Abstract
AIM
To investigate possible age-related changes in glycosaminoglycans (GAGs) in the human cornea. The substances today called GAGs were previously referred to as mucopolysaccharides.
METHODS
Samples of human cornea were taken from 12 younger (age 21 ± 1.2) and 12 older (age 72 ± 1.6) male subjects. Samples were weighed, homogenized, and used for biochemical and molecular analyses. All the quantitative results were statistically analyzed.
RESULTS
The human cornea appears to undergo age-related changes, as evidenced by our biochemical and molecular results. The total GAG and hyaluronic acid counts were significantly higher in the younger subjects than in the older subjects. The sulfated heavy GAGs, such as chondroitin, dermatan, keratan, and heparan sulfate, were lower in the younger subjects than in the older subjects.
DISCUSSION
GAGs of the human cornea undergo numerous age-related changes. Their quantity is significantly altered in the elderly in comparison with younger subjects. GAGs play an important role in age-related diseases of the human cornea.
Keywords: mucopolysaccharides, glycosaminoglycans, mucoproteins, proteoglycans, age-related changes, human cornea
Introduction
Glycosaminoglycans (GAGs) are the most abundant heteropolysaccharides in the human eye. These molecules are long, unbranched polysaccharides containing a repeating disaccharide unit. The disaccharide units contain one of two modified sugars (N-acetylgalactosamine or N-acetylglucosamine) and a uronic acid, such as glucuronate or iduronate. GAGs are highly negatively charged molecules, with an extended conformation that imparts high viscosity to solutions.
Their biophysical functions depend on their unique properties: the ability to fill a space, to bind and organize water molecules, and to repel negatively charged molecules. Because of high viscosity and low compressibility, they are ideal lubricants in the eyes. On the other hand, their rigidity provides cells with structural integrity and resistance to deformation, and allows cell migration.
Finally, GAGs are a major component of the extracellular matrix (ECM), the “filler” substance existing between cells in an organism. Here they form larger complexes, binding to proteoglycans, to hyaluronan, and to fibrous matrix proteins, such as collagen. They have also been shown to bind with cations (such as sodium, potassium, and calcium) and with water, and their role in regulating the movement of molecules through or within the ECM has also been demonstrated. Individual functions of proteoglycans can be attributed to either the protein core or the attached GAG chain.1,2
For all these reasons, GAGs are considered to be the “glue” of the cornea, responsible for providing plasticity and the structural support needed for successful corneal function. Along with other molecules, GAGs form the solid portion of the cornea (22%), the remainder being water.3 The corneal transparency is due to the uniform distribution of collagen fibrils, which is regulated by proteoglycans.4
The first studies on the role of GAGs in corneal disorders date back to 1969.5
In the years that followed, numerous studies were performed on corneal cells and the role of GAGs was investigated in various species, such as chicks6 and rabbits,7,8 and also in humans.9,10 At the same time, the biosynthetic mechanisms of sulfated GAGs were particularly studied,11 as were their metabolism and functions.12 Finally, biochemical measurement in tissue extracts has also been performed.13,14 Sulfated GAGs were considered to be pathognomonic factors in some corneal diseases, including corneal explant.15–18
In fact, the α-keratan sulfate proteoglycan appears to play a key role in the transparency of the cornea, while the major keratan sulfate proteoglycan, present in large quantities in the corneal stroma, seems to regulate the organization of collagen fibrils and is involved in wound repair of epithelial tissues of the human cornea.19,20 Proteoglycans, mucous proteins, and mucopolysaccharides, as stated, are the historic terms for the chemical substances today referred to as GAGs.21,22
Mucopolysaccharides are present in excess in a group of diseases named mucopolysaccharidoses.23 Hurler’s mucopolysaccharidosis starts with a corneal clouding that worsens with other dangerous illness, as reported by Iozzo.16 This author demonstrated the involvement of GAGs within the corneal structure and in the maintenance of its transparency.
