Abstract
Recent studies have identified recurrent mutations in SETBP1, the gene that encodes SET-binding protein 1, in several types of myeloid malignancies, including chronic myeloid and acute myeloid leukemias. The identified mutations frequently target the SKI-homologous domain, although the exact pathogenic mechanisms remain unknown.
Myeloid malignancies are a broad class of blood disorders, including acute myeloid leukemia (AML), chronic myelomonocytic leukemia (CMML) and myelodysplastic/myeloproliferative neoplasm (MDS/MPN), in which the events leading to oncogenic transformation and disease progression are not completely understood. Although previous efforts have identified genetic perturbations in components of key signaling pathways (including alterations affecting JAK2, KRAS, NRAS and FLT3)1, as well as in regulators of histones and DNA methylation (including EZH2, KDM6A, MLL, TET2, IDH1, IDH2 and DNMT3A)2, the continued identification of additional driver lesions improves understanding of the molecular basis of these diseases.
SETBP1 mutations in myeloid disease
With genetic changes identified in three recent papers from Gambacorti-Passerini3, Maciejewski4 and Kojima5 and their respective colleagues (Fig. 1a), as well as mutations identified elsewhere6–9, it is now clear that somatic mutations in SETBP1 are an important genetic event in several classes of myeloid malignancies.
Figure 1.
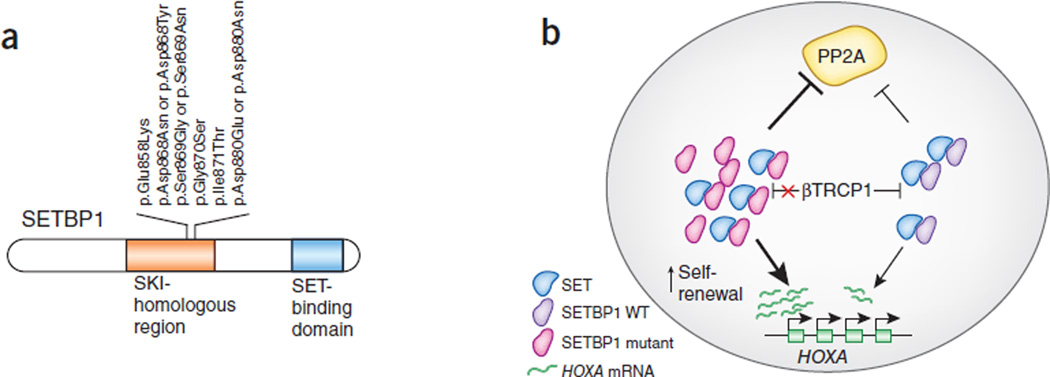
SETBP1 alterations and their proposed effects on myeloid cells. (a) SETBP1 alterations in myeloid malignancies affecting a specific putative degron in the SKI-homologous region of the protein. (b) Alterations lead to higher stability of the protein and, as a result, higher expression levels of the HOXA gene cluster as well as greater inhibition of PP2A action through the activity of the SET protein. WT, wild type.
Piazza et al.3 initially reported recurrent p.Gly870Ser substitutions in atypical chronic myeloid leukemia (aCML). The underlying mutations are identical to germline lesions found in Schinzel-Giedion syndrome, a disorder marked by severe mental retardation and high tumor incidence10. Using targeted resequencing of a large cohort of aCMLs and other myeloid malignancies, the authors identified additional SETBP1 mutations in 24.3% of aCMLs, 10% of unclassified MDS/MPNs and 4% of CMMLs, with all mutations targeting the SKI-homologous region of the protein.
Similarly, Makishima et al.4 surveyed SETBP1 in a cohort of 727 myeloid malignancies and identified mutations affecting the SKI-homologous domain in 52 cases. This study reported high rates of recurrent somatic mutations in SETBP1 in secondary AML (16.8%) and CMML (14.5%). Finally, Sakaguchi et al.5 also reported mutations targeting the SKI-homologous domain in 7.6% of JMML, a myeloid tumor in which somatic and germline mutations in the RAS pathway are nearly obligatory. Both Makashima et al. and Sakaguchi et al. have provided evidence that mutations in SETBP1 likely occur after the initial establishment of disease and contribute to tumor progression or evolution, and these findings constitute an important point that has not been proposed by others3,6–9. In addition to these three reports, studies from several other groups have confirmed that SETBP1 is an independent prognostic factor in myeloid disease, such that somatic mutations are associated with significantly shorter survival and higher white blood cell counts6–9.
Oncogenic functions of SETBP1
Previous evidence suggested a pro-oncogenic role for SETBP1, as its overexpression in myeloid progenitors led to enhanced self-renewal11, and a gene fusion involving SETBP1 and NUP98 was identified in T cell acute lymphoblastic leukemia12.
