Cardiovascular disease and musculoskeletal frailty are prominent in our patients with CKD,1 synergistically enhanced by concurrent diabetes that drives ESRD in approximately 40% of patients receiving RRT.2 As London et al. first demonstrated,3 the presence and extent of arterial calcium accrual in patients undergoing dialysis, be it in atherosclerotic intimal disease or medial artery calcification, convey significant morbidity and mortality risk.3 Importantly, these researchers established that individuals with low-turnover bone disease were at greatest risk for extensive vascular calcium load.4 Conversely, in community-dwelling older men, the presence of peripheral arterial disease (PAD), routinely defined as an ankle-brachial blood pressure index ratio (ABIx) of <0.9 or >1.3, conveys increased risk of hip fracture.5 While atherosclerotic calcification lowers ABIx with vessel occlusion, the medial arterial calcification of diabetes and CKD results in elevated ABIx values and equally significant clinical consequences, including limb ischemia via vascular stiffening;6–8 thus, vascular calcium metabolism and musculoskeletal health have emerged as being physiologically linked.8,9 Consistent with this, women with lower bone mineral density have greater coronary artery calcification scores,10 and this portends greater probability of future coronary events.11,12 Guzman applied tibial artery calcification scoring to diabetic and non-diabetic patients with PAD.13 Intriguingly, the tibial artery calcification score receiver-operating characteristics outperformed the current clinical standard of the ABIx in predicting future progression to critical limb ischemia and lower-extremity amputation.13 Thus, the appellation CKD–mineral and bone disorder was established1 to emphasize the endocrinology, integrative physiology, and therapeutic implications of disordered bone-vascular interactions that cause cardiovascular and musculoskeletal disease in CKD. However, a better understanding of these interactions is clearly needed in all clinical contexts.
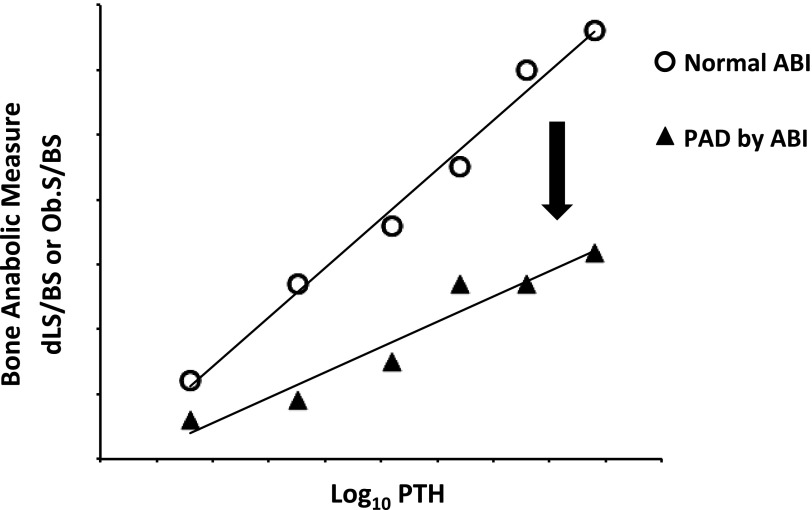
In the present issue of JASN,14 London and colleagues once again blaze the trail by illuminating the physiologic relationships between osteoblast bone anabolic function, parathyroid hormone (PTH) levels, and clinically relevant PAD. In this cohort of 65 well phenotype patients receiving RRT, approximately one third had elevated ABIx values as consistent with prevalent medial artery calcification, 17% had reduced ABIx values indicating atherosclerotic calcification, and half possessed normal indices.14 The authors then analyzed the relationship between intact PTH levels and direct measure of osteoblast anabolic function by dynamic bone histomorphometry, comparing individuals with and without PAD. They reasoned that the slope of the regression relationship between PTH—the prototypic bone anabolic hormone—to direct histologic measures of osteoblast anabolic function (dLS/BS) would provide an index of PTH sensitivity.14 Via this enlightened analysis, the authors demonstrated that patients with PAD exhibited a significantly shallower slope in the bone formation–PTH relationship; this indicates a reduced bone anabolic response at prevailing PTH tone in those individuals with PAD (Figure 1). Because PTH exerts important bone anabolic actions in part via the inhibition of osteoprogenitor apoptosis,15 independent assessment of the PTH–osteoblast surface relationship also revealed a distinctly shallower slope in patients with PAD.14 These relationships persisted after adjustment for C-reactive protein as an index of inflammation. Most important, in stepwise regression dLS/BS—the direct histologic measure of osteoblast anabolic function—continued to significantly contribute, along with inflammation and RRT duration, to the risk for PAD diagnosis. Thus, the authors conclude that in nondiabetic patients on RRT, PAD is associated with lower turnover bone disease and significant reductions in the skeletal anabolic response.14
Figure 1.