GAGs were particularly studied in relation to age- related changes in human plasma24–26 and also regarding their role in the trabecular meshwork of the human eye.27,28 The aim of this work is to evaluate possible age-related changes in the human cornea, particularly in relation to the role played by GAGs.
Materials and Methods
Samples of human cornea taken from 12 younger (age 21 ± 1.2 years) and 12 older (age 72 ± 1.6 years) male subjects were harvested from autopsies, without any aesthetic damage to the corpses. As post-mortem phenomena may produce early morphological modifications of the corneal structures, our samples were harvested as early as possible after death (12–18 hours).
The Ethics Committees of the Hospitals participating in the study gave their approval for the study, and the relatives of the sampled subjects gave their written informed consent for the harvesting and use of the samples. Experiments were in accordance with the ethical standards of the responsible committee and with the Declaration of Helsinki.
Samples of the human cornea (3 mg weight) were homogenized using a modified Potter-Elvehjem homogenizer (glass/glass), in an ice-cold buffer solution 1/10 w/v of veronal-acetate (pH 7.4).
Each homogenate was centrifuged in a 0.656 M sucrose gradient solution containing 1 mM-ethylenediaminetetraacetic acid (EDTA). The tubes were centrifuged for 30 minutes at 47.000g (21.500 rpm) in the Spinco SW 25.1 rotor. Six fractions were obtained: (1) supernatant A, (2) membranes, (3) supernatant B, (4) mitochondria, (5) cytosol, and (6) pellet. Layers 1, 3, and 5 constituted the liquid phases, while layers 2, 4, and 6 comprised the solid phases. The liquid phases were collected and used for the separation of GAGs.29
Tissue protein concentrations in all our samples were determined using the method described by Lowry et al,30 using bovine serum albumin (BSA) as standard, and Folin phenol was used as the reagent.
Elution and concentration of total GAGs
GAGs were isolated from the supernatants using ion-exchange chromatography on Diethylaminoethyl–Sephacel, according to De Muro.31 The fraction containing GAGs was collected, lyophilized, and stored at −20 °C until used for biochemical analysis.
Preparation of GAG subfractions
Samples of total GAGs were subjected to enzymatic digestion, capable of specifically eliminating particular GAG types. The following GAG digestion factors were used: chondroitinase ABC and/or nitrous acid.
Digestion with chondroitinase A
Cleavage with chondroitinase A, which specifically degraded chondroitin-sulfate, allowed dermatan sulfate and heparan sulfate, or hyaluronic acid, to be retained. The digestion process was performed using only chondroitinase A (0.05 M) in Tris-HCl buffer (50 nM, pH 7.3).
Digestion with nitrous acid
Nitrous acid was used in order to remove HS/H. Samples of serum GAGs subjected earlier to enzymatic depolymerization with chondroitinase ABC or AC, respectively, were additionally treated with nitrous acid, according to Lagunoff and Warren.32
All the subfractions containing the different types of GAGs were collected, lyophilized, and stored at −20 °C until used for biochemical analysis.
Assay of GAGs and/or their subfractions
The amount of total GAGs and/or their subfractions was quantified by hexuronic acid biochemical assay, according to Blumenkrantz and Asboe-Hansen,33 taking into account modifications proposed by Slim et al.34 The concentrations were expressed as milligrams of hexuronic acid per gram of protein.
Statistics
Mean values, maximum and minimum limits (experimental values), variations, standard deviation (SD), standard error of the mean (SEM), and correlation coefficients were calculated.
A correlative analysis of molecular and biochemical data was performed by comparing the significant differences in each group with the corresponding values in other homogenous groups. The significance of differences between age groups was assessed using Duncan’s multiple range test.35
Results
The clinically relevant data of the subjects from whom the corneas were harvested are shown in Table 1. The corneas of the 12 eyes of young people, and those of 12 elders, did not have any macroscopic or microscopic abnormalities. Moreover, no donor was suffering from other general diseases that might have impacted on the local diseases of the eye and/or of the cornea.
Table 1.