SETBP1 is known to bind the SET nuclear oncoprotein13, and the resulting complex has an apparent inhibitory effect on protein phosphatase type 2a (PP2A)14, a putative tumor suppressor15 (Fig. 1b). With respect to this function, both Piazza et al. and Makishima et al. have shown that mutant SETBP1 alleles conferred overall diminished PP2A activity. However, it is not clear if this is the main mechanism underlying SETBP1 oncogenic activity.
Indeed, others have suggested a role for SETBP1 in the direct transcriptional activation of the HOXA9 and HOXA10 genes in both human and mouse myeloid progenitors11 (Fig. 1b). Makishima et al.4 verified this finding by showing that overexpression of either wild-type or mutant SETBP1 immortalized mouse myeloid progenitors and resulted in upregulation of Hoxa9 and Hoxa10. Notably, cells expressing mutant SETBP1 had higher proliferative capacity and greater ability to form colonies in vitro compared to controls expressing wild-type SETBP1. Additionally, the authors verified that this oncogenic phenotype was dependent on Hoxa9 and Hoxa10 expression, as ablation of either gene halted the ability of myeloid progenitors to form colonies in vitro.
Another possibility is that the mutations affecting the SKI-homologous domain may alter SETBP1 protein function by increasing its stability. Piazza et al. proposed that these mutations disrupt a phosphodegron that is a key signal for recognition by the E3 ubiquitin ligase βTRCP1, thereby allowing SETBP1 to avoid ubiquitination and subsequent proteasomal degradation3 (Fig. 1b). This mechanism supports the idea that mutant SETBP1 can evade post-translational control, thereby sustaining its oncogenic potential in a manner that could persist in the absence of transcriptional overexpression. However, Makishima et al.4 report that mutant SETBP1 protein is no more stable than wild-type protein and that its mRNA transcript tends to be overexpressed in mutant cases as well as in a subset of cases with wild-type SETBP1.
Together, these three reports implicate SETBP1 as a new player in the pathogenesis of a wide spectrum of myeloid malignancies. Although SETBP1 mutational status has been suggested as a prognostic marker, its potential value as a target for therapeutic intervention will become clearer when the molecular functions of both the wild-type and mutant proteins are better understood. The consideration of SETBP1 as an oncogenic factor is especially interesting owing to its apparent functional duality as both a negative regulator of PP2A activity and a transcriptional regulator. Whereas regulation of the HOXA gene cluster by the SET-SETBP1 complex has previously been reported, Piazza et al. also demonstrated that many transforming growth factor (TGF)-β–responsive genes were upregulated in SETBP1-mutant cases. This interesting observation suggests that SETBP1 may have a direct role in the transcriptional regulation of other genes, a hypothesis worthy of further study. Continued investigation into the physiological activity of SETBP1 will be needed to gain a complete understanding of how each of its proposed functions can contribute to disease. Finally, the development of animal models using SETBP1 mutations combined with other lesions, such as mutations affecting ASXL1 and CBL, may allow for further insights into myeloid tumors.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Gilliland DG. Curr. Opin. Hematol. 2001;8:189–191. doi: 10.1097/00062752-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Shih AH, Abdel-Wahab O, Patel JP, Levine RL. Nat. Rev. Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
- 3.Piazza R, et al. Nat. Genet. 2013;45:18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makishima H, et al. Nat. Genet. 2013;45:942–946. doi: 10.1038/ng.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi H, et al. Nat. Genet. 2013;45:937–941. doi: 10.1038/ng.2698. [DOI] [PubMed] [Google Scholar]
- 6.Meggendorfer M, et al. Leukemia. 2013 Apr 30; published online; [Google Scholar]
- 7.Damm F, et al. Leukemia. 2013;27:1401–1403. doi: 10.1038/leu.2013.35. [DOI] [PubMed] [Google Scholar]
- 8.Laborde RR, et al. Leukemia. 2013 Apr 5; published online; [Google Scholar]
- 9.Thol F, et al. Leukemia. 2013 May 7; published online; [Google Scholar]
- 10.Hoischen A, et al. Nat. Genet. 2010;42:483–485. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 11.Oakley K, et al. Blood. 2012;119:6099–6108. doi: 10.1182/blood-2011-10-388710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panagopoulos I, et al. Br. J. Haematol. 2007;136:294–296. doi: 10.1111/j.1365-2141.2006.06410.x. [DOI] [PubMed] [Google Scholar]
- 13.Minakuchi M, et al. Eur. J. Biochem. 2001;268:1340–1351. doi: 10.1046/j.1432-1327.2001.02000.x. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Makkinje A, Damuni Z. J. Biol. Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 15.Gallipoli P, Abraham SA, Holyoake TL. Hematol. Oncol. Clin. North Am. 2011;25:951–966. doi: 10.1016/j.hoc.2011.09.001. [DOI] [PubMed] [Google Scholar]