Abstract
The P2C2 HIV Study is a prospective natural history study initiated by the National Heart, Lung, and Blood Institute in order to describe the types and incidence of cardiovascular and pulmonary disorders that occur in children with vertically transmitted HIV infection (i.e., transmitted from mother to child in utero or perinatally). This article describes the study design and methods. Patients were recruited from five clinical centers in the United States. The cohort is composed of 205 infants and children enrolled after 28 days of age (Group I) and 612 fetuses and infants of HIV-infected mothers, enrolled prenatally (73%) or postnatally at age <28 days (Group II). The maternal-to-infant transmission rate in Group II was 17%. The HIV-negative infants in Group II (Group IIb) serves as a control group for the HIV-infected children (Group IIa). The cohort is followed at specified intervals for clinical examination, cardiac, pulmonary, immunologic, and infectious studies and for intercurrent illnesses. In Group IIa, the cumulative loss-to-follow-up rate at 3 years was 10.5%, and the 3-year cumulative mortality rate was 24.9%. The findings will be relevant to clinical and epidemiologic aspects of HIV infection in children.
Keywords: HIV, pulmonary complications, cardiovascular complications, pediatric AIDS, prospective study, vertical transmission
INTRODUCTION
In 1982, a year after the initial description of the acquired immunodeficiency syndrome (AIDS) in adults, cases were described in children [1–4]. Subsequent reports clearly indicated that pulmonary and cardiac disease contributed significantly to the morbidity and mortality experienced by children with human immunodeficiency virus (HIV) infection [5–8]. In response to the growing epidemic, the National Heart, Lung, and Blood Institute (NHLBI) initiated a study designed to describe the cardiovascular and pulmonary disorders that occur in children in association with vertical transmission of HIV infection from mother to child during gestation or during the perinatal period. This article describes the organization, hypotheses, study plan, and methods of the ongoing Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted Human Immunodeficiency Virus (P2C2 HIV) Infection Study.
PROTOCOL DEVELOPMENT
Design Process
Five clinical centers from different geographic areas began protocol development in May 1989. The study protocol was developed by a Steering Committee consisting of the principal investigators from the clinical centers, the Clinical Coordinating Center (CCC), and the project officer and staff from the NHLBI. Input into the design of the protocol was given by subcommittees of investigators specializing in cardiology, pulmonology, immunology, infectious disease, radiology, nuclear medicine, biostatistics, and pathology.
The five clinical centers are as follows: (1) Baylor College of Medicine/Texas Children’s Hospital (Houston, TX), (2) Children’s Hospital/Harvard Medical School (Boston, MA), (3) Mount Sinai School of Medicine (New York, NY), (4) Presbyterian Hospital/Columbia University College of Physicians and Surgeons (New York, NY), and (5) UCLA School of Medicine (Los Angeles, CA). The CCC was established at the Cleveland Clinic Foundation (Cleveland, OH). A full list of participants is provided in Appendix 1.
Objectives
The overall goals of the study are as follows:
To determine the prevalence, incidence, and types of cardiovascular and pulmonary complications in the fetus, newborn infant, and child with vertically transmitted HIV infection and to describe the course and outcome of these disorders
To determine whether early detection of cardiovascular and pulmonary complications associated with vertically transmitted HIV infection can be accomplished in utero by cardiologic methods and postnatally by sensitive cardiologic and pulmonary surveillance before they become clinically evident
To determine whether immunologic dysfunction or co-infections (acquired before or after birth) in patients with vertically transmitted HIV infection lead to progressive pulmonary and/or cardiovascular disease
Time Table
During the initial 1-year design phase, the study protocol, manual of operations, data collection forms, and distributed data entry system were developed, and training sessions were held. Recruitment began in May 1990 and continued through January 1994. The cohort is being followed for an additional 3 years beyond the end of recruitment; follow-up times will range from 2.5 to 6.6 years. An analysis phase encompasses the final 6 months of the study, through July 1997.
STUDY POPULATION
The cohort of subjects being studied includes two groups. Group I is composed of infants and children with documented vertically transmitted HIV infection. Children in Group I must have been born after April 1, 1985, except where vertical transmission of HIV infection can be documented with reasonable medical certainty, and be more than 28 days old. Vertical transmission to the child is defined by HIV infection in the mother or maternal death due to AIDS where there is no evidence of sexual abuse of the child. Children in Group I who were classified as P-2, subclass E (secondary cancers) at enrollment by the 1987 Revised Center for Disease Control (CDC) Classification System [9], as well as children in either group for whom informed consent could not be obtained, were excluded. Group II is composed of infants born to HIV-infected mothers, enrolled during gestation or on or before 28 days of age postnatally. Children in Group II who are later determined to be infected with HIV are designated as Group IIa, and uninfected children in Group II are designated Group IIb.
The conventional tests for HIV antibody (enzyme-linked immunosorbent assay [ELISA] and Western blot) are used to establish a diagnosis of HIV infection in pregnant women. HIV cultures are performed on Group II children at birth (not cord blood), and 3 and 6 months postnatally. Two positive cultures are necessary to assign a patient to Group IIa (infected). Two negative cultures, one of which must be when the child is 5 months of age or older, are necessary to assign a patient to Group IIb (noninfected). An ELISA and, if necessary, Western blot are performed on all Group IIb patients at a minimum age of 15 months to confirm their HIV-negative status.
Sample Size, Recruitment, and Randomization
Recruitment goals were 200 Group I children and 600 Group II infants and children. The sample size of 200 Group I patients was justified on the basis of precision of estimating pulmonary and cardiovascular complication rates. At the time the original study protocol was written, it was estimated that the transmission rate was 30%. The recruitment goal was changed from 500 to 600 Group II patients in February 1993 when it became apparent that the actual transmission rate was 20% or less [10]. A subset of approximately 200 Group IIb children is randomly selected to remain on study as controls, where randomization is stratified by clinical center and the mother is used as the unit of randomization to allow for mothers with more than one child enrolled in the study. Children not selected as controls undergo no further study procedures, but return for confirmation of HIV-negative status at age ≥ 15 months via serology tests.
The sample size for Group I was determined on the basis of its adequacy for estimating incidence and prevalence rates of complications, either for the entire Group I cohort or for subgroups stratified by factors such as age or immunologic status at enrollment. Complication rates for Group I expressed as proportions can be estimated with 95% confidence interval half-widths of ≤7 or ≤10%, based on sample sizes of 200 and 100. These half-widths are conservative estimates (upper bounds) obtained assuming a complication rate of 50% and will be narrower if the rate is closer to 0 or 1.