Clinical data of donor subject from which small fragments of cornea were harvested.
| NUMBER OF PATIENTS | AGE RANGE | SEX | EYE AND CORNEA |
|---|---|---|---|
| 12 | 20 ± 1.2* | Male | No ocular and/or cornea diseases |
| 12 | 72 ± 1.6* | Male | No ocular and/or cornea diseases |
Note:
Results are reported in years ± SD.
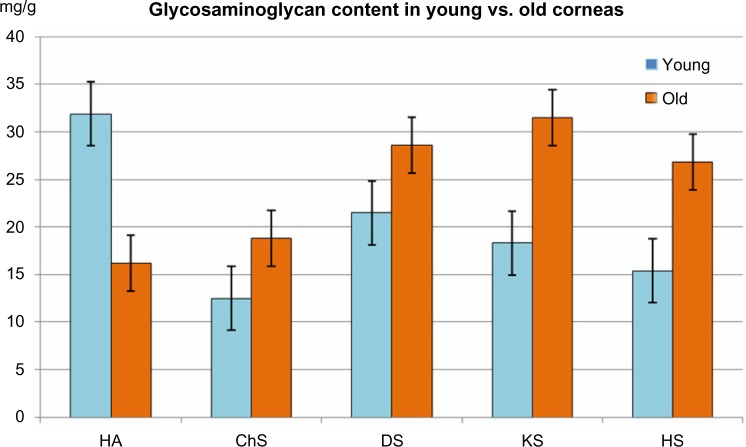
Table 2 contains the biochemical values related to the quantity of GAGs in the corneal homogenates of the two age groups of subjects. As can be seen, the total GAGs count was 97.4 ± 6.2 mg of hexuronic acid per gram of protein. In the younger subjects, the GAG count was 127.3 ± 8.5 mg of hexuronic acid per gram of protein, while in the older subjects, there was a significant increase of about 30% compared to younger corneas (Fig. 1).
Table 2.
Quantitative analysis of GAGs in the corneal tissues of young and old subjects.
| FINDINGS | YOUNG (n = 12) | OLD (n = 12) | P* |
|---|---|---|---|
| Total GAGs | 97.4 ± 6.2 | 127.3 ± 8.5 | <0.05 |
| Hyaluronic acid | 31.9 ± 1.3 | 16.2 ± 1.5 | <0.05 |
| Chondroitin sulphate | 12.5 ± 1.4 | 18.8 ± 1.6 | <0.05 |
| Dermatan sulphate | 21.5 ± 1.8 | 28.6 ± 1.8 | <0.05 |
| Keratan Sulphate | 18.3 ± 1.6 | 31.5 ± 1.4 | <0.05 |
| Heparan sulphate | 15.4 ± 1.2 | 26.8 ± 1.2 | <0.05 |
Notes: All results are expressed as μM of hexuronic acids / g proteins.
P was assessed comparing the values of young versus of corneas and considered significant if P < 0.05. Duncan’s multiple range test was calculated comparing young and old corneas.
Figure 1.
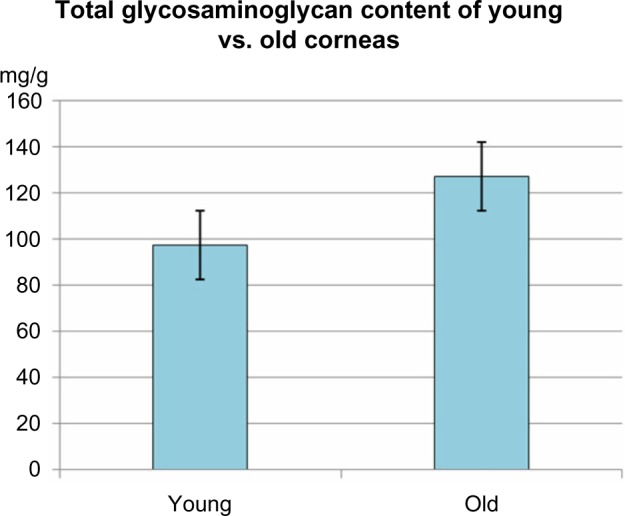
Comparison of total GAG content of young and old human corneas: Total GAG content was significantly higher in old corneas as compared to young corneas (<0,05).
