Abstract
Purpose
To compare rates of topographic change in ocular hypertensive eyes that do or do not develop primary open angle glaucoma (POAG) and to identify factors that influence the rate of change.
Design
Longitudinal, randomized clinical trial.
Methods
441 participants (832 eyes) in the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study were included. POAG was defined as repeatable visual field and/or photography-based optic disc changes. The rate of topographic change in the 52 participants (66 eyes) who developed POAG was compared to participants who did not develop POAG using multivariable mixed effects models.
Results
In both univariate and multivariable analyses, the rate of rim area loss was significantly faster in eyes developing POAG than in eyes that did not (univariate mean: −0.0131 mm2/yr and −0.0026 mm2/yr, respectively). The significantly faster rate of rim area loss in African Americans found in univariate analysis did not remain significant when baseline disc area was included in the model. In multivariable analyses, the rate of rim area loss and other topographic parameters was also significantly faster in eyes with worse baseline visual field PSD and higher IOP during follow-up. Moreover, a significant rate of rim area loss was detected in eyes that did not develop POAG (p<0.0001). The rate of rim area loss in eyes developing an optic disc endpoint was significantly faster than those developing a visual field endpoint.
Conclusions
The rate of rim area loss is approximately 5 times faster in eyes that developed POAG compared to eyes that did not. These results suggest that measuring the rate of structural change can provide important information for the clinical management of ocular hypertensive patients. Additional follow-up is needed to determine whether the statistically significant change in the eyes that did not develop POAG represents normal aging or glaucomatous change not detected by conventional methods.
INTRODUCTON
Detection of glaucomatous change is one of the most challenging aspects of the clinical assessment of ocular hypertensive and glaucoma patients. The ability to differentiate between eyes that are progressing rapidly and eyes that are progressing slowly is important for the appropriate management of patients with glaucoma. Patients with rapidly progressing glaucoma may require adjustments to the treatment regimen to prevent the development of significant visual impairment depending on their age and life expectancy. Alternatively, patients with slowly progressing disease may require less aggressive treatment, when significant visual dysfunction is not expected in their lifetimes.
New image analysis techniques have improved our ability to identify structural change and, most importantly, to measure the rate of optic disc and retinal nerve fiber layer changes. Although imaging instruments have been available for almost 20 years, there is a paucity of information on the rate of structural change in patients with ocular hypertension.[1–5] Because glaucoma is a slowly progressing disease that occurs in a relatively small proportion of ocular hypertensive patients, studies investigating structural change over time in ocular hypertensive patients require extensive follow-up and a large population. The Confocal Scanning Laser Ophthalmoscopy (CSLO) Ancillary Study to the Ocular Hypertensive Treatment Study (OHTS) was initiated in 1995 with annual CSLO imaging through 2008 to provide the long-term follow-up necessary to characterize structural change over time in ocular hypertensive patients.[6–9] The purpose of this report is to compare rates of change in topographic optic disc parameters in eyes that developed primary open angle glaucoma (POAG) to those eyes that did not develop POAG and to evaluate factors that influence the rate of structural change.
METHODS
Participants included in this report met the OHTS inclusion and exclusion criteria[10] and participated in the CSLO Ancillary Study to the OHTS with at least one good quality Heidelberg Retina Tomograph image during follow-up. Seven OHTS clinics participated in the CSLO Ancillary Study to the OHTS: Hamilton Glaucoma Center, University of California, San Diego, California; New York Eye and Ear Infirmary, New York, New York; Devers Eye Institute, Portland, Oregon; Henry Ford Medical Center, Troy Michigan; Jules Stein Eye Institute, University of California, Los Angeles, California: University of California, Davis, California; and Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania. The OHTS Clinical Trial Registration Number (clinical trials.gov) is NCT00000125. The CSLO Ancillary study was conducted in compliance with the Institutional Review Board and HIPAA requirements at each study center. Written informed consent for participation in this ancillary study was obtained from all participants prior to enrollment.
OHTS eligibility criteria required participants to have an IOP ranging from 24 mm Hg to 32 mm Hg in at least one eye and 21 mm Hg to 32 mm Hg in the fellow eye, as well as 2 normal, reliable automated achromatic 30-2 full threshold visual fields (Carl-Zeiss-Meditec, Dublin, California) together with normal appearing optic discs based on clinical examination and review of stereoscopic optic disc photographs[10] The Optic Disc Reading Center graders assessed photographs and estimated horizontal and vertical cup-to-disc ratios by contour.
The development of POAG, the primary endpoint for OHTS, was defined as the development of a confirmed visual field abnormality or a confirmed clinically significant stereophotograph-based optic disc deterioration attributed to POAG by a masked Endpoint Committee.[10] Specifically, the Endpoint Committee reviewed all confirmed visual field abnormalities and confirmed disc progression to determine whether the change was “most probably due to POAG”, “most probably not due to POAG” or, in the case of disc progression, whether the progression was “not clinically significant” or an artifact. Endpoint committee members, masked as to treatment history, reviewed baseline and follow-up case report forms, visual fields and stereoscopic disc photographs of both eyes. The first date of three consecutive abnormal visual fields or the first date of two consecutive sets of stereophotographs that classified the eye as reaching a POAG endpoint was used as the date for a POAG endpoint in all analyses.
As described previously,[6–9] three 10-degree images were obtained on both eyes and three 15-degree Heidelberg Retina Tomograph (HRT; Heidelberg Engineering, GmbH, Heidelberg, Germany) images were obtained on the right eye at the annual OHTS dilated fundus examination. If both the 10-degree and the 15-degree good quality images were available, the 10-degree images were used in this analysis. The scans were obtained using the HRT 1 Classic instrument throughout the study and analyzed using software version 3.0. Corneal curvature measurements were used to correct images for magnification error. Corrective lenses were used during image acquisition when astigmatism was greater than one diopter. The mean of 3 images was used for statistical analyses. As described previously, the CSLO Reading Center at the University of California, San Diego conducted all quality assessment and image processing and certified all operators at every site according to standard protocols.[9] In brief, CSLO Reading Center staff reviewed each image series (images at 32 consecutive focal planes) for clarity, appropriate focus and depth adjustment, and minimal eye movement. In addition, each mean topography image was monitored for adequate reproducibility (standard deviation of the mean image <50 µm). Out of a total of 7556 right and left eye testing sessions, data for 461 (6.1%) sessions were excluded from the analysis due to poor quality images.
Because the CSLO Ancillary Study to the OHTS was funded after the initiation of enrollment in OHTS, 77% of participants completed their first CSLO examination visit after their OHTS baseline, randomization visit.[6, 7] For this reason, 7 participants with documented optic disc deterioration or visual field abnormality that was subsequently confirmed and attributed to POAG at or before their first CSLO imaging session were excluded from the analysis. The current report includes all good quality images from the first CSLO visit to the closure date for the OHTS (March 2009) or to the first suspicious date of POAG, whichever was first.
The rate of topographic change was measured using the following CSLO stereometric parameters rim area and volume, cup area and volume, rim-to-disc area ratio, mean cup depth, retinal nerve fiber layer (RNFL) thickness and cross-sectional area, and cup shape. Contour lines outlining the disc margin of the baseline image, necessary for calculating stereometric parameters were drawn by a certified operators at the University of California, San Diego CSLO Reading Center while viewing a copy of stereoscopic optic disc photographs from the OHTS Optic Disc Reading Center.[9] HRT software automatically places the contour line on all follow-up images. Reference plane based stereometric parameters were measured relative to the standard reference plane calculated 50 microns posterior to the mean height contour along a small temporal section of the contour line. The standard deviation of the mean image was used as a measure of image quality and included as a covariate with repeated measures in multivariable models. Measurements of rim area were also evaluated in six sectors. The 6 sectors defined using standard HRT software are not of equal size; the temporal inferior, temporal superior, nasal superior, nasal inferior sectors are each 45 degrees, while the temporal and nasal sectors are each 90 degrees.