Sample sizes in Group II were justified on the basis of the ability to detect differences in pulmonary and cardiovascular complication rates between infected (IIa) and noninfected (IIb) children, assuming the rate will be low (e.g., 1 to 5%) in the uninfected children. At ages less than 6 months, with approximately 90 Group IIa and 450 Group IIb children, differences in complication rates (IIb vs. IIa) of 1 vs. 9% or 5% vs. 17% will be detectable with 90% power using a two-sided test with significance level 0.05. At age 1 year, assuming sample sizes of 90 in IIa and 200 in IIb, differences in rates of 1 vs. 11% or 5 vs. 19% can be detected with 90% power. When comparing means for continuous measurements, a difference in Group IIa and IIb means of 0.37 standard deviations can be detected with 90% power age ≤ 6 months, and a difference in means of 0.41 standard deviations can be detected at 1 year of age.
Recruitment Strategies
Recruitment of Group I patients occurred in pediatric clinics (hospital and nonhospital based) and inpatient wards. Recruitment of Group II patients was achieved with the help of collaborating obstetrical co-investigators working in high-risk obstetric clinics. These obstetricians, using the existing allied health professionals (e.g., research nurse, social worker), informed the principal investigator or nurse coordinator of the pending or recent delivery of a baby born to an HIV-positive mother. If HIV-infected children were enrolled in other clinical trials, the assistance of the participating investigators was solicited for recruitment.
Cohort Management and Retention
Each clinical center has its own administrative structure for managing its cohort and retaining the participants. The nurse coordinators are the primary contact persons for the patients and the participating personnel from the various disciplines. Along with social workers, the nurse coordinators maintain contact with families and assist them in keeping clinic appointments, in obtaining social welfare benefits, and with transportation to the hospitals. Study personnel generate records of scheduled and missed appointments on a weekly basis. These listings are used to make reminder calls, assure accurate scheduling, and provide follow-up for missed appointments. Subject compliance with appointments is also promoted by providing reimbursement for meals and transportation at each visit. Monthly reports of tests not done are generated by the CCC in order to identify and resolve problems on an ongoing basis. The centers have social gatherings, such as a Christmas party, to help foster compliance.
Examinations and Study Variables
The parent or legal guardian provided informed consent. A questionnaire regarding the mother’s medical and social history was administered. HIV infection in the Group II mothers was confirmed by ELISA and Western blot. Urine was cultured for cytomegalovirus (CMV) and a throat washing (saliva) was cultured for Epstein–Barr virus (EBV). Serologic tests for CMV and EBV were also obtained. Baseline history included information on prescribed medication and street drug use, anti-HIV and anti-Pneumocystis therapy, past diagnoses, and pregnancy history.
The specific evaluations performed at the initial and subsequent evaluations in the children are as follows:
History, physical examination at enrollment
Complete blood count (CBC) and erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), serum immunoglobulins, other laboratory tests (sodium, chloride, potassium, blood urea nitrogen [BUN], creatinine, total protein, albumin)— yearly for Group I, every 6 months for Group II
Determinations of lymphocyte subsets (CD4, CD8, and CD 3)—yearly for Group 1, every 6 months for Group II
CMV culture and serology—every 6 months for Group II only
EBV culture and serology—every 6 months for Group II only
Pulmonary physical examination and oxygen saturation by pulse oximetry—every 3 months in the first year, every 6 months thereafter
Delayed hypersensitivity skin testing using tuberculin, Candida, and tetanus—yearly after 1 year of age
-
Pulmonary function tests—every 6 months, with Group IIb not tested at ages 24, 30 months
Under 2 years: Total respiratory system compliance and resistance, functional residual capacity, forced expiratory flow from partial forced expiratory flow volume curves
Three to 5 years: Peak expiratory flow rates, compliance, and resistance
Five years and over: Spirometry (forced vital capacity, 1-sec forced expiratory flow, forced expiratory flow between 25 and 75% of the vital capacity)
-
Cardiac function tests
Electrocardiogram and Holter monitoring—yearly
Cardiac assessment and echocardiogram—every 4 months for Groups I, IIa, and every 6 months for Group IIb
Fetal echocardiogram—Group II fetuses prenatally enrolled
Chest roentgenogram—yearly for Group I, at ages 3, 12, 18 months for Group IIb, at ages 3, 12, 18 months, yearly thereafter for Group IIa
Technetium-99m diethylenetrianene pentacetate (99mTc-DTPA) clearance studies—every 6 months after age 6 months; stopped after age 18 months in Group IIb
Serum is drawn and frozen for use in future studies. Immediate past history is recorded during follow-up visits. Complications other than pulmonary or cardiovascular are recorded at the follow-up visits. For Group IIb patients who are randomized off the study, follow-up after 6 months consists of ELISA and if necessary Western blot age ≥ 15 months to confirm that there is no HIV infection.
INTERCURRENT ILLNESSES
Pulmonary complications are recorded (see Appendix 2 for definitions). A respiratory evaluation is triggered by any one of the following symptoms, signs, and tests:
Unexplained cough for more than 5 days
Oral temperature >38°C for more than 5 days or rectal temperature >38.5°C for more than 5 days
Tachypnea
Crackles on auscultation
Appearance of digital clubbing
Oxygen saturation <96%
Abnormal chest roentgenogram
Primary caretakers were alerted to these symptoms and signs and instructed to contact the clinical study center should they develop. Unless an extrapulmonary diagnosis can be made, a respiratory symptom evaluation will be made, which includes the following:
Interval visit questionnaire and physical examination
Chest roentgenogram
Arterial blood gas
Blood culture
Respiratory viral cultures
CBC, ESR, LDH
An intercurrent illness algorithm was developed (Fig. 1) as a guideline to be followed after the respiratory symptom evaluation visit. The algorithm is intended to maximize the possibility of making a diagnosis while minimizing invasive studies that might decrease patient adherence. If the symptom evaluation visit reveals a new abnormality on chest X-ray or tachypnea, further evaluation is dependent on the presence or absence of hypoxemia. If a blood culture is positive with a new radiographic abnormality, the patient is treated for a bacterial pneumonia with antibiotics. If a clinical response is not observed within 72 hr, a bronchoalveolar lavage (BAL) is performed. If there is a negative culture and focal consolidation, and empiric antibiotic therapy is used with no response after 5 days, a BAL is done. An open lung biopsy is encouraged within 24 hr if a radiograph shows diffuse interstitial lung disease with a negative BAL and the patient remains hypoxemic.