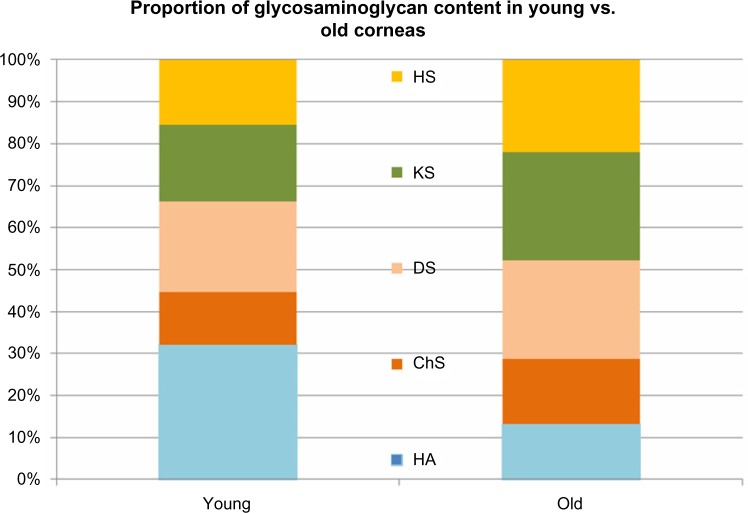
These changes in total GAG content were associated with significant modification of both the quantity and proportion of each component of GAG. In contrast, lighter GAGs, such as hyaluronic acid, strongly decreased in the older subjects (16.2 ± 1.5 mg hexuronic acid per milligram of protein) versus young donors (31.9 ± 1.3 mg of uronic acid per milligram of protein).
By contrast, the sulfated heavy GAGs, such as chondroitin, dermatan, keratan, and heparan sulfate, strongly increased in the older subjects, by about 30–40% with the exception of the heparan sulfate, which increased by about 80%. All the data present in Table 2 were statistically significant (P < 0.05) (Figs. 2 and 3).
Figure 2.
Comparison of the GAG content of young and old human corneas: the proportion of hyaluronic acid (HA) was higher in the young corneas, while the proportion of sulfated GAGs (ChS, DS, KS, HS) was lower in young corneas as compared to old corneas.
Figure 3.
Comparison of GAG composition of young and old human corneas: hyaluronic acid (HA) content is significantly higher in young corneas (<0,05), while chondroitin-sulfate (ChS), dermatan sulfate (DS), heratan sulfat (KS) and heparan sulfate (HS) content is significantly lower (<0,05) as compared to old corneas.
Discussion
The human cornea presented age-related changes, as evidenced by our biochemical and molecular results. The total GAGs and sulfated heavy GAGs, such as chondroitin, dermatan, keratan and heparan sulfate, were higher in the older subjects than in the younger subjects; at the same time, the hyaluronic acid count was significantly higher in the younger subjects than in the older subjects. Hyaluronic acid count was significantly higher in the younger subjects than in the older subjects. The sulfated heavy GAGs, such as chondroitin, dermatan, keratan, and heparan sulfate, were lower in the younger subjects than in the older subjects.
