Abstract
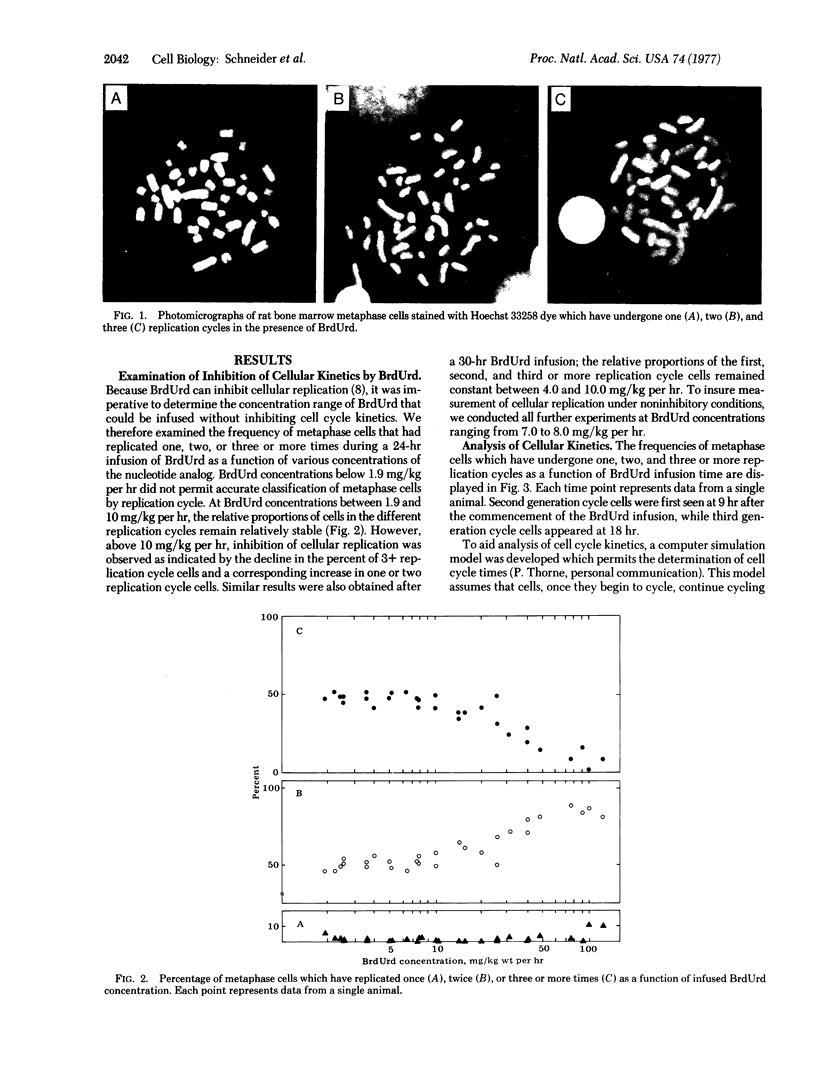