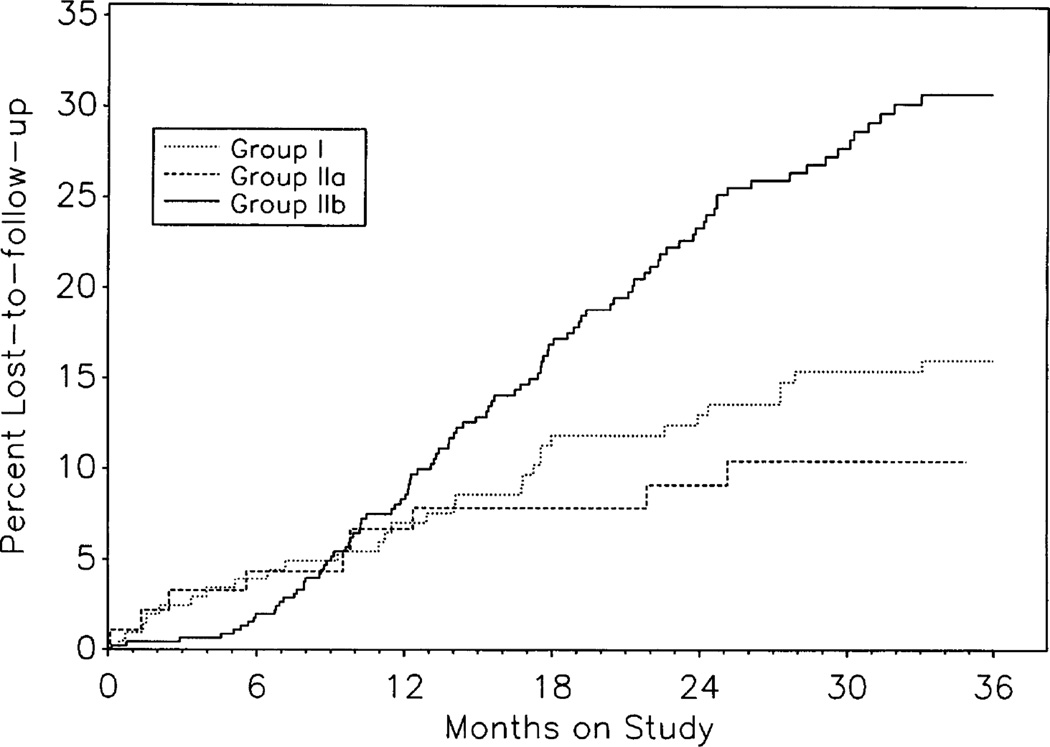
FIGURE 1.
Cumulative rates of loss to follow-up, estimated by the Kaplan-Meier method, are plotted for Groups I, IIa, and IIb using information available through of March 31, 1996. The cumulative loss-to-follow-up rate for all Group II children (not shown) is higher than for Groups IIa or IIb because the rates for Groups IIa and IIb exclude HIV-indeterminate children who are lost to follow-up before their HIV status can be determined. Loss-to-follow-up rates for all Group II children combined, including HIV indeterminates, are 26.9%, and 38.3% at 24 and 36 months, respectively.
A chronic lung disease algorithm was developed for the evaluation of an intercurrent illness if an abnormal chest X-ray or tachypnea persists for more than 2 weeks. When following the chronic lung disease algorithm, if the patient has negative BAL with persistent infiltrate for 6 months’ duration on radiograph, a thin-cut computerized tomography (CT) scan is recommended for diagnosis of bronchiectasis.
In the presence of an intercurrent illness a cardiac evaluation algorithm was developed so that assessment could be undertaken in a timely fashion. These guidelines were instituted because cardiovascular involvement is often clinically occult, may be life threatening, and signs and symptoms may erroneously be attributed to other organ systems. An assessment (physical examination, echocardiogram, electrocardiogram [ECG], and Holter monitor) is triggered if respiratory symptoms that are not attributed to lung disease are unresponsive for more than 7 days, if there are persistent respiratory symptoms with documented lung disease for more than 2 weeks, or if there are signs or symptoms that may be attributable to cardiovascular disease.
QUALITY ASSURANCE PROCEDURES
This study has implemented a number of procedures to ensure that the data obtained are of high quality. Prior to beginning recruitment, nurse coordinators and data management personnel from the clinical centers attended a 3-day training session at the CCC to review the protocol, manual of operations, and data management system. Personnel were certified in the following four areas: (1) forms completion, (2) data entry, (3) data transmission, and (4) query data reports. Monitoring of data submission, patient retention, and protocol compliance is accomplished by monthly reports, and through periodic site visits to the clinical centers. Additional steps are taken, as summarized below.
Laboratory
Since all of the participating centers in the study are members of the National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group (ACTG), all centers have quality control procedures for HIV testing and certain immunologic tests (e.g., leukocyte markers and flow cytometry). A central EBV laboratory performs all EBV culture and serology tests. To assure standardized performance of CMV results, an external quality control system has been implemented whereby every 6 months, known culture and serologic specimens are sent to all participating laboratories for blinded analysis, and results are compared with the known standards.
Cardiology
In addition to being measured by clinical center cardiologists, all fetal echocardiograms have been reviewed and reanalyzed by a single individual, to ensure uniformity. Similarly, to ensure uniformity of echocardiographic measurements, all postnatal echocardiograms are sent to a central location for offline digitization, to include measurements of systolic and diastolic ventricular function. Any Holter monitor report showing a disturbance of rhythm or conduction other than sinus tachycardia or sinus bradycardia is sent to a central Holter monitor reviewer for evaluation. Quality control of electrocardiograms is accomplished through a central review of a random sample of electrocardiographic tracings.
Pulmonary
Quality assurance of chest X-ray readings has been accomplished by periodically distributing a random sample of normal and abnormal films from each center to two other study radiologists for review in a blinded fashion, followed by review of all films by the radiologist at the CCC. All centers use the same equipment and software for pulmonary function testing. To assess intertechnician variability, random samples of pulmonary function test studies are analyzed by technicians from all pulmonary function testing sites on a periodic basis. Quality control of Tc99m DTPA studies is accomplished through a central review of all studies by the chair of the Nuclear Medicine Subcommittee, development of an atlas distributed to the nuclear medicine physicians, and follow-up conference calls. Quality control of spirometry is accomplished by examination of reproducibility of the two best measurements of forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC), out of five efforts recorded.
All cases of pneumonia in which no specific organism was isolated or pathological diagnosis was made (pneumonia, not otherwise specified) are reviewed at 6-month intervals. The investigators review the clinical course, chest X-rays, and laboratory findings to determine whether a specific diagnosis can be made.
DATA MANAGEMENT
Data are recorded on paper forms, and are entered on a personal computer into a FoxPro database [11] by personnel at each of the five clinical centers. Extensive use of required fields, range checks, and built-in skips are utilized at the time of key entry to minimize the possibility of data entry errors, and all data are re-key verified. In the first years of the study, a random sample of approximately 5% of the forms was also sent to the CCC for duplicate key entry; the rate of key-entry errors was found to be extremely low (0.1% or less). Data are transmitted nightly via modem to the main study computer at the CCC and further data inconsistencies are referred to the clinical centers for resolution. These queries are tracked in the main study database. To ensure confidentiality the CCC identifies subjects by study number only.
ANALYSIS PLANS
Incidence, prevalence, and recurrence rates of cardiac and pulmonary complications will be summarized using proportions or from Kaplan-Meier [12] analyses of cumulative incidence or recurrence. In Groups IIa and IIb, time from birth will be the natural time scale in time-to-event analyses. Cox proportional hazards regression models [13] will be used to examine the effects of multiple factors (e.g., treatment[s] received, immune status, maternal characteristics, coinfections) on time to complications or disease incidence and survival. Time-dependent covariates can be used to allow for persons changing treatments or receiving multiple treatments over the course of follow-up. Cumulative incidence rates at various time points (e.g., birth, 1 year) will be calculated for Groups IIa and IIb, as well as for selected subgroups. Logistic regression [14] will be used to determine factors that are associated with complications and to compare groups (e.g., IIa and IIb) after adjusting for confounding factors.