The corneas of the elderly are more susceptible to oxidative injury in comparison to the corneas of younger people.36–38 Moreover, the activities of all antioxidant enzymes were dramatically decreased in aged eyes. The decrease in enzymes that normally metabolize and detoxify hydrogen peroxide and other free radicals can account for the alteration in levels of GAGs in the elderly.38
The cornea is a peculiar and multifunctional structure of the eyeball, as its transparency permits light perception by the retina; its shape contributes to the refractive power responsible for appropriate sight; and together with the sclera, it forms a protective envelop for delicate vascular and neuronal intraocular structures. All these functions depend mainly on the integrity of the corneal stroma, a unique transparent connective tissue layer, which represents approximately nine-tenth of the corneal thickness.39
From the late ‘80s, but particularly in the last ten years, significantly increased clinical interests on corneal microsurgery as the refractive surgery, ie, surgical interventions on the curvature of the cornea in myopia, in hypermetropy and astigmatism, and even more in presbyopia, have become more and more common worldwide and have opened new perspectives to improve visual performance at any age. Furthermore, new microsurgery techniques have been developed for corneal transplantation of any part of the cornea. These interventions cause damage to the corneal stroma and particularly to the innervation of the cornea. Even if this damage is progressively less extensive by using laser technology, the wound healing of the stroma highly depends on the GAG as their composition profoundly modify the all post-operative functions of the cornea for both short and long terms.40–42
Taking together our findings on age-related changes in the cornea and the rapidly growing prevalence of corneal surgery we may conclude: any intervention on the cornea should be customized considering also the age of subject; specifically, how the age will influence the outcome of surgery, and any intervention on the cornea should consider future aging; specifically, how the intervention will influence the physiological aging of the cornea. Further studies are certainly needed to reach deeper knowledge on the corneal structure and pathophysiology to planning customized interventions, in particular, regarding aging and age-related changes.
Our results provide direct evidence that GAGs play an important role in age-related diseases of the human cornea; however, there is no direct experiment to show this in the current manuscript. Their quantity is significantly affected in the elderly in comparison with younger subjects.
A distinction must be made between conditions considered within the boundaries of normal aging and those of true disease processes affecting the cornea in the elderly.
Conclusions
GAGs perform numerous vital functions within the human cornea. They provide corneal hydration, structural integrity, transparency and thickness. Aging results in an altered composition of GAGs in the cornea. For this reason, the corneas of the elderly are more susceptible to oxidative damage.
Acknowledgments
We extend thanks to Professor Janos Feher for his contribution to revisions of this paper.
Footnotes
ACADEMIC EDITOR: Joshua Cameron, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CC, EP, FP. Analyzed the data: CC, EP, FP, GDP. Wrote the first draft of the manuscript: CC, EP, GDP. Contributed to the writing of the manuscript: FP, PT, GDP, FRP. Agree with manuscript results and conclusions: EP, FP, CC, PT, GA, GDP, FRP. Jointly developed the structure and arguments for the paper: EP, FP, CC, PT, GA, GDP, FRP. Made critical revisions and approved final version: EP, FP, CC, PT, GA, GDP, FRP. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Brooks SA, Dwek MV, Schumacher U. Functional and Molecular Glycobiology. Oxford: BIOS Scientific Publishers; 2002. [Google Scholar]