PAD in CKD is associated with reductions in the skeletal anabolic–PTH “modulus.” In the current issue of JASN, London and colleagues establish that compared with those without PAD, patients with PAD diagnosed by ABIx exhibit a regression relationship between osteoblast anabolic function and intact PTH that characterizes reduced PTH sensitivity.14 This elegant analysis frames for the first time the functional endocrine relationships between PTH and the bone-vascular axis in humans. PAD emerges as a PTH-regulated cause, consequence, or concomitant of the reduced anabolic state of the skeleton.9 See text for details. ABI, ankle-brachial index; dLS/BS, doubled labeled surface to bone surface ratio; Ob.S/BS, osteoblast surface to bone surface ratio.
Why is this report so important? This elegant analysis frames for the first time the functional endocrine relationships between PTH and the bone-vascular axis in humans.14 PAD in this setting emerges as a PTH-regulated cause, consequence, or concomitant of the reduced anabolic state of the skeleton.9 As Fadini et al. highlight,16 the skeleton elaborates multiple cell types that circulate and affect vascular structure and function, including calcification. Thus, skeletal resistance to PTH may give rise to altered bone-derived cellular17 or endocrine18 cues that affect cardiovascular function. Given that osteoblast PTH/PTHrP receptor (PTH1R) signaling directly controls the size of the hematopoietic niche,19 this mechanism of bone-vascular interaction is no doubt an important contributor to arteriosclerotic disease. Resistance to PTH may also limit vascular remodeling, realignment, and perfusion necessary for bone health.20–22 However, the vascular smooth muscle cell (VSMC) also expresses the PTH1R and is a direct target of both PTHrP and PTH activation and tachyphylaxis.23 In vitro24 and in vivo,25,26 activation of the PTH1R reduces VSMC-directed arterial calcification. Indeed, we demonstrated that a transgenic mouse expressing the constitutively active Janssen PTH1R variant in VSMC is resistant to the arterial calcification, fibrosis, and vascular stiffness arising from diabetes.25 Thus, the relationships elucidated by London and colleagues between skeletal PTH resistance and PAD probably also reflect the global insufficiency in PTH1R signaling that arises in uremia,27 wherein reduced VSMC PTH1R signal transduction directly predisposes to arteriosclerotic disease.9 On the basis of the recent data of Raison et al,28 paracrine PTH1R activation by PTHrP in conduit arteries is likely to be significantly perturbed in CKD in ways that impair vasodilatation, vascular structure, and tissue blood flow. Unfortunately, no direct measure currently exists to quantify or establish normal vascular PTH1R signaling tone or sensitivity. Once such a measure is identified, however, it will be possible to assay vascular PTH1R pharmacokinetic-pharmacodynamic relationships, similar to London’s skeletal anabolic–PTH “modulus” (Figure 1), and thus optimize vascular PTH1R signaling tone to maintain arterial structure and function with diabetes, aging, and uremia.
Disclosures
D.A.T. serves as a consultant for Merck & Co., Daiichi-Sankyo, and Eli Lilly.