Longitudinal data on continuous outcomes such as cardiac function measurements from offline echocardiogram analysis (fractional shortening, contractility, afterload) or from pulmonary function testing (compliance, resistance, flow at functional residual capacity [FRC]) will be modeled using mixed-effects models [15] or unbalanced repeated measures models [16], which allow for data to be collected at irregular time points. These methods will allow, for example, estimation and comparison of mean profiles by age for Groups I, IIa, and IIb. Careful interpretation of longitudinal profiles or average growth curves for continuous outcomes such as height, weight, or cardiac or pulmonary function measurements of HIV-infected children will be required, recognizing that the patients in the cohort still being followed at a given age are survivors who may have had different growth profiles than the patients who are no longer being followed. When external normative data are available, data from Groups I, IIa, and IIb will be compared to nonstudy data by calculating and analyzing z scores. Mean profiles of original measurements as well as age or size-corrected z scores will be examined. These will require careful interpretation, because the Group IIa and IIb children are expected to differ in size owing to growth retardation, and standardization of many cardiac or pulmonary function measurements is based on height or body surface area. Also, analyses involving Group I children must be interpreted cautiously since this group is not a population-based sample and selection biases may have operated in the formation of this group; e.g., symptomatic children may have been more likely to be enrolled than nonsymptomatic children who have not come to medical attention. In addition, detailed information on Group I children between birth and time of enrollment is not available.
RESULTS OF DESIGN STRATEGY
Enrollment of participants began in May 1990 and continued through April 1993 in Group I and through January 1994 for Group II. The last Group II birth occurred in August 1994. We enrolled 206 infants and children in Group I and 612 fetuses and infants resulting from 599 separate pregnancies among 563 mothers in Group II. Of the 612 Group II enrollees, 444 (73%) were enrolled prenatally. Twelve (3%) of the prenatal enrollments were fetal deaths. As of March 1, 1996, 62 (10%) of the 612 Group II fetuses and infants were HIV indeterminate, and of the remainder, 95 (17%) were HIV infected (Group IIa) and 455 (83% were noninfected (Group IIb). Two hundred and sixteen Group IIb children from 204 mothers have been randomized to remain on study as controls. The 62 indeterminates either died (n = 17, 27%) or were lost to follow-up (n = 45; 73%) before HIV status could be determined. Selected characteristics of the mothers and children are shown in Tables 1 and 2,16a respectively.
TABLE 1.
Characteristics of mothers
| Characteristic | Group I (n = 205) | Group II (n = 588)a |
|---|---|---|
| Age (years) at birth of child: median (range) | 27.1 (16.5–43.5) | 26.6(15.5–41.8) |
| CD4%: mean ± SD | — | 27.3 ± 11.2 |
| CD4 cells/mm3: mean ± SD | — | 494 ± 386 |
| CD8%: mean ± SD | — | 50.7 ± 12.7 |
| CD4:CD8 ratio: mean ± SD | — | 0.64 ± 0.54 |
| Race (%) | ||
| Black non-Hispanic | 42.6% (86/202) | 49.6% (282/569) |
| Hispanic | 39.6% (81/202) | 32.2% (183/569) |
| White non-Hispanic | 15.3% (31/202) | 15.8% (90/569) |
| Other | 2.5% (5/202) | 2.5% (14/569) |
| HIV risk factors (%) | ||
| Used IV drugs | 34.4% (65/189) | 23.5% (129/549) |
| Sex with drug users | 62.9% (109/174) | 54.5% (266/488) |
| Sex with homosexual men | 7.7% (11/142) | 8.1% (35/431) |
| Blood transfusion prior to 1985 | 12.6% (22/174) | 5.0% (27/554) |
| Smoked while pregnant (%)b | 41.0% (73/178) | 38.3% (213/556) |
| Drank alcohol while pregnant (%)b | 24.1% (42/174) | 20.9% (115/551) |
| Used street drugs while pregnant (%)b | 33.7% (61/181) | 31.5% (176/558) |
| Used cocaine/crack while pregnant (%)b | 27.6% (50/181) | 23.7% (132/558) |
| AZT used during pregnancy (%) | — | 34.1% (192/563) |
| Medical history of anemia (%) | 37.0% (67/182) | 33.2% (185/557) |
Sample size is number of pregnancies.
Self-report by mothers.
TABLE 2.
Characteristics of children and infants at birth (Group II) or enrollment (Group I)
| Characteristic | Group I (n = 205) | Group II (n = 600)a |
|---|---|---|
| Age (months) at enrollment: median (range) | 22.9 (1.7–166) | — |
| Percent male | 46.3% (95/205) | 52.7% (316/600) |
| CDC pediatric HIV disease classification (1987 version) | ||
| P1: Asymptomatic infection | 11.7% (24/205) | — |
| P2: Symptomatic infectionb | 88.3% (181/205) | — |
| Nonspecific findings only (subclasses A, D3) | 25.4% (52/205) | — |
| Progressive neurologic (subclass B) | 20.5% (42/205) | — |
| LIP (subclass C) | 4.9% (10/205) | — |
| Nonneurologic (subclasses D1, D2) | 44.9% (92/205) | — |
| Other (subclass F) | 28.3% (58/205) | — |
| Number of past hospitalizations (%) | ||
| 0 | 24.9% (50/201) | — |
| 1 | 30.3% (61/201) | — |
| ≥2 | 44.8% (91/201) | — |
| Gestational age <37 weeks (%) | 24.5% (45/184) | 18.9% (111/587) |
| Birthweight (g)c,d: mean ± SD | 2872 ± 687 | 3018 ± 655 |
| Birthweight z scorec,d: mean ± SD | −0.55 ± 1.49 | −0.32 ± 1.18 |
| Birthweight < 2500 (%) | 28.6% (52/182) | 16.5% (93/562) |
| Birth length z scorec,d: mean ± SD | — | −0.20 ± 1.32 |
| Head circumference z scorec,d: mean ± SD | — | −0.64 ± 1.28 |
| Apgar (1-min): mean ± SD | — | 7.89 ± 1.46 |
| Apgar (5-min): mean ± SD | — | 8.78 ± 0.89 |
| Urine test positive for illicit drugs (n = 212 tested) (%) | — | 55.8% |
| Urine test positive for cocaine/crack (n = 212 tested) (%) | — | 39.6% |
Excludes n = 12 fetal deaths.
Numbers reported for subclasses B, C (Dl, D2), F are not exclusive.
Excludes n = 24 twin births.
z Scores calculated on basis of gestational age [16a].