- 2.Alberts B. Cell biology: the endless frontier. Mol Biol Cell. 2010;21(22):3785. doi: 10.1091/mbc.E10-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millodot M. Dictionary of Optometry and Visual Science. 7th ed. Oxford: Butterworth-Heinemann; 2008. [Google Scholar]
- 4.Maurice DM. Epithelial potential of the cornea. Exp Eye Res. 1967;6(2):138–40. doi: 10.1016/s0014-4835(67)80065-4. [DOI] [PubMed] [Google Scholar]
- 5.Anseth A. Studies on corneal polysaccharides 8. Changes in the glycosaminoglycan in some human corneal disorders. Exp Eye Res. 1969;8(4):438–41. doi: 10.1016/s0014-4835(69)80010-2. [DOI] [PubMed] [Google Scholar]
- 6.Conrad GW, Dorfman A. Synthesis of sulfated mucopolysaccharides by chick corneal fibroblast in vitro. Exp Eye Res. 1974;18(5):421–33. doi: 10.1016/0014-4835(74)90079-7. [DOI] [PubMed] [Google Scholar]
- 7.Yue BY, Baum JL. The synthesis of glycosaminoglycans by cultures of rabbit corneal endothelial and stromal cells. Biochem J. 1976;158(5):567–73. doi: 10.1042/bj1580567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goes RM, Laicine EM, Porcionatto MA, Bonciani Nader H, Haddad A. Glycosaminoglycans in components of the rabbit eye: synthesis and characterization. Curr Eye Res. 1999;19:146–53. doi: 10.1076/ceyr.19.2.146.5324. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X, Pinsky PM. Mechanisms of self-organization for the collagen fibril lattice in the human cornea. J R Soc Interface. 2013;10(87):20130512. doi: 10.1098/rsif.2013.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isnard N, Fodil I, Robert L, Renard G. Modulation of cell-phenotype during in vitro aging. Glycosaminoglycan biosynthesis by skin fibroblast and corneal keratocytes. Exp Gerontol. 2002;37:1377–85. doi: 10.1016/s0531-5565(02)00120-1. [DOI] [PubMed] [Google Scholar]
- 11.Sugahara K, Yamada S, Kitagawa H. Biosynthetic mechanism of sulphated glycosaminoglycans. Seikagaku. 2001;73:458–70. [PubMed] [Google Scholar]
- 12.Sugahara K, Kitagawa H. Recent advances in the study of the biosynthesis and functions of sulphated glycosaminoglycans. Curr Opin Struct Biol. 2000;10:518–27. doi: 10.1016/s0959-440x(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 13.Rusova TV, Matyeeva FL, Talashova IA. Measurement of sulphated glycosaminoglycans in tissue extracts. Klin Lab Diagn. 2000;7:17–8. [PubMed] [Google Scholar]
- 14.Gealy EC, Kerr BC, Young RD, et al. Differential expression of the keratan sulphate proteoglycan, keratocan, during chick corneal embryogenesis. Histochem Cell Biol. 2007;128(6):551–5. doi: 10.1007/s00418-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 15.François J, Victoria-Troncoso V, Maudgal PC, Victoria-Ihler A. Study of the lysosomes by vital stains in normal keratocytes and keratocytes from macular dystrophy of the cornea. Invest Ophtahalmol. 1976;15(8):599–605. [PubMed] [Google Scholar]
- 16.Friberg TR, Guibord NM. Corneal endothelial cell loss after multiple vitreoretinal procedures and the use of silicone oil. Ophthalmic Surg Lasers. 1999;30(7):528–34. [PubMed] [Google Scholar]
- 17.Plaas AH, West LA, Thonar EJ, et al. Altered fine structures of corneal and skeletal keratan sulfate and chondroitin/dermatan sulfate in macular corneal dystrophy. J Biol Chem. 2001;276(43):39788–96. doi: 10.1074/jbc.M103227200. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi E, Scarinci F, Ripandelli G. Retinal pigment epithelium, age-related macular degeneration and neurotrophic keratouveitis. Int J Mol Med. 2013;31:232–42. doi: 10.3892/ijmm.2012.1164. [DOI] [PubMed] [Google Scholar]
- 19.Davies Y, Lewis D, Fullwood NJ, et al. Proteoglycans on normal and migrating human corneal endothelium. Exp Eye Res. 1999;68(3):303–11. doi: 10.1006/exer.1998.0609. [DOI] [PubMed] [Google Scholar]
- 20.Guo N, Li X, Mann MM, Funderburgh ML, Du Y, Funderburgh JL. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta. J Biol Chem. 2010;285(42):32012–9. doi: 10.1074/jbc.M110.127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma R, Varma RS. Simultaneous determination of neutral sugars and hexosamines in glycoproteins and acid mucopolysaccharides (glycosaminoglycans) by gas-liquid chromatography. J Chromatogr. 1976;128(1):45–52. doi: 10.1016/s0021-9673(00)84029-7. [DOI] [PubMed] [Google Scholar]