In addition, the rate of change of the Glaucoma Probability Score (GPS) was also calculated. This measure does not require a reference plane and is operator independent as it does not depend on an operator drawn contour line to outline the disc margin. As described previously, the GPS is based on a geometric model of 5 parameters (cup size, cup depth, rim steepness, horizontal retinal nerve fiber layer curvature, and vertical retinal nerve fiber layer curvature) that describes the shape of the optic disc/parapapillary retina (globally and locally).[7] These parameters are then used as inputs to a relevance vector machine classifier, and the resulting output summarized as the probability (between 0% and 100%) that the eye is glaucomatous (based on fit to training data from healthy and glaucoma eyes).
Statistical analysis
The final OHTS and CSLO analysis data sets include all OHTS and CSLO data and POAG endpoints with initial suspicious dates that were confirmed and entered into the database by March 2009. The primary analysis compares the rate of topographic change in eyes that developed POAG endpoints and that did not develop endpoints as determined by the Endpoint Committee. In addition, the rate of HRT topographic rate of change was estimated in those eyes that developed 1) an initial optic disc POAG endpoint only, 2) an initial visual field POAG endpoint, and 3) demonstrated reproducible optic disc change as determined by the Optic Disc Reading Center, including those not considered as POAG endpoints by the endpoint committee. Specifically, eyes determined to have optic disc progression by the Optic Disc Reading Center included eyes classified by the Endpoint Committee as an OHTS optic disc POAG endpoint (“most probably due to POAG”), as well as eyes not considered a optic disc POAG endpoint (“most probably not due to POAG” or, “not clinically significant” or an artifact). For participants that developed unilateral POAG, only the POAG eye was included in the analysis.
All descriptive tables (means and percentages) of ophthalmic measures include the right and left eyes separately as each eye was used in the multivariable analysis. To compare patient-specific categorical variables (race, gender, family history of glaucoma and randomization assignment to treatment arm) of participants who did/did not develop POAG, we used Fisher’s exact test. Continuous variables (age, baseline visual field pattern standard deviation (PSD), baseline visual field mean deviation (MD), central corneal thickness, baseline IOP, and baseline rim area) in eyes who did/did not develop POAG were compared using the Wilcoxon Rank Sum Test.
Mixed effects modeling[11] was used to evaluate the relationship between HRT measurements and POAG status over time in univariate and multivariable models. Initially, in what we are considering “univariate” analysis, we compared rates of topographic change in POAG versus non-POAG eyes without adjusting for any covariates. These “univariate” or single covariate models included time, POAG and an interaction term (time × POAG). Subsequently, we built 8 multivariable mixed-effects models which evaluated the influence of the following 8 covariates: Randomization to medication, race, baseline age, central corneal thickness baseline visual field PSD, IOP (as repeated measures), disc area, and image quality (standard deviation of the mean topography) on the rate of rim area change (Table 5). IOP and standard deviation of the mean image height were included as covariates with repeated measures in the multivariable models. These covariates were chosen for analysis based on their importance in previous publications on their effect on the development of glaucoma[6, 10, 12] and their statistical significance in the univariate models. In multivariable models, appropriate two-way (e.g. covariate * time) and three-way interactions (covariate*time*poag) were studied to understand any multifactorial relationships. Mixed effects models with random intercepts and random slopes have been used previously in this setting to adjust for within-patient correlation in measurements between eyes from the same participant and to account for repeated measurements over time.[13–16] Eyes that had a single eligible HRT visit were included in the models.
Table 5.
Multivariable Longitudinal Mixed Effects Models Examining the Effect of Demographic and Ophthalmic Covariates on the Rate of Global Rim Area Change Over Time in the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study
| Main Effects | |||||
|---|---|---|---|---|---|
| Multivariable Models |
COVARIATES | TIME | COVARIATE | Association of covariates on the rate of rim area change (covariate * time) |
|
| #1 | Randomization to Medication | Estimate | −0.0025 | −0.0130 | <0.0001 |
| p-value | 0.0011 | 0.6070 | 0.9270 | ||
| #2 | Race | Estimate | −0.0025 | 0.1534 | −0.0007 |
| p-value | <0.0001 | <0.0001 | 0.3448 | ||
| #3 | Baseline Age | Estimate | −0.0011 | −0.0023 | <0.0001 |
| p-value | 0.4965 | 0.0962 | 0.3438 | ||
| #4 | Central Corneal Thickness | Estimate | −0.0026 | 0.0120 | −0.0003 |
| p-value | <0.0001 | 0.3358 | 0.1702 | ||
| #5 | Baseline Visual Field Pattern Standard Deviation | Estimate | −0.0026 | −0.0116 | −0.0009 |
| p-value | <0.0001 | 0.1734 | 0.0001 | ||
| #6 | IOP as a repeated measure | Estimate | −0.0028 | 0.0061 | −0.0017 |
| p-value | <0.0001 | 0.0133 | <0.0001 | ||
| #7 | Disc Area | Estimate | −0.0026 | 0.1647 | −0.0009 |
| p-value | <0.0001 | <0.0001 | 0.0005 | ||
| #8 | Standard Deviation of the Mean Topography as a repeated measure | Estimate | −0.0025 | 0.0014 | −0.0008 |
| p-value | <0.0001 | 0.4728 | 0.0009 | ||
IOP = Intraocular Pressure
A p-value < 0.05 was considered statistically significant. Multiple testing corrections were not applied. Statistical analyses were performed in SAS (version 9.2, SAS Cary, NC) and R (version 2.10.0, http://www.r-project.org/).
Results
Four hundred forty-one participants (832 eyes) in the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the OHTS met the inclusion criteria for this report. Baseline clinical and ocular factors of the 441 CSLO Ancillary Study participants included in this analysis are presented in Tables 1 and 2. The mean baseline age (95% CI) of the participants was 54.4 years (53.5 to 55.2 years).
Table 1.
Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study baseline demographic and clinical characteristics by Primary Open Angle Glaucoma status* (n=number of participants)
| Not at POAG endpoint* |
POAG endpoint |
All | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Race | ||||||
| Other | 327 | 89.1 | 40 | 10.9 | 367 | 100% |
| African American | 62 | 83.8 | 12 | 16.2 | 74 | 100% |
| Sex | ||||||
| Female | 234 | 91.4 | 22 | 8.6 | 256 | 100% |
| Male | 155 | 83.8 | 30 | 16.2 | 185 | 100% |
| Parent/sibling family history of glaucoma | ||||||
| No | 256 | 85.6 | 43 | 14.4 | 299 | 100 |
| Yes | 133 | 93.7 | 9 | 6.3 | 142 | 100 |
| Randomization to Medication | ||||||
| No | 193 | 89.4 | 23 | 10.6 | 216 | 100 |
From Ocular Hypertension Treatment Study baseline visit.
POAG = Primary Open Angle Glaucoma
Table 2.