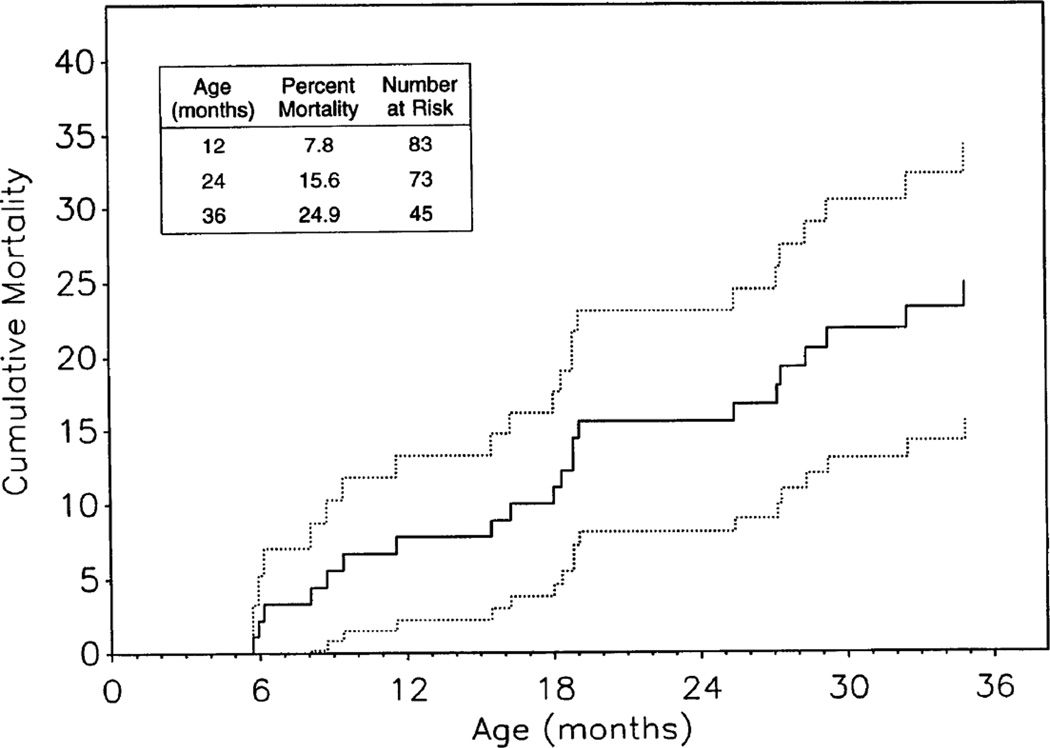
Retention of the cohorts of infected children (Groups I and IIa) was good, with only 16.0% (Group I) and 10.5% (Group IIa) lost to follow-up over 3 years (Fig. 1). Retention of noninfected Group IIb children was lower (30.6% lost to follow-up over 3 years). Kaplan-Meier cumulative mortality rates (± SE) through 2 and 3 years of life were 15.6% (± 3.8%) and 24.9% (± 4.9%) in Group IIa, respectively (Fig. 2), whereas the observed 3-year mortality was 0% in Group IIb. Cumulative mortality rates through 2 and 3 years since enrollment in Group I were 17.0% (± 2.7%) and 20.7% (± 2.9%), respectively.
FIGURE 2.
The cumulative mortality curve for the Group IIa children, estimated by the Kaplan-Meier method, is shown with 95% confidence limits (dotted lines).
DISCUSSION
This study prospectively follows a cohort of children born to HIV-infected mothers beginning during fetal life or within 28 days of birth (Group II) and another group of predominantly symptomatic children with HIV infection who presented after the neonatal period (Group I). Because the subjects have been receiving either standard care or were part of clinical drug trials, the study describes the treated disease process rather than the true natural history of the pulmonary and cardiac complications. The types and incidence of these complications as well as the factors associated with their occurrence can be determined by this design. HIV-infected mothers are enrolled into the study during pregnancy for characterization of clinical, immunologic, and viral factors during pregnancy, which provides a unique opportunity for relating the course of pulmonary and cardiac complications of HIV infected children to maternal factors. Group I allows the study of the course of pulmonary and cardiovascular complications at more advanced stages of infection.
The children born to HIV-infected mothers had a high rate of prematurity (gestational age <37 weeks) and low birth weight (weight <2500 g). However, these rates are similar to previously reported rates for low-income African-Americans and Hispanics in the United States [17–19].
The P2C2 recruitment efforts resulted in a Group I cohort with asymptomatic and symptomatic children with a wide range of disease severity (Table 2). Group IIa is more representative of the natural history of HIV infection because recruitment took place prenatally or at birth. The cumulative mortality rate of Group IIa at 36 months was 25%. This is comparable to the 19% mortality rate observed by the French Pediatric HIV Infection Study Group at 36 months [20,21].
A broad range of pulmonary and cardiac disorders is associated with HIV infection. Previous studies have either retrospectively described pulmonary and cardiovascular disorders in a cohort of HIV-infected patients or described a cross-section of patients who were not necessarily followed from birth [22–24]. Prospectively collected data regarding progression of disease, correlations with immunologic and infectious factors, and evaluation of common respiratory infections in HIV-infected patients have not been available. The children in this study cohort are characterized by clinical features, immunologic tests, and viral exposures that previous studies have indicated may play a role in development of a specific disease or be predictive of subsequent development of disease [7,8,25–28]. The design will help elucidate the role of these factors in prediction, diagnosis, and pathophysiology of pulmonary and cardiovascular disease. The longitudinal nature of this study permits a description of the causes and outcomes of specific disorders and an assessment of the impact of other factors in the course of the disorder. In addition, the effect of specific diseases on progression of HIV disease may also be assessed. Such data are important for program planning and resource allocation.
The majority of infants born to infected mothers do not acquire HIV infection; noninfected infants cannot be identified at birth using presently available techniques. This uninfected group (Group IIb) serves as a control in the study. The advantage of this group as a control is that it consists of children with similar environmental and psychosocial risks. However, it is not known whether these children who are exposed to HIV in utero or at time of delivery differ from unexposed normal children. The possibility exists that maternal HIV affects the immune system of exposed but apparently uninfected individuals. Evidence indicates that seropositive infants (i.e., those with passively acquired antibody but not infected) have slightly lower CD4 T lymphocyte counts in the first year as compared to normal controls [29]. If differences exist between Group IIb and normal controls, they may be ascribed either to the effects of developing in the womb of an HIV-infected mother or to factors such as maternal smoking, alcohol or drug abuse that are prevalent among Group IIb mothers. If no differences in this study are found in a comparison between Groups IIa and IIb, one cannot tell with certainty if the groups are similar because of exposure to maternal HIV infection, or because of shared psychosocial or other factors. However, comparisons can be made of Group IIb with normal data taken from populations with similar demographics (e.g., echocardiographic variables, infant pulmonary function, or growth). It would have been ideal to have an additional group of children followed from birth whose mothers were uninfected but were from socioeconomic backgrounds similar to those in Groups I and II, but this was not feasible for economic reasons.
