitz B, Bush A: Eplerenone for gitelman syndrome in pregnancy. Nephrology (Carlton) 16: 349, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Liaw LC, Banerjee K, Coulthard MG: Dose related growth response to indometacin in Gitelman syndrome. Arch Dis Child 81: 508–510, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colussi G, Rombolà G, De Ferrari ME, Macaluso M, Minetti L: Correction of hypokalemia with antialdosterone therapy in Gitelman’s syndrome. Am J Nephrol 14: 127–135, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Murdoch DL, Forrest G, Davies DL, McInnes GT: A comparison of the potassium and magnesium-sparing properties of amiloride and spironolactone in diuretic-treated normal subjects. Br J Clin Pharmacol 35: 373–378, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberger MH, Roniker B, Krause SL, Weiss RJ: Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens 15: 709–716, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Brown NJ: Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 9: 459–469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzdorf C, Kurtz A, Höcherl K: COX-2 activity determines the level of renin expression but is dispensable for acute upregulation of renin expression in rat kidneys. Am J Physiol Renal Physiol 292: F1782–F1790, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Seyberth HW, Schlingmann KP: Bartter- and Gitelman-like syndromes: Salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol 26: 1789–1802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchner KA: Indomethacin antagonizes furosemide’s intratubular effects during loop segment microperfusion. J Pharmacol Exp Ther 243: 881–886, 1987 [PubMed] [Google Scholar]
- 23.Kirchner KA, Brandon S, Mueller RA, Smith MJ, Bower JD: Mechanism of attenuated hydrochlorothiazide response during indomethacin administration. Kidney Int 31: 1097–1103, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Kammerl MC, Nüsing RM, Richthammer W, Krämer BK, Kurtz A: Inhibition of COX-2 counteracts the effects of diuretics in rats. Kidney Int 60: 1684–1691, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Breyer MD, Harris RC: Cyclooxygenase 2 and the kidney. Curr Opin Nephrol Hypertens 10: 89–98, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Stichtenoth DO, Frölich JC: COX-2 and the kidneys. Curr Pharm Des 6: 1737–1753, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Palmer BF: Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 43: 516–533, 1995 [PubMed] [Google Scholar]
- 28.Passmore AP, Copeland S, Johnston GD: The effects of ibuprofen and indomethacin on renal function in the presence and absence of frusemide in healthy volunteers on a restricted sodium diet. Br J Clin Pharmacol 29: 311–319, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandhu GK, Heyneman CA: Nephrotoxic potential of selective cyclooxygenase-2 inhibitors. Ann Pharmacother 38: 700–704, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Schlondorff D: Renal complications of nonsteroidal anti-inflammatory drugs. Kidney Int 44: 643–653, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, Shekelle P: A metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. J Rheumatol 29: 804–812, 2002 [PubMed] [Google Scholar]
- 32.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, Bombardier C, Cannon C, Farkouh ME, FitzGerald GA, Goss P, Halls H, Hawk E, Hawkey C, Hennekens C, Hochberg M, Holland LE, Kearney PM, Laine L, Lanas A, Lance P, Laupacis A, Oates J, Patrono C, Schnitzer TJ, Solomon S, Tugwell P, Wilson K, Wittes J, Baigent C, Coxib and traditional NSAID Trialists’ (CNT) Collaboration : Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 382: 769–779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García Rodríguez LA, Tacconelli S, Patrignani P: Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 52: 1628–1636, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Famularo G, Gasbarrone L, Minisola G: Hypomagnesemia and proton-pump inhibitors. Expert Opin Drug Saf 12: 709–716, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Bobrie G, Frank M, Azizi M, Peyrard S, Boutouyrie P, Chatellier G, Laurent S, Menard J, Plouin PF: Sequential nephron blockade versus sequential renin-angiotensin system blockade in resistant hypertension: A prospective, randomized, open blinded endpoint study. J Hypertens 30: 1656–1664, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Leplège A, Ecosse E, Verdier A, Perneger TV: The French SF-36 Health Survey: Translation, cultural adaptation and preliminary psychometric evaluation. J Clin Epidemiol 51: 1013–1023, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Blanchard A, Azizi M, Peyrard S, Stern N, Alhenc-Gelas F, Houillier P, Jeunemaitre X: Partial human genetic deficiency in tissue kallikrein activity and renal calcium handling. Clin J Am Soc Nephrol 2: 320–325, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen G, Blanchard A, Curis E, Bergerot D, Chambon Y, Hirose T, Caumont-Prim A, Tabard SB, Baron S, Frank M, Totsune K, Azizi M: Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension 63: 297–302, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Senn S: Cross-Over Trials in Clinical Research, Chichester, United Kingdom, Wiley, 2000 [Google Scholar]
- 41.Aickin M, Gensler H: Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am J Public Health 86: 726–728, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.