Characteristics of Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study participants
| Not at POAG endpoint | POAG endpoint | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | (95% CI) | N | Mean | (95% CI) | N | Mean | (95% CI) | ||
| Follow-up (years) | 766 | 8.9 | (8.5, 9.2) | 66 | 5.8 | (5.1, 6.5) | 832 | 8.6 | (8.3, 8.9) | |
| Number of HRT visits | 766 | 7.0 | (6.7, 7.3) | 66 | 4.8 | (4.2, 5.4) | 832 | 6.8 | (6.6. 7.1) | |
| Baseline Variables | ||||||||||
| Age (years) | 389 | 54.1 | (53.2, 55.0) | 52 | 56.4 | (53.9, 58.8) | 441 | 54.4 | (53.5, 55.2) | |
| IOP mm Hg* | OD | 384 | 25.1 | (24.8, 25.3) | 34 | 25.8 | (24.8, 26.8) | 418 | 25.1 | (24.9, 25.4) |
| OS | 382 | 24.9 | (24.7, 25.2) | 32 | 25.8 | (24.9, 26.8) | 414 | 25.0 | (24.8, 25.2) | |
| Central corneal thickness µm* | OD | 352 | 577.0 | (573.0, 581.0) | 33 | 562.0 | (550.7, 573.3) | 385 | 575.7 | (572.0, 579.4) |
| OS | 351 | 577.1 | (573.2, 581.0) | 30 | 560.3 | (546.6, 574.0) | 381 | 575.7 | (572.0, 579.5) | |
| Visual field pattern standard deviation, dB* | OD | 384 | 1.91 | (1.88, 1.94) | 34 | 1.95 | (1.88, 2.02) | 418 | 1.91 | (1.89, 1.93) |
| OS | 382 | 1.89 | (1.86, 1.92) | 32 | 1.99 | (1.90, 2.08) | 414 | 1.90 | (1.87, 1.93) | |
| Visual field mean deviation, dB* | OD | 384 | 0.43 | (0.32, 0.54) | 34 | 0.08 | (−0.31, 0.47) | 418 | 0.40 | (0.29, 0.51) |
| OS | 382 | 0.44 | (0.32, 0.56) | 32 | 0.09 | (−0.36, 0.54) | 414 | 0.42 | (0.31, 0.53) | |
| Disc area (mm2) | OD | 384 | 1.92 | (1.88, 1.96) | 34 | 1.92 | (1.79, 2.05) | 418 | 1.92 | (1.88, 1.96) |
| OS | 382 | 1.93 | (1.89, 1.97) | 32 | 1.92 | (1.77, 2.07) | 414 | 1.93 | (1.89, 1.97) | |
| Rim area (mm2) | OD | 384 | 1.41 | (1.38, 1.44) | 34 | 1.18 | (1.09, 1.27) | 418 | 1.39 | (1.36, 1.42) |
| OS | 382 | 1.41 | (1.38, 1.44) | 32 | 1.18 | (1.09, 1.27) | 414 | 1.39 | (1.36, 1.42) | |
| Rim to disc area ratio | OD | 384 | 0.75 | (0.74, 0.76) | 34 | 0.63 | (0.60, 0.70) | 418 | 0.74 | (0.73, 0.75) |
| OS | 382 | 0.75 | (0.74, 0.76) | 32 | 0.63 | (0.58, 0.68) | 414 | 0.74 | (0.73, 0.75) | |
From Ocular Hypertension Treatment Study baseline visit.
IOP = Intraocular Pressure
POAG = Primary Open Angle Glaucoma
HRT = Heidelberg Retina Tomograph
Fifty-two participants (11.8%) (66 eyes) developed POAG and 389 (88.2%) participants (766 eyes) did not develop POAG during the follow-up period. Rates of change were calculated for all available visits for eyes not developing POAG, and until the first suspicious finding for those eyes that developed POAG. The median (1st quartile to 3rd quartile) length of follow-up was 11.0 years (5.2 to 12.2 years) for participants not developing POAG, and 5.6 years (4.0 to 8.0 years) to the time participants developed POAG. The median (1st quartile to 3rd quartile) number of HRT examinations was 8.0 visits (3 to 10 visits) for participants not developing POAG, and 4.5 (3 to 6 visits) for participants who developed POAG. Of the 52 participants who developed POAG, 14 developed bilateral POAG and 38 developed unilateral POAG after the initial CSLO measurement. For participants that developed unilateral POAG, only the POAG eye was included in the analysis. Of the 66 POAG eyes, 20 (30.3%) eyes initially reached endpoint based on visual fields alone, 45 (68.2%) eyes initially on stereophotographs alone and 1 (1.5%) eye based on concurrent visual fields and stereophotographs. Among the 74 African American participants, 12 (16%) participants (13 eyes) developed POAG.
It should be noted that of the 45 eyes that were initially classified as POAG only on the basis of stereophotographs, 8 (17.8%) eyes went on to develop visual field damage attributable to POAG and 8 (40%) of the 20 eyes classified initially as POAG based on only visual fields later developed optic disc changes attributable to POAG. To summarize, of the 66 POAG eyes, a total of 17 (25.8%) developed both optic disc and visual field changes attributable to POAG during the study follow-up period. The primary statistical modeling classifies the eyes as developing POAG or not without regard to whether the POAG endpoint(s) for an eye was determined by visual fields, optic disc or both.
Based on the univariate mixed effects model, the mean rate of global rim area change (95% CI) was significantly faster (p<0.0001) in eyes that developed POAG compared to eyes that did not (−0.0131 mm2/yr (−0.0174, −0.0089 mm2/yr) and −0.0026 mm2/yr (−0.0036, −0.0015 mm2/yr), respectively (Table 3). There was a broad distribution in rim area slopes, ranging from −0.150 mm2/yr to 0.088 mm2/yr in eyes that developed POAG, and from −0.170 mm2/yr to 0.1 mm2/yr in eyes that did not develop POAG (Figure 1). Among eyes that were in the fastest quartile of rim area loss (between −.009 mm2/yr to −0.170 mm2/yr), 22% developed POAG, compared to between 3 to 5% in the slower 3 quartiles. In addition, the rate of rim area change was significantly faster in each of the 6 rim area regions (temporal, temporal inferior, temporal superior, nasal, nasal inferior and nasal superior) in eyes that developed POAG compared to eyes that did not (p-value for each region was < .05) (Figure 2).
Table 3.
Rate of rim area change over time: Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study Results of the longitudinal mixed effects regression model (assuming random intercept and random slope)
| Parameter | Estimate | Standard Error | p-value |
|---|---|---|---|
| Intercept | 1.41 | 0.013 | <.0001 |
| POAG* | −0.200 | 0.039 | <.0001 |
| Time (in years) | −0.003 | 0.0005 | <.0001 |
| POAG*Time | −0.011 | 0.0022 | <.0001 |
POAG (Primary Open Angle Glaucoma): variable indicating development of POAG endpoint (1 yes, 0 no)
Figure 1.