HIV transmission from mother to infant can be significantly reduced by zidovudine treatment of the mother antepartum and intrapartum and by treatment of the infants postpartum, reducing transmission rates to as low as 8% [30]. Therefore, a large number of children must be enrolled into a clinical study to recruit a sufficient number of HIV-infected children. During the course of the study, advances in technology became available that allowed us to make earlier diagnosis of HIV infection in infants. We then were able to reduce costs significantly by randomly selecting a subset of the HIV-negative children for long-term follow-up.
Cost efficiency can also be achieved by optimizing retention of recruited participants. Our data show that we are dealing with a population that not only has significant health problems related to HIV, but also has significant alcohol and drug-related problems that could potentially interfere with study participation. Nevertheless, by having study nurse recruiters acting as case managers, we have demonstrated that excellent retention in the study over 48 months can be achieved in families with infected children. We were less successful in retaining those who were not infected and had few health problems. Other incentives to retain these subjects may be necessary if similar recruitment strategies are used in future studies.
In summary, this study has enrolled participants from a wide geographic area and represents a population that is likely to be typical of the national population of infants and children born to HIV-infected mothers. Because HIV-infected children are a growing segment of the population, careful documentation of disease patterns is essential. The systematic accumulation of information regarding cardiovascular and pulmonary complications and the assessment of factors relating to their development will be useful in the long-term management of patients and allocation of resources.
Acknowledgments
This study was supported by Contracts N01-HR-96037 through N01-HR-96043 from the National Heart, Lung, and Blood Institute, National Institutes of Health, and in part by NIH General Clinical Research Center Grants RR-00188, RR-02172, RR-00533, RR-00071, RR-00645, RR-00865, and RR-00043.
APPENDIX 1. Participants in the P2C2 HIV Study
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE
Hannah Peavy, M.D. (Project Officer), Anthony Kalica, Ph.D., Carol Kasten-Sportes, M.D., Elaine Sloand, M.D., George Sopko, M.D., M.P.H., Carol Vreim, Ph.D., Constance Weinstein, Ph.D., Margaret Wu, Ph.D.
CHAIRMAN OF THE STEERING COMMITTEE
Robert Mellins, M.D.
CLINICAL CENTERS
Baylor College of Medicine (Houston, TX)
William Shearer, M.D., Ph.D., a Stuart Abramson, M.D., Ph.D., Nancy Ayres, M.D., Carol Baker, M.D., J. Timothy Bricker, M.D., Gail Demmler, M.D., Marilyn Doyle, M.D., Maynard Dyson, M.D., Janet Englund, M.D., Nancy Eriksen, M.D., Arthur Garson, M.D., Bernard Gonik, M.D., Hunter Hammill, M.D., Thomas Hansen, M.D., I. Celine Hanson, M.D., Peter Hiatt, M.D., Keith Hoots, M.D., Robert Jacobson, M.D., Debra Kearney, M.D., Mark Kline, M.D., Claudia Kozinetz, Ph.D., M.P.H., Claire Langston, M.D., C. Lapin, M.D., Achi Ludomirsky, M.D., Warren Moore, M.D., Lawrence Pickering, M.D., Howard Rosenblatt, M.D., Edward Singleton, M.D., Larry Tabor, M.D., Theresa Aldape, MSW, Deborah Brinsdon, R.N., Nancy Calles, R.N., B.S.N., Madeline Cantini, R.N., B.S.N., Linda Davis, R.N., B.S.N., Kim Evans, P.N.P., Paula Feinman, David Flores, Alison Istas, M.P.H., Sharon Haymore, MSW, Suzanne Kirkpatrick, PNP, Jill Laflen, M.S., R.N., Lisa Luedtke, R.N., B.S.N., Mary Beth Mauer, R.N., B.S.N., Cheryl Maurice, P.A.C., Chuck Mazac, B.S., C.N.M.T., Ruth McConnell R.N., B.S.N., Laurence McKinney, CSW, Debra Mooneyham, R.N., Cathy Murtagh, PA., Valerie Nichols, R.N., B.S.N., Kelly O’Donnell, Sherryon Sterling, R.N., Teresa Tonsberg, R.N., Denise Treece, RRT, Pam Weaver, R.N., B.S.N.
The Children’s Hospital, Boston / Harvard Medical School (Boston, MA)
Steven Lipshultz, M.D., a Inas Al-Attar, M.D., Robert Cleveland, M.D., Steven Colan, M.D., Andrew Colin, M.D., Ellen Cooper, M.D., William Cranley, M.D., Thorn Griscom, M.D., Lisa Hornberger, M.D., John Kasznica, M.D., Kenneth McIntosh, M.D., Tracie Miller, M.D., E. John Orav, Ph.D., Stephen Pelton, M.D., Antonio Perez-Atayde, M.D., Stephen Saunders, M.D., Marcy Schwartz, M.D., Suzanne Steinbach, M.D., S. Ted Treves, M.D., Ruth Tuomala, M.D., Mary Ellen Wohl, M.D., Cynthia Barber, M.P.H., Roxellen Bayer, R.N., Ann Marie Boller, B.A., Nancy Borden, B.S., Pamela D’Arcy, R.N., Rachel Diness, L.C.S.W., Helen Donovan, Sartreina Dottin, L.C.S.W., Julie Druker, M.H.S., Mary Ford, R.N., Cheryl Gothing, R.N., Nina Greenbaum, M.P.H., C.H.E.S., Lisa Heughan, Janice Hunter, M.S., Karen Lewis, R.N., Kathleen Masterson, B.A., Ellen McAuliffe, B.S.N., Suzanne Mone, M.S., Nandini Moorthy, M.B.B.S., Amy Nguyen, Patricia Ray, B.S., Sheryl Ross, R.N., Andrew Schiavoni, M.S., Chris Thayer, B.S., Pamela Torre, M.S.W., Samten Williams, R.N., B.S.N., Joan Winchester, B.A.
Mount Sinai School of Medicine (New York, NY)
Meyer Kattan, M.D., a Asher Barzalai, M.D., Richard Bonforte, M.D., Renata Dishe, M.D., Stephen Heaton, M.D., David Hodes, M.D., Chun Kim, M.D., Neal Kotin, M.D., Wyman Lai, M.D., Elisabeth Luder, Ph.D., Karen Norton, M.D., Vicky Peters, M.D., Samuel Ritter, M.D., Joel Rosen, M.D., Kumundini Shah, M.D., Rhoda Sperling, M.D., Andrew Ting, M.D., Robin Berry, Debbie Benes, M.S., R.N., Alice Betita, B.S., Diane Carp, M.S.N., R.N., Geraldo Ellis, R.N., Alexia Karaefthimoglu, Jennifer Kas, R.N., Donna Lewis, Mary Lyons, M.S., R.N., Sue Mone, M.S., Diane Ranieri, P.A., Deo Religioso, Elizabeth Salazar, B.A., Aurora Valones, B.S., Mary Ann Worth, R.N., Gloria Xanthos, R.N.