- 22.Michelacci YM. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res. 2003;36(8):1037–46. doi: 10.1590/s0100-879x2003000800009. [DOI] [PubMed] [Google Scholar]
- 23.Dorfman A, Matalon R. The mucopolysaccharides (a review) Proc Natl Acad Sci U S A. 1976;73(2):630–7. doi: 10.1073/pnas.73.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calatroni A, Donelly PV, Di Ferrante N. The glycosaminoglycans in human plasma. J Clin Invest. 1969;48:32. doi: 10.1172/JCI105989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larking PW. Total glycosaminoglycans in plasma of adults: effects of age and gender, and relationship to plasma lipids: a preliminary study. Biochem Med Metabol Biol. 1989;42:192–7. doi: 10.1016/0885-4505(89)90055-8. [DOI] [PubMed] [Google Scholar]
- 26.Carreras FJ, López Caballero JJ, Porcel D. A gel of glycosaminoglycans lining the anterior and posterior chambers in man: histochemical evidence at light and electron microscopy levels. Eye (Lond) 1992;6(pt 6):574–82. doi: 10.1038/eye.1992.125. [DOI] [PubMed] [Google Scholar]
- 27.Ascott TS, Westcott M, Passo MS, van Buskirk ME. Trabecular meshwork glycosaminoglycans in human and cynomolgus monkey eye. Invest Ophthalmol Vis Sci. 1985;6:1320–9. [PubMed] [Google Scholar]
- 28.Cavallotti C, Feher J, Pescosolido N, Sagnelli P. Glycosaminoglycans in human trabecular meshwork: age-related changes. Ophthalmic Res. 2004;36(4):211–7. doi: 10.1159/000078779. [DOI] [PubMed] [Google Scholar]
- 29.Takegawa Y, Araki K, Fujitani N. Anal Chem. 2011;83(24):9443–9. doi: 10.1021/ac2021079. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 31.De Muro P. Quali-quantive analysis of urinary glycosaminoglycans for monitoring glomerular inflammatory activity. Scand J Urol Nephrol. 2007;41(3):230–6. doi: 10.1080/00365590601017105. [DOI] [PubMed] [Google Scholar]
- 32.Lagunoff D, Warren G. Determination of 2-deoxy-2-sulphioaminohexose content of mucopolysaccharides. Arch Biochem Biophys. 1962;99:396–400. doi: 10.1016/0003-9861(62)90285-0. [DOI] [PubMed] [Google Scholar]
- 33.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–9. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 34.Slim GC, Furneaux RH, Yorke SC. A procedure for analysis of glycosaminoglycans mixtures based on digestion by specific enzymes. Carbohydr Res. 1994;255:285–93. doi: 10.1016/s0008-6215(00)90985-6. [DOI] [PubMed] [Google Scholar]
- 35.Castino M, Roletto E. Statistica applicata. Padova: Piccin; 1992. [Google Scholar]
- 36.Kitayama K, Hayashida Y, Nishida K, Akama TO. Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J Biol Chem. 2007;282(41):30085–96. doi: 10.1074/jbc.M703695200. [DOI] [PubMed] [Google Scholar]
- 37.Feher J, Kovacs I, Pacella E, Keresz S, Spagnardi N, Balacco-Gabrieli C. Pigment Epithelium Derived Factor (PEDF) attenuated capsaicin induced neurotrophic keratouveitis. Invest Ophthalmol Vis Sci. 2009;50:5173–80. doi: 10.1167/iovs.08-1852. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Kobayashi M, Hirano K, Kobayashi K, Hoshino T, Awaya S. Assembly of 100 nm periodic fibrils (type VI collagen) in human infant corneal stroma. Jpn J Ophthalmol. 1992;36(4):458–64. [PubMed] [Google Scholar]
- 39.Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19(4–5):275–85. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- 40.Feher J. Pathophysiology of the Eye 3. Transparency and Refraction of the Cornea. Akademiai Kiadó Budapest. 1996:11–24. [Google Scholar]
- 41.Salomao MQ, Wilson SE. Corneal molecular and cellular biology update for the refractive surgeon. J Refract Surg. 2009;25:459–66. doi: 10.3928/1081597x-20090422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azar DT, Chang JH, Han KY. Wound healing after keratorefractive surgery: review of biological and optical considerations. Cornea. 2012;31(suppl 1):S9–19. doi: 10.1097/ICO.0b013e31826ab0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]