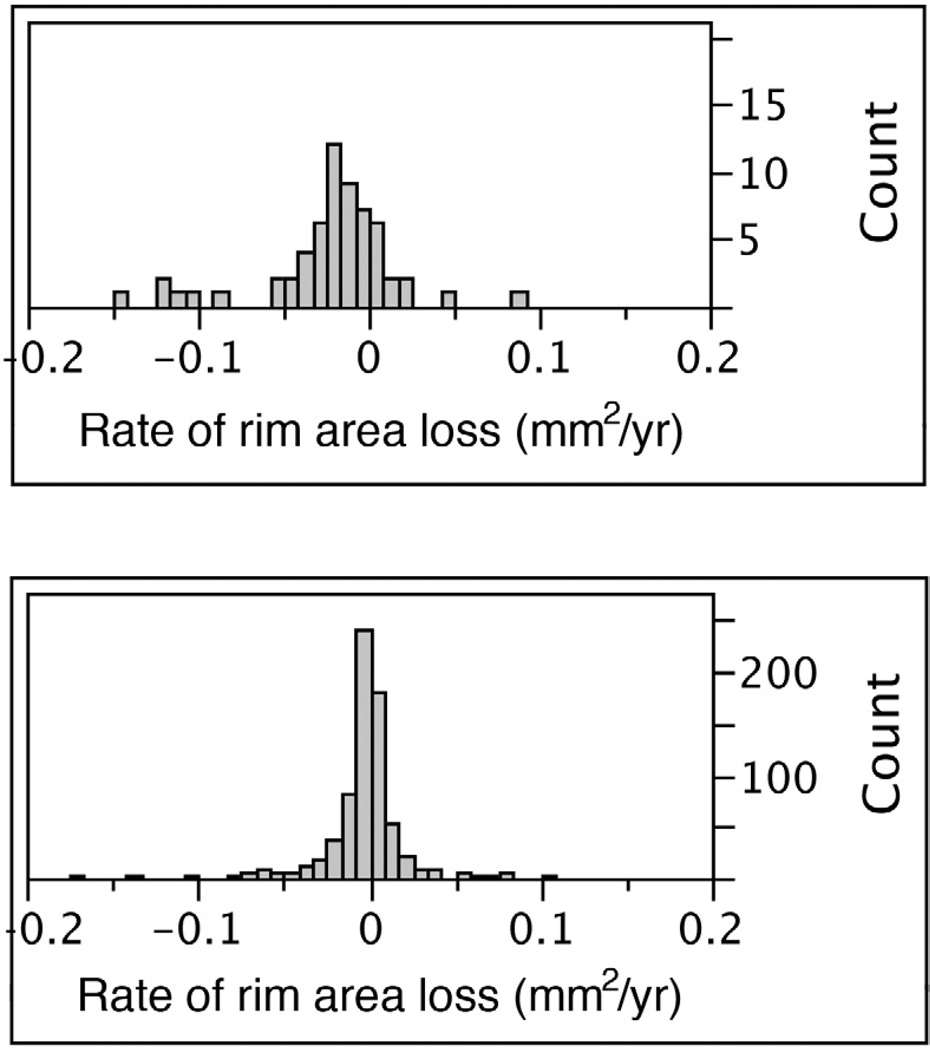
The distribution in the rate of rim area change in Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Study eyes that developed primary open angle glaucoma (POAG) (Figure top) and eyes that did not develop POAG (Figure bottom).
Figure 2.
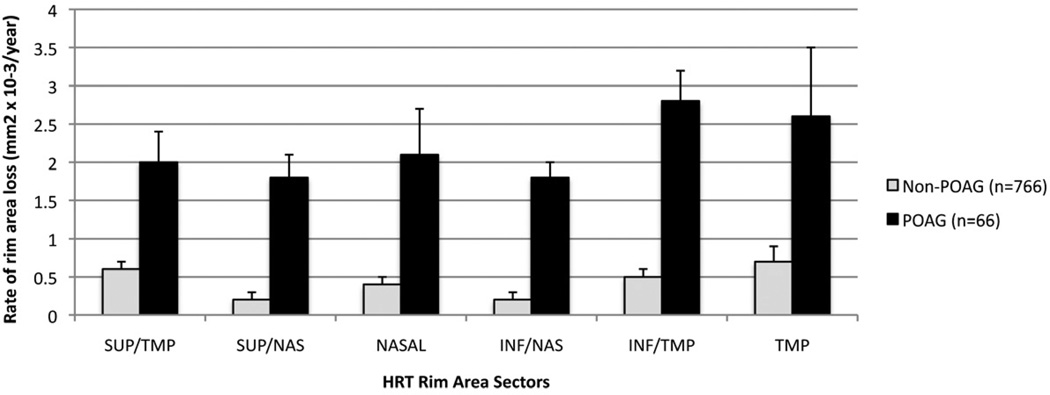
Mean (se) rate of rim area loss among Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Study eyes that developed primary open angle glaucoma (POAG) (dark bars) and eyes that did not develop POAG (light bars) by HRT sector. Error bars= standard error.
We also investigated the rate of change as a percentage of the baseline values. Eyes that developed POAG had a mean baseline rim area of 1.12 mm2 in eyes and a mean (95% CI) percentage decrease from baseline rim area was 0.99%/yr (0.74% to 1.23% / year); in eyes that did not develop POAG (mean baseline rim area of 1.39 mm2) this rate represents an overall mean (95% CI) percentage rim area decrease of 0.18%/year (0.14% to 0.21%/year).
In eyes that did not develop POAG, we also found statistically significant rates of rim area decrease over time globally as well as in each of the six regions. Specifically, in univariate models, the average rate of change in global rim area (−0.0026 mm2/yr, p<0.0001), and rim area in the temporal inferior (−0.0005 mm2/yr, p<0.0001), nasal inferior (−0.0002 mm2/yr, p=0.0027), temporal superior (−0.0006 mm2/yr, p<0.0001), nasal superior (−0.0002 mm2/yr, p<0.0001), temporal (−0.0007 mm2/yr, p=0.0034) and nasal regions (−0.0004 mm2/yr, p=0.0061) were significantly less than zero.
Among participants who developed POAG, the mean (95%CI) rate of rim area change over time was significantly (p=0.0262) faster in African American participants −0.0182, (−0.0256 to −0.0107mm2/yr) compared to other participants −0.0116, (−0.0166 to 0.0065 mm2/yr)), (Figure 3). Among those who did not develop POAG during the study period, the rate of rim area change was similar in African American and other participants (−0.0024 mm2/yr (−0.0048 to −0.0000)) and (−0.0026 mm2/yr (−0.0038 to −0.0014)), respectively (Figure 3). To see this difference, refer to figure 3: among eyes with POAG, African Americans had a faster rate of rim area loss. However, when both race and disc area were included in the same multivariable model to explain the rate of change over time, only disc area remained significantly associated with the rate of rim area change; the p-value for the interaction term of time with disc area was p=0.0004, and p=0.6655 for the interaction term of time with race (Table 4).
Figure 3.
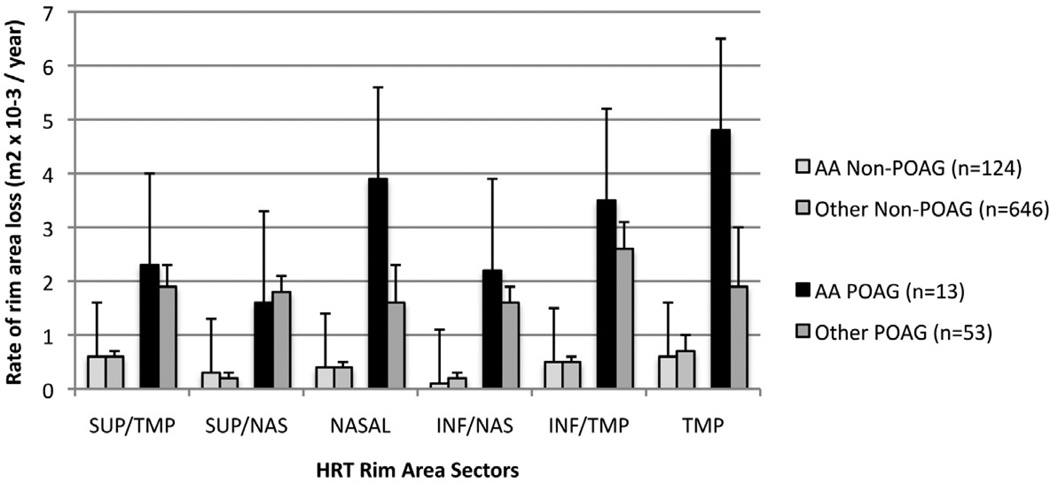
Mean (se) rate of rim area loss among Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Study eyes that developed primary open angle glaucoma (POAG) (dark bars) and eyes that did not develop POAG (light bars) in African American and other participants. Error bars= standard error.
Table 4.
Rate of rim area change over time: Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study Results of the longitudinal mixed effects regression model with both disc area and race in the model
| Parameter | Estimate | Standard Error | p-value |
|---|---|---|---|
| Intercept | 1.4043 | 0.0130 | <.0001 |
| POAG* | −0.216 | 0.0414 | <.0001 |
| Time | −0.0024 | 0.0003 | <.0001 |
| Disc Area | 0.1684 | 0.0099 | <.0001 |
| Race | 0.0330 | 0.0333 | 0.3213 |
| POAG*Time | −0.0099 | 0.0016 | <.0001 |
| POAG*Race | 0.0730 | 0.0901 | 0.4182 |
| POAG*Disc Area | −0.093 | 0.0309 | 0.0028 |
| Time * Area | −0.0009 | 0.0003 | 0.0004 |
| Time* Race | 0.0003 | 0.0007 | 0.6655 |
POAG (Primary Open Angle Glaucoma): variable indicating development of POAG endpoint (1 yes, 0 no)
We evaluated the effect of 8 covariates (randomization assignment to treatment arm, race, baseline age, central corneal thickness, baseline visual field PSD, IOP as a longitudinal measure, disc area, and standard deviation of the mean image) individually on the rate of rim area loss in 8 separate multivariable model that included terms that specifically measured the contribution of each of these covariates on the rate of change over time. (Table 5) Specifically, the interaction term (covariate * time) was included to assess whether the covariate influences the rate of change. We found that when evaluated in separate single variable models, larger disc area (p=0.0005), worse baseline visual field PSD (p=0.0001), higher IOP (as a longitudinal measure) (p<0.0001) and higher standard deviation of the mean image (p=0.0009) were each individually associated with a faster rate of rim area loss. Randomization assignment to treatment arm (p=0.927,), race (p=0.3448), central corneal thickness (p=0.1702), and baseline age (p=0.3438) were not significantly associated with the rate of rim area loss (Table 5).
We also evaluated whether the rate of change of other topographic parameters was faster in eyes that developed POAG compared to eyes that did not develop POAG (Table 6). In univariate analysis, the rate of change of rim volume, rim area as a percent of baseline rim area, rim to disc ratio, cup to disc ratio, mean cup depth, cup volume below surface, GPS probably score, were significantly faster in eyes that developed POAG compared to eyes that did not. The rate of change of RNFL thickness and RNFL cross sectional area were not significantly faster in eyes that developed POAG compared to eyes that did not develop POAG. Among eyes that did not develop POAG, the rate of change of all topographic parameters except RNFL thickness and RNFL cross-sectional area were statistically significant (Table 6).
Table 6.
Global rate of change of topographic optic disc parameters by Ocular Hypertension Treatment Study Primary Open Angle Glaucoma status*: Univariate analysis
| Not at POAG endpoint (n=766 eyes) |
POAG endpoint (66 eyes) |
Difference between POAG and Non- POAG |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | p-value* | Mean | (95% CI) | p-value* | Mean | (95% CI) | p-value | |
| Rim area (mm2 /year) | −0.0026 | −0.0036, −0.0015 | <0.0001 | −0.0131 | −0.0174, −0.0089 | <0.0001 | −0.0105 | −0.0149, −0.0062 | <0.0001 |
| Rim Area (%/yr) | −0.1723 | −0.081, −0.1365 | <0.0001 | −0.8948 | −1.1172, −0.6724 | <0.0001 | −0.7225 | −0.9478, −0.4972 | <0.0001 |
| Rim to disc area ratio | −0.0013 | −0.0019, −8 × 10−4 | <0.0001 | −0.0064 | −0.0085, −0.0042 | <0.0001 | −0.005 | −0.0073, −0.0028 | <0.0001 |
| Cup to disc area ratio | 0.0013 | 8 × 10−4, 0.0019 | <0.0001 | 0.0064 | 0.0042, 0.0085 | <0.0001 | 0.005 | 0.0028, 0.0073 | <0.0001 |
| Mean cup depth (µm/yr) | 0.0015 | 0.0012, 0.0017 | <0.0001 | 0.0028 | 0.0018, 0.0039 | <0.0001 | 0.0013 | 3.0 × 10−4, 0.0024 | 0.0156 |
| RNFL thickness (µm/yr) | 3.0 × 10−4 | −1.0 × 10−4, 7.0 × 10−4 | 0.2018 | −0.0012 | −0.003, 7 × 10−4 | 0.215 | −0.0014 | −0.0033, 5 × 10−4 | 0.1369 |
| RNFL cross sectional area (µm2/yr) | 0.0014 | −7.0 × 10−4, 0.0034 | 0.1852 | −0.006 | −0.0152, 0.0031 | 0.1954 | −0.0074 | −0.0167, 0.0019 | 0.1207 |
| Cup Shape | 0.0022 | 0.0018, 0.0026 | <0.0001 | 0.0041 | 0.0024, 0.0059 | <0.0001 | 0.0019 | 0.0001, 0.0037 | 0.0383 |
| Rim Volume above reference (µm3/yr) | 0.0023 | 0.0004, 0.0016 | <0.0001 | −0.003 | −0.0062, 0.0002 | 0.0638 | −0.0053 | −0.0086, −0.0021 | 0.0013 |
| Cup volume below surface (µm3/yr) | 7.0 × 10−4 | 3 × 10−4, 0.001 | <0.0001 | 0.0054 | 0.0039, 0.007 | <0.0001 | 0.0047 | 0.0032, 0.0063 | <0.0001 |
| Glaucoma probability score (%/yr) | 0.26 | 0.11, 0.42 | 0.001 | 1.49 | 0.77, 2.22 | <0.0001 | 1.23 | 0.49, 1.97 | 0.0011 |
POAG = Primary Open Angle Glaucoma
RNFL = Retina Nerve Fiber Layer
In addition, we compared the rate of topographic change in eyes that developed a visual field POAG endpoint compared to eyes that developed an optic disc POAG endpoint (Table 7). We found that the mean (95% CI) rate of rim area change was significantly (p=0.042) faster in eyes that reached an optic disc POAG endpoint (−0.0169 mm2/yr (−0.0225 to −0.0113) compared to eyes that reached a visual field POAG endpoint (−0.0079 mm2/yr (−0.0157 to −0.0001). Moreover, we measured the rate of rim area change in eyes that were considered as progressing by the Optic Disc Reading Center that were not considered as a POAG endpoint by the endpoint committee and found that the mean (95% CI) change (−0.0094 mm2/yr (−0.0151 to −0.0027) tended to be faster than the rate of change among eyes that reached a visual field POAG endpoint, but slower than the rate of change in eyes that reached an optic disc POAG endpoint. These differences did not reach statistical significance.
Table 7.
Differences in Rate of Rim Area Change in eyes with a Ocular Hypertension Treatment Study Visual Field Primary Open Angle Glaucoma Endpoint compared to eyes with an Optic Disc Primary Open Angle Glaucoma Endpoint only or Optic Disc Change (not necessarily a Primary Open Angle Glaucoma endpoint)
| Visual Field and Op.c Disc Change Criteria | N (eyes) | Slope (95% CI) (mm2/yr) |
P-Value# | P-Value comparing slopes to VF POAG Endpoint slope |
P-Value comparing slopes to Not at POAG endpoint slope |
|---|---|---|---|---|---|
| Not at POAG Endpoint | 731 | −0.0021 (−0.0031, −0.0011) | <0.0001 | 0.1655 | NA |
| Visual Field POAG Endpoint | 21 | −0.0079 (−0.0157, −0.0001) | 0.0513 | NA | 0.1655 |
| Optic Disc POAG Endpoint Only* | 45 | −0.0169 (−0.0225, −0.0113) | <0.0001 | 0.0426 | 0.0001 |
| Optic Disc Reading Center Change (POAG and not POAG)* | 72 | −0.0131 (−0.0178, −0.0084) | <0.0001 | 0.1433 | 0.0001 |
| Optic Disc Reading Center Change NOT POAG Endpoint* | 27 | −0.0094 (−0.0161, −0.0027) | 0.0063 | 0.54 | 0.0012 |
Does not include the 1 eye that had both a POAG visual field endpoint and a POAG optic disc
p-value to evaluate whether the slope is significantly different from zero
POAG: Primary Open Angle Glaucoma
Discussion
In this report, the rate of neuroretinal rim area loss as measured using the Heidelberg Retina Tomograph was approximately five times faster in ocular hypertensive eyes that developed POAG compared to eyes that did not. Moreover, we found that the rate of neuroretinal rim area loss was significantly different from zero in eyes that did not develop POAG during the follow-up period.
Other investigators have also reported that the rate of neuroretinal rim area change is faster in ocular hypertensive eyes that develop glaucomatous visual field loss than eyes that do not. Strouthidids et al[2] reported that the rate of rim area change (mm2/ year) in the inferior temporal sector was 3.2 times faster in eyes with visual field progression compared to those without. In the Diagnostic Innovations in Glaucoma Study (DIGS), glaucoma suspects (with elevated IOP or optic disc damage at baseline) and glaucoma patients with documented glaucomatous visual field or optic disc progression had between 5 times and 8 times faster rates of retinal nerve fiber layer thinning measured with the GDx VCC and GDx ECC (Carl Zeiss Meditec Inc., Dublin, CA) than eyes that were not progressing.[14, 15, 17–19] In addition, See et al.[20] reported that the rate of global HRT neuroretinal rim area change was over 4× faster in glaucoma eyes than normal eyes when measured in absolute units (medians, −5.33×10−3 mm2/year and −1.25×10−3 mm2 /year, respectively; P = 0.006) or as a percentage of baseline rim area (medians, −0.42%/year and −0.07%/year, respectively; P = 0.001). Leung et al,[21] reported a mean rate of rim area % change (−1.06%/yr) in glaucoma patients that was similar to the rate of change reported in eyes developing POAG in the current study (.99%/yr).
In univariate analysis, we found that race was significantly associated with the rate of neuroretinal rim change in eyes that developed POAG; African Americans have a faster rate of neuroretinal rim area loss than other participants. However, the faster rate of loss in African Americans compared to other racial groups who developed POAG is largely explained by the larger optic disc size in the African American participants. After adjusting for optic disc area in the multivariable model, the racial differences in the rate of neuroretinal rim area loss are reduced, and no longer statistically significant. These results are consistent with our previous report[8] and reports of other investigators[22–24] demonstrating that differences in the risk of developing POAG between African American and other OHTS participants are explained, at least in part, by other factors including thinner central corneal thickness, baseline cup-to-disc ratio and larger disc area of the African Americans.[8, 23–25]
It is interesting to note that in previous reports of the CSLO Ancillary Study to the OHTS, baseline optic disc area was not predictive of the development of a POAG endpoint in multivariable Cox proportional hazards models.[6, 7] In the current analysis, larger disc area was associated with a faster rate of rim area loss (Table 4) in eyes that developed POAG. One might expect that if a larger disc area was associated with a faster rate of rim area loss, then it also would be associated with the development of POAG. There are several possible explanations for the inconsistency of these results. It may be that the rate of rim area loss is measured more reproducibly in large optic discs, thereby increasing the possibility of detecting significant rates of change. This finding may also be explained in part because eyes with larger discs have relatively larger rim area and more likely to have more absolute rim area loss, but not a larger % rim area loss from baseline. Specifically, we divided the disc area in tertiles, and found that the mean (95%CI) percentage decrease from baseline rim area in eyes with relatively large discs (highest tertile > 2.07 mm2) was similar to the percentage decrease from baseline rim area in the small discs (lowest tertile <1.73 mm2), −0.65% (−.27% to −0.04%), and −0.43% (−0.74 to −0.12%), respectively. Alternatively, it may be that the disc size influenced the ability to detect glaucomatous changes in the stereophotograph based assessment of the optic disc; perhaps a larger disc made it more difficult to detect change or to determine that it was “clinically significant structural change”, in which case progression in relatively large optic discs might have been underestimated by the OHTS Optic Disc Reading Center.
Our results also confirm previous reports on ocular hypertensive eyes, glaucoma suspect eyes and eyes with early to moderate glaucoma finding that the rate of change varies by region, with the fastest neuroretinal rim change in the inferotemporal sector[2, 20] (Figure 1). We also found that there is considerable rim area change detected in the temporal and nasal regions, particularly in African American participants and in eyes with large discs. Other investigators have also reported significant neuroretinal rim area change measured with the HRT in the temporal region in both glaucoma eyes and normal eyes.[20] It should be noted that the standard HRT temporal and nasal sectors are larger (90 degrees each) than the other 4 sectors (45 degrees each) and may contain more non-neural tissue (blood vessels) than the other sectors.[2, 26] For these reasons, the temporal and nasal rim area loss measured in absolute units (mm2 / year) may be greater than other sectors due in part to the larger sector size, whereas when measured in relative units (%/yr) the temporal, and especially nasal sector[26] rate of change may be smaller compared to other sectors. Therefore, comparisons of the amount of change in temporal and nasal rim area to the other 4 sectors in this study should be made with caution. However, See et al.[20] measured neuroretinal rim area loss in 12 equal 30-degree sectors and found significant change in the temporal regions in both glaucoma and normal eyes during the 7+ years of study follow-up. Investigation of the rate of temporal rim area changes using different imaging modalities such as spectral domain optical coherence tomography may elucidate whether the temporal changes are related to issues specific to CSLO imaging or to previously underestimated temporal change that occur due to progressive glaucomatous optic neuropathy.
There are several possible explanations for our findings of a statistically significant but slow rate of neuroretinal rim loss in eyes that did not develop POAG. First, these changes may represent age-related loss of retinal ganglion cells. Several investigators have documented small, but significant loss of retinal nerve fiber layer and neuroretinal rim area with age from cross-sectional[27–29] and more recently from longitudinal studies.[20] Second, it is likely that the HRT is detecting topographic changes in some of the approximately 55% of eyes that were not classified as clinically significant or POAG by the endpoint committee, but were classified as progressive change based on masked assessment of stereophotographs by the OHTS Optic Disc Reading Center (personal communication Gordon M 2011). The OHTS appropriately used highly specific criteria to define a reproducible change and an endpoint committee to attribute the reproducible change to POAG. In addition, reproducible changes on stereophotographs also had to be clinically significant. As shown in Table 7, the mean (95%CI) rate of rim area change in the 27 eyes that were classified as changing by the Optic Disc Reading Center but were not considered a POAG endpoint by the Endpoint Committee was significantly faster (−0.0094 mm2/yr (−0.0161 to −0.0001) than eyes not reaching a POAG endpoint (−0.0021mm2/yr (−0.0031 to −0.0011). Third, the HRT may be identifying early changes in some eyes that were not yet detectable on stereophotographs. Finally, some of the structural changes detected may represent false positive results. The above explanations may also explain why only 22% of eyes in the fastest quartile of rim area change developed a POAG endpoint; some of this change may be age-related, and some eyes may have shown change that the endpoint committee did consider a POAG endpoint.
Although the OHTS demonstrated that treatment can significantly delay and/or prevent the onset of glaucoma, in this report, randomization to treatment was not significantly associated with the rate of rim area change in multivariate mixed effects models. There are several possible reasons for the lack of association of the rate of rim area change with ocular hypotensive treatment. First, this study included follow-up of participants during both phases of the OHTS; through both OHTSI (1994–2002) that included participants randomized to treatment and observation and OHTSII (2002–2008) at which time, all participants were offered treatment.[30] Therefore, almost all participants were treated during the study follow-up period. As the results of OHTSII suggest, delaying treatment of ocular hypertension did not significantly increase the risk of developing POAG in participants with a low probability of developing POAG, a group that constitutes the majority of participants in the OHTS. In addition, we found that higher IOP throughout the follow-up was associated with an increase in the rate of rim area loss.
This indicates that in this analysis, measured IOP serves as more sensitive indicator of the modifying effect of treatment on glaucomatous structural change. It should be noted that at baseline, the neuroretinal rim area was smaller in eyes that developed POAG compared to eyes that did not. This suggests that some eyes were included in the OHTS that may have had early pre-clinical or pre-perimetric glaucoma loss that was not identified by the OHTS Optic Disc Reading Center as “glaucomatous”. Pre-perimetric glaucoma eyes may be more likely to progress than ocular hypertensive eyes, and thus show a faster rate of rim area loss.
Smaller central corneal thickness was predictive of the development of POAG in previous OHTS studies,[6, 7, 10] but showed a weak association with the rate of neuroretinal rim area loss in the current study (the interactions with time did not reach statistical significance (p=0.10). The lack of a significant association between the rate of rim area loss and central corneal thickness is unexpected and may be due to the relatively small numbers of POAG eyes included in this analysis and/or the complex relationship between the CCT, IOP and rim area measurements and their variability over time. We know that in some eyes changes in IOP can result in measurable changes in HRT topographic measurements, and that eyes with thinner CCT tend to have lower measured IOP.[23, 31–33] Consequently, it is possible that the complex association between IOP, CCT and HRT topographic measurements, and the wide variation of CCT among OHTS participants made detection of the relationship between CCT and the rate of rim area slope difficult to detect in this study population. Further evaluation of this issue in different study populations is needed.
The number of subjects in this report is slightly higher than in our previous reports.[6–9] At study closeout in 2009, study centers transferred HRT images of participants that had not been previously sent to the UCSD CSLO Reading Center and therefore had not been included in earlier published reports.
Limitations to this study include the relatively small number of eyes that developed POAG, particularly among the African American participants. The small numbers result in relatively large confidence intervals around the rate of change estimates particularly when results are presented as stratified by race and sector. Despite the small numbers of eyes that developed POAG and therefore limited statistical power in this report, important trends were documented and rates of change estimated. To our knowledge this report represents the longest follow-up of the largest group of ocular hypertensive subjects using optical imaging instruments. The HRT is the only optical imaging technology whose image acquisition technique has remained stable since its initial commercialization. It should be noted that we included only good quality HRT images and therefore our results may not be completely generalizable to the general ophthalmic community. Even within this group of good quality images, we found that a higher standard deviation of the mean baseline image (a poorer quality image) was significantly associated with a faster rate of change. This result may be due in part that glaucoma eyes tend to have a higher standard deviation of the mean topography image than non-glaucomatous eyes.[34, 35] However, including the standard deviation in the full model had almost no effect on the estimated rates of rim area change over time. It is possible that if we had included poor quality images, then we might expect more variability in the estimates of the stereometric parameters and a decreased likelihood that significant rates of change would be detected.
In summary, the overall rate of neuroretinal rim area loss is approximately 5 times faster in eyes that developed POAG compared to eyes that did not with approximately 22% of eyes in the fastest quartile of rim area loss developing POAG. Worse baseline visual field PSD, higher IOP during follow-up and larger disc area were associated with a faster rate of rim area loss. Moreover, neuroretinal rim area loss was detected in eyes that did not develop POAG as well as eyes that developed POAG. These results suggest that measuring of the rate of structural change using CSLO can provide important information for the clinical management of ocular hypertensive patients. Further investigation is needed to determine how best to apply rate of change information to the individual ocular hypertensive patient.
Acknowledgements
| Disclosures | ||
| a. | Funding support: | |
| a. | NIH/NEI grants, EY11158 (RNW), (EY09341, EY09307 (MOG, MAK)), Horncrest Foundation awards, NIH Vision Core Grant P30 EY02687, Merck Research Laboratories, White House Station, New Jersey, Pfizer Inc., New York, NY and unrestricted grants from the Research to Prevent Blindness Inc, New York, NY | |
| b. | Financial Disclosures | |
| i. | LM Zangwill, Instrument support from : Carl Zeiss Meditec, Inc., Heidelberg Engineering, GmbH, Optovue, Inc., Topcon Medical Systems, Inc. | |
| ii. | S Jain, None | |
| iii. | F He, None | |
| iv. | RN Weinreb, | |
| i. Consultant : Alcon Laboratories, Inc., Allergan, Inc., Carl Zeiss Meditec, Inc,. | ||
| ii. Research Support: Glaxo., Heidelberg Engineering, GmbH, Novartis, Optovue, Inc., Pfizer, Inc., Topcon Medical Systems, Inc. | ||
| v. | GL Trick, Consultant: Allergan, Inc. | |
| vi. | JD Brandt, Consultant: Alcon, Allergan, Pfizer | |
| vii. | GA Cioffi, Consultant: Allergan, Pfizer | |
| viii. | AL Coleman, None | |
| ix. | JM Liebmann, | |
| i. Consultant: Alcon Laboratories, Inc., Allergan, Inc., Diopsys, Inc, Optovue Inc., Glaukos, Inc., Quark, Inc., Merz Laboratories, Inc. | ||
| ii. Research support: Carl Zeiss Meditec, Inc., Diopsys, Inc., Optovue, Inc., Pfizer, Inc., Alcon Laboratories, Inc., Merck, Linc. Allergan, Inc.,Topcon Medical Systems, Inc., Glaukos, Inc. | ||
| x. | JR Piltz-Seymour None | |
| xi. | K Dirkes, None | |
| xii. | MO Gordon, None | |
| xiii. | MA Kass Consultant : Pfizer | |
| c. | Contributions to Authors: | |
| a. | Design of the study (MOG, MAK, LMZ, RNW) | |
| b. | Conduct of the study (LMZ, KD, JDB, GAC, ALC, JML, JRP, MOG, MAK, RNW) | |
| c. | Collection and management of the data (LMZ, KD, FH, SJ, JDB, GAC, ALC, JML, JRP, MOG, MAK, RNW) | |
| d. | Analysis and interpretation of the data (LMZ, SJ, FH JDB, GAC, ALC, JML, JRP, MOG, MAK, RNW | |
| e. | Preparation of the manuscript (LZ, SJ, FH) | |
| f. | Review or approval of the manuscript (LMZ, KD, JDB, GAC, ALC, JML, JRP, MOG, MAK, RNW) | |
| d. | Other Acknowledgements: None |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mansouri K, Leite MT, Medeiros FA, Leung CK, Weinreb RN. Assessment of rates of structural change in glaucoma using imaging technologies. Eye (Lond) 2011;25(3):269–277. doi: 10.1038/eye.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strouthidis NG, Gardiner SK, Sinapis C, Burgoyne CF, Garway-Heath DF. The spatial pattern of neuroretinal rim loss in ocular hypertension. Invest Ophthalmol Vis Sci. 2009;50(8):3737–3742. doi: 10.1167/iovs.08-2844. [DOI] [PubMed] [Google Scholar]
- 3.Philippin H, Unsoeld A, Maier P, Walter S, Bach M, Funk J. Ten-year results: detection of long-term progressive optic disc changes with confocal laser tomography. Graefes Arch Clin Exp Ophthalmol. 2006;244(4):460–464. doi: 10.1007/s00417-005-0094-4. [DOI] [PubMed] [Google Scholar]
- 4.Kamal DS, Garway-Heath DF, Hitchings RA, Fitzke FW. Use of sequential Heidelberg retina tomograph images to identify changes at the optic disc in ocular hypertensive patients at risk of developing glaucoma. Br J Ophthalmol. 2000;84(9):993–998. doi: 10.1136/bjo.84.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamal DS, Viswanathan AC, Garway-Heath DF, Hitchings RA, Poinoosawmy D, Bunce C. Detection of optic disc change with the Heidelberg retina tomograph before confirmed visual field change in ocular hypertensives converting to early glaucoma. Br J Ophthalmol. 1999;83(3):290–294. doi: 10.1136/bjo.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123(9):1188–1197. doi: 10.1001/archopht.123.9.1188. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb RN, Zangwill LM, Jain S, et al. Predicting the onset of glaucoma: The Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Ophthalmology. 2010;117(9):1674–1683. doi: 10.1016/j.ophtha.2010.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zangwill LM, Weinreb RN, Berry CC, et al. Racial differences in optic disc topography: baseline results from the confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study. Arch Ophthalmol. 2004;122(1):22–28. doi: 10.1001/archopht.122.1.22. [DOI] [PubMed] [Google Scholar]
- 9.Zangwill LM, Weinreb RN, Berry CC, et al. The confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study: study design and baseline factors. Am J Ophthalmol. 2004;137(2):219–227. doi: 10.1016/j.ajo.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 11.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 12.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123(10):1351–1360. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50(4):1675–1681. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Weinreb RN. The Relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116(6):1125–1133. e1121–e1123. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Susanna R, Jr, Weinreb RN. Impact of atypical retardation patterns on detection of glaucoma progression using the GDx with variable corneal compensation. Am J Ophthalmol. 2009;148(1):155–163. e151. doi: 10.1016/j.ajo.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros FA, Alencar LM, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127(10):1250–1256. doi: 10.1001/archophthalmol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci. 2010;51(7):3531–3539. doi: 10.1167/iovs.09-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alencar LM, Zangwill LM, Weinreb RN, et al. Agreement for Detecting Glaucoma Progression with the GDx Guided Progression Analysis, Automated Perimetry, and Optic Disc Photography. Ophthalmology. 2010;117(3):462–470. doi: 10.1016/j.ophtha.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medeiros FA, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149(6):908–915. doi: 10.1016/j.ajo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 20.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116(5):840–847. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Leung CK, Liu S, Weinreb RN, et al. Evaluation of retinal nerve fiber layer progression in glaucoma a prospective analysis with neuroretinal rim and visual field progression. Ophthalmology. 2011;118(8):1551–1557. doi: 10.1016/j.ophtha.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Girkin CA, McGwin G, Jr, Xie A, Deleon-Ortega J. Differences in optic disc topography between black and white normal subjects. Ophthalmology. 2005;112(1):33–39. doi: 10.1016/j.ophtha.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108(10):1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 24.Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122(6):813–820. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]
- 25.Gordon MO, Gao F, Beiser JA, Miller JP, Kass MA. The 10-year incidence of glaucoma among patients with treated and untreated ocular hypertension. Arch Ophthalmol. 2011;129:1630–1631. doi: 10.1001/archophthalmol.2011.337. [DOI] [PubMed] [Google Scholar]
- 26.Jonas JB, Fernandez MC. Shape of the neuroretinal rim and position of the central retinal vessels in glaucoma. Br J Ophthalmol. 1994;78(2):99–102. doi: 10.1136/bjo.78.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowd C, Zangwill LM, Blumenthal EZ, et al. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender. J Opt Soc Am A Opt Image Sci Vis. 2002;19(1):197–207. doi: 10.1364/josaa.19.000197. [DOI] [PubMed] [Google Scholar]
- 28.Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009;116(6):1119–1124. doi: 10.1016/j.ophtha.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garway-Heath DF, Wollstein G, Hitchings RA. Aging changes of the optic nerve head in relation to open angle glaucoma. Br J Ophthalmol. 1997;81(10):840–845. doi: 10.1136/bjo.81.10.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 31.Bowd C, Weinreb RN, Lee B, Emdadi A, Zangwill LM. Optic disk topography after medical treatment to reduce intraocular pressure. Am J Ophthalmol. 2000;130(3):280–286. doi: 10.1016/s0002-9394(00)00488-8. [DOI] [PubMed] [Google Scholar]
- 32.Brandt JD, Gordon MO, Beiser JA, Lin SC, Alexander MY, Kass MA. Changes in central corneal thickness over time: the ocular hypertension treatment study. Ophthalmology. 2008;115(9):1550–1556. 1556 e1551. doi: 10.1016/j.ophtha.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Tan JC, Hitchings RA. Reversal of disc cupping after intraocular pressure reduction in topographic image series. J Glaucoma. 2004;13(5):351–355. doi: 10.1097/01.ijg.0000133151.82804.e3. [DOI] [PubMed] [Google Scholar]
- 34.Weinreb RN, Lusky M, Bartsch DU, Morsman D. Effect of repetitive imaging on topographic measurements of the optic nerve head. Arch Ophthalmol. 1993;111(5):636–638. doi: 10.1001/archopht.1993.01090050070031. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan BC, LeBlanc RP, McCormick TA, Rogers JB. Test-retest variability of topographic measurements with confocal scanning laser tomography in patients with glaucoma and control subjects. Am J Ophthalmol. 1994;118(1):9–15. doi: 10.1016/s0002-9394(14)72836-3. [DOI] [PubMed] [Google Scholar]