Presbyterian Hospital in the City of New York / Columbia University (New York, NY)
Robert Mellins, M.D., a Fred Bierman, M.D. a (through May 1991), Philip Alderson, M.D., Walter Berdon, M.D., Michael Bye, M.D., Renata Dische, M.D., M. Fleischman, M.D., Harold Fox, M.D., Welton Gersony, M.D., Sarmistha Hauger, M.D., Anastassios Koumbourlis, M.D., Scott Miller, M.D., Jane Pitt, M.D., Lynne Quittel, M.D., Paul Shurin, M.D., Thomas Starc, M.D., Philip Ursell, M.D., Robert Winchester, M.D., Patricia Boyle, R.N., B.S.N., Anthony Brown, Bismania Burgos, Maritza Cardona, C.S.W., Ray Cases, Margaret Challenger, Brian Currid, Jane DeLuca, M.S., R.N., Kim Geromanos, M.S., R.N., Darcy Gulbin, R.N., Alice Higgins, M.P.H., R.N., Lena Jackson, Regina Jennings, C.S.W., Andrea Jurgrau, P.N.P., David Montague, B.S., George Nevrodis, Gloria Ramirez-Scully, R.N., Caroline Rostant, C.S.W.
UCLA School of Medicine (Los Angeles, CA)
Samuel Kaplan, M.D., a Y. Al-Khatib, M.D., Ines Boechat, M.D., Pamela Boyer, M.D., Ph.D., Yvonne Bryson, M.D., David Chen, M.D., Joseph Church, M.D., R. Dorio, M.D., Robin Doroshow, M.D., Stacey Drant, M.D., Robert Elashoff, Ph.D., Alistair Fyfe, M.D., Ph.D., Meena Garg, M.D., Barbara George, M.D., Randall Hawkins, M.D., Carol Hoh, M.D., Arno Hohn, M.D., Josephine Isabel-Jones, M.D., Andrea Kovacs, M.D., Charles Marboe, M.D., Barry Marcus, M.D., John Miller, M.D., Arnold Platzker, M.D., Robert Settlage, M.D., Timothy Triche, M.D., Linda Vachon, M.D., Roberta Williams, M.D., Marilyn Woo, M.D., Beverly Wood, M.D., Sandra Blaauw, R.N., Jeffrey Chen, M.S., Helene Cohen, P.N.P., R.N., Norma Dolmo, Marie Earle, Lynn Fukushima, M.S.N., R.N., Eileen Garratty, S.R.A., Sharon Golden, R.D.M.S., Lucy Kunzman, R.N., M.S., C.P.N.P., Jennifer Kwon, Elaine Moore, Mary Pica, Karen Qi, M.S., Kevin Saiki, B.S., Mary Schultze, R.N., Myung Sim, M.S., Karen Simandle, R.D.M.S., Angie Williams, Ah-Lin Wong, R.D.M.S.
CLINICAL COORDINATING CENTER
The Cleveland Clinic Foundation (Cleveland, OH)
Mark Schluchter, Ph.D., a James Boyett, Ph.D. (through July 1991), a Barbara Baetz-Greenwalt, M.D., Gerald Beck, Ph.D., Johanna Goldfarb, M.D., Michael McHugh, M.D., Atul Mehta, M.D., Moulay Meziane, M.D., Douglas Moodie, M.D., Amrik Shah, Sc.D., Richard Sterba, M.D., George Williams, Ph.D., Kenneth Abraham, B.S., Jeanette Bradley, B.S., Cindy Chen, M.S., Kirk Easley, M.S., Scott Husak, B.S., Victoria Konig, ART, Kevin McCarthy, RCPT, Joseph McPherson, B.S., Venita Midcalf, M.B.A., Lisa Rybicki, M.S., Paul Sartori, B.S., Lori Schnur, B.S., Susan Sunkle, B.A.
Consultants: Case Western Reserve University (Cleveland OH)
Harold Houser, M.D., Richard Martin, M.D.
CENTRAL LABORATORY FOR EPSTEIN-BARR VIRUS TESTING
The University of Texas Health Sciences Center at San Antonio (San Antonio, TX)
Ciro V. Sumaya, M.D. (through December 1992), a Hal Jenson, M.D.,a Yasmin Ench, B.S.
aPrincipal Investigator.
APPENDIX 2. Criteria for diagnosis of specific pulmonary complications
| Complication | Criteria |
|---|---|
| a. Upper respiratory illness | Rhinitis, pharyngitis, or otitis diagnosed by appropriate history and physical examination |
| b. Fungal pulmonary infection | Clinical evidence of pneumonia, new CXR abnormality, and tracheal secretion positive for fungal organism (by stain or culture) |
| c. Viral pneumonia | Clinical evidence of pneumonia, new CXR abnormality, and virus recovered from lavage fluid or lung biopsy without bacterial or PCP infection. Histologic evidence of invasive infection required for diagnosis of CMV |
| d. Bacterial pneumonia | Clinical evidence of pneumonitis (i.e., cough, tachypnea, rales, and purulent sputum), new chest X-ray abnormality, leukocytosis (> 15,000 WBCs, predominantly polymorphonuclear cells) with intracellular organisms from respiratory secretions or positive blood culture, or positive cold agglutinins |
| e. Mycobacterial pulmonary infection | New CXR abnormality, mycobacteria recovered from BAL or lung biopsy, or positive gastric aspirate |
| f. Pneumocystis carinii pneumonia | Evidence of organisms from BAL fluid or open lung biopsy |
| g. Lymphoproliferative interstitial pneumonia | Evidence of interstitial lung disease by CXR, BAL negative for PCP, open lung biopsy histologically consistent for LIP or PLHa |
| h. Other interstitial lung disease | Evidence of either interstitial lung disease by CXR, or pathology from open lung biopsy consistent with interstitial lung disease. BAL negative for PCPb |
| i. Chronic obstructive lung disease | Clinical findings, including expiratory airway obstruction on physical examination and/or chest roentgenographic evidence and evidence of hyperinflation in absence of clinical and/or laboratory finds of infection |
| j. Airway hyperreactivity | Obstructive airway disease with clinical response to bronchodilators |
| k. Upper airway obstruction | Abnormality by direct inspection and/or appropriate clinical history |
| l. Pulmonary vascular disease | Histologic evidence on open lung biopsy,b or abnormality defined on cardiac catheterization or pulmonary angiography |
| m. Bronchiolitis | Clinical findings consistent with bronchiolitis and absence of lobar consolidation on chest radiograph |
POLICY, DATA, AND SAFETY MONITORING BOARD
Henrique Rigatto, M.D. (Chairman), Edward B. Clark, M.D., Robert B. Cotton, M.D., John Johnson, M.D., Vijay V. Joshi, M.D., Paul S. Levy, Sc.D., Norman S. Talner, M.D., Patricia Taylor, Ph.D., Robert Tepper, M.D., Ph.D., Janet Wittes, Ph.D., Robert H. Yolken, M.D., Peter E. Vink, M.D.