Acknowledgments
D.A.T. is supported by HL069229, HL081138, and HL114806 from the National Institutes of Health, the Sanford-Burnham Medical Research Institute, and the Florida Hospital Translational Research Institute for Metabolism and Diabetes.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Ankle-Brachial Index and Bone Turnover in Patients on Dialysis,” on pages 476–483.
References
- 1.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G; Kidney Disease: Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Debella YT, Giduma HD, Light RP, Agarwal R: Chronic kidney disease as a coronary disease equivalent—a comparison with diabetes over a decade. Clin J Am Soc Nephrol 6: 1385–1392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 4.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE: Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation 119: 2305–2312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilly SM, Qasim AN, Mulvey CK, Churchill TW, Reilly MP, Eraso LH: Non-compressible arterial disease and the risk of coronary calcification in type-2 diabetes. Atherosclerosis 230: 17–22, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Ix JH, Miller RG, Criqui MH, Orchard TJ: Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg 56: 721–727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M: Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16: 978–983, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Thompson B, Towler DA: Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol 8: 529–543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SH, An JH, Lim S, Koo BK, Park SE, Chang HJ, Choi SI, Park YJ, Park KS, Jang HC, Shin CS: Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf) 71: 644–651, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P: Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 303: 1610–1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HT, Shin J, Min SY, Lim YH, Kim KS, Kim SG, Kim JH, Lim HK: Relationship between bone mineral density and a 10-year risk for coronary artery disease in a healthy Korean population: The Korea National Health and Nutrition Examination Survey 2008-2010 [published online ahead of print August 20, 2014]. Coron Artery Dis doi:10.1097/MCA.0000000000000165 [DOI] [PubMed] [Google Scholar]
- 13.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X: Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol 51: 1967–1974, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London GM, Marchais SJ, Guerin AP, de Vernejoul MC: Ankle-brachial index and bone turnover in dialysis patients. J Am Soc Nephrol 26: 476–483, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jilka RL, O’Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC: Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone 44: 275–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S: Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation 125: 2772–2781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napoli C, William-Ignarro S, Byrns R, Balestrieri ML, Crimi E, Farzati B, Mancini FP, de Nigris F, Matarazzo A, D’Amora M, Abbondanza C, Fiorito C, Giovane A, Florio A, Varricchio E, Palagiano A, Minucci PB, Tecce MF, Giordano A, Pavan A, Ignarro LJ: Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat Clin Pract Cardiovasc Med 5: 571–579, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Yuan Q, Sato T, Densmore M, Saito H, Schuler C, Erben RG, Lanske B: FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J Bone Min Res 26: 2026–2035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT: Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lafage-Proust MH, Prisby R, Roche B, Vico L: Bone vascularization and remodeling. Joint Bone Spine 77: 521–524, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Brenneise CV, Squier CA: Blood flow in maxilla and mandible of normal and atherosclerotic rhesus monkeys. J Oral Pathol 14: 800–808, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, Demer LL, Tintut Y: Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Min Res 26: 1197–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyby MD, Hino T, Berger ME, Ormsby BL, Golub MS, Brickman AS: Desensitization of vascular tissue to parathyroid hormone and parathyroid hormone-related protein. Endocrinology 136: 2497–2504, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Jono S, Nishizawa Y, Shioi A, Morii H: 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 98: 1302–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA: Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res 107: 271–282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA: Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem 278: 50195–50202, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki Y, Yamato H, Nii-Kono T, Fujieda A, Uchida M, Hosokawa A, Motojima M, Fukagawa M: Insufficiency of PTH action on bone in uremia. Kidney Int Suppl 102(Suppl): S34–S36, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Raison D, Coquard C, Hochane M, Steger J, Massfelder T, Moulin B, Karaplis AC, Metzger D, Chambon P, Helwig JJ, Barthelmebs M: Knockdown of parathyroid hormone related protein in smooth muscle cells alters renal hemodynamics but not blood pressure. Am J Physiol Renal Physiol 305: F333–F342, 2013 [DOI] [PubMed] [Google Scholar]