References
- 1.Centers for Disease Control. MMWR. Vol. 31. New York, New Jersey, California: 1982. Unexplained immunodeficiency and opportunistic infections in infants; pp. 665–667. [PubMed] [Google Scholar]
- 2.Oleske J, Minnefor A, Cooper R, et al. Immune deficiency in children. JAMA. 1983;249:2345–2349. [PubMed] [Google Scholar]
- 3.Rubinstein A, Sicklick M, Gupta A, et al. Acquired immunodeficiency with reversed T4/T8 ratios in infants born to promiscuous and drug addicted mothers. JAMA. 1983;249:2350–2356. [PubMed] [Google Scholar]
- 4.Scott AB, Buck BE, Letterman JG, et al. Acquired immunodeficiency syndrome in infants. N Engl J Med. 1984;310:76–81. doi: 10.1056/NEJM198401123100202. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein A, Morecki R, Silverman B, et al. Pulmonary disease in children with acquired immunodeficiency syndrome and AIDS-related complex. J Pediatr. 1986;108:489–503. doi: 10.1016/s0022-3476(86)80822-8. [DOI] [PubMed] [Google Scholar]
- 6.Marolda J, Pace B, Bonforte RJ, et al. Outcome of mechanical ventilation in children with acquired immunodeficiency syndrome. Pediatr Pulmonol. 1989;7:230–234. doi: 10.1002/ppul.1950070408. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz S, Chanock S, Sanders SP, et al. Cardiac manifestations of human immunodeficiency virus infection in infants and children. Am J Cardiol. 1989;63:1489–1497. doi: 10.1016/0002-9149(89)90014-3. [DOI] [PubMed] [Google Scholar]
- 8.Luginbuhl LM, Orav EJ, McIntosh K, et al. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269:2869–2875. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Classification system for human immunodeficiency virus (HIV) infection in children under 13 years of age. MMWR. 1987;36:225–236. [PubMed] [Google Scholar]
- 10.Pitt J, Goldfarb J, Schluchter M, et al. for the P2C2 HIV Study Group. Transmission rate determinations are subject to differing definitions and therefore different rates; First National Conference on Human Retroviruses and Related Infections, Program and Abstracts, abstract no. 662; 1993. Dec 12–16, [Google Scholar]
- 10a.Joshi VV, Oleske JM, Minnefor AB, et al. Pathologic pulmonary findings in children with the acquired immunodeficiency syndrome: A study of ten cases. Hum Pathol. 1985;16:241–246. doi: 10.1016/s0046-8177(85)80009-5. [DOI] [PubMed] [Google Scholar]
- 10b.Edelson JD, Hyland RH. Pulmonary complications of AIDS: A clinical strategy. Can Med Assoc J. 1989;140:1281–1287. [PMC free article] [PubMed] [Google Scholar]
- 11.FoxPro Relational Database Management System. Perrysburg, Ohio: Fox Software, Inc.; 1989. [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;8:699–712. [Google Scholar]
- 13.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34B:187–220. [Google Scholar]
- 14.Hosmer DW, Jr, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 15.Laird NM, Ware JE. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 16.Jennrich RI, Schluchter MD. Unbalanced repeated measures models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 16a.Babson SG, Benda GI. Growth graphs for the clinical assessment of infants of varying gestational age. J Pediatr. 1976;89:814–820. doi: 10.1016/s0022-3476(76)80815-3. [DOI] [PubMed] [Google Scholar]
- 17.Collins JW, Shay DK. Prevalence of low birth weight among Hispanic infants with United States and foreign-born mothers: The effect of urban poverty. Am J Epidemiol. 1994;139:184–192. doi: 10.1093/oxfordjournals.aje.a116980. [DOI] [PubMed] [Google Scholar]
- 18.Collins JW, David RJ. The differential effect of traditional risk factors on infant birthweight among blacks and whites in Chicago. Am J Public Health. 1990;80:679–681. doi: 10.2105/ajph.80.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventura SJ, Martin JA, Taffel SM, Matthews TJ, Clarke SC. Monthly Vital Statistics Report. Vol. 43. U.S. Department of Health and Human Services; 1994. Advance report of final natality statistics, 1992. 5(S) [PubMed] [Google Scholar]
- 20.The European Collaborative Study. Natural history of vertically acquired human immunodeficiency virus-1 infection. Pediatrics. 1994;94:815–819. [PubMed] [Google Scholar]
- 21.Blanche S, Mayaux MJ, Rouzioux C, et al. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N Engl J Med. 1994;330:308–312. doi: 10.1056/NEJM199402033300502. [DOI] [PubMed] [Google Scholar]
- 22.Marolda J, Pace B, Bonforte RJ, et al. Pulmonary manifestations of HIV infection in children. Pediatr Pulmonol. 1991;10:231–235. doi: 10.1002/ppul.1950100402. [DOI] [PubMed] [Google Scholar]
- 23.Scott GB, Hutto C, Makuch RW, et al. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein LJ, Bye MR, Rubinstein A. Prognostic factors and life expectancy in children with acquired immunodeficiency syndrome and Pneumocystis carinii pneumonia. Am J Dis Child. 1989;143:775–778. doi: 10.1001/archpedi.1989.02150190025013. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitz E, Regaud M, Pollack H, et al. Pneumocystis carinii pneumonia in infants infected with the human immunodeficiency virus with more than 450 CD4+ lymphocytes pet cubic millimeter. N Engl J Med. 1990;323:531–533. doi: 10.1056/NEJM199008233230807. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs A, Frederick T, Church J, et al. CD4 T-lymphocyte counts and Pneumocystis carinii pneumonia in pediatric HIV infection. JAMA. 1991;265:1698–1703. [PubMed] [Google Scholar]
- 27.Andiman WA, Eastman R, Martin K, et al. Opportunistic lymphoproliferations associated with Epstein-Barr viral DNA in infants and children with AIDS. Lancet. 1985;2:1390–1393. doi: 10.1016/s0140-6736(85)92557-7. [DOI] [PubMed] [Google Scholar]
- 28.Chayt KJ, Harper ME, Marselle LM, et al. Detection of HTLV-III RNA in lungs of patients with AIDS and pulmonary involvement. JAMA. 1986;256:2356–2359. [PubMed] [Google Scholar]
- 29.Gesner M, Papaevangelou V, Kim M, et al. Alteration in the proportion of CD4 T-lymphocytes in a subgroup of human immunodeficiency virus-exposed uninfected children. Pediatrics. 1994;93:624–630. [PubMed] [Google Scholar]
- 30.Conner EM, Sperling RS, Gelber R, et al. for the Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. Reduction of maternal-infant transmission of human immunodeficiency virus type I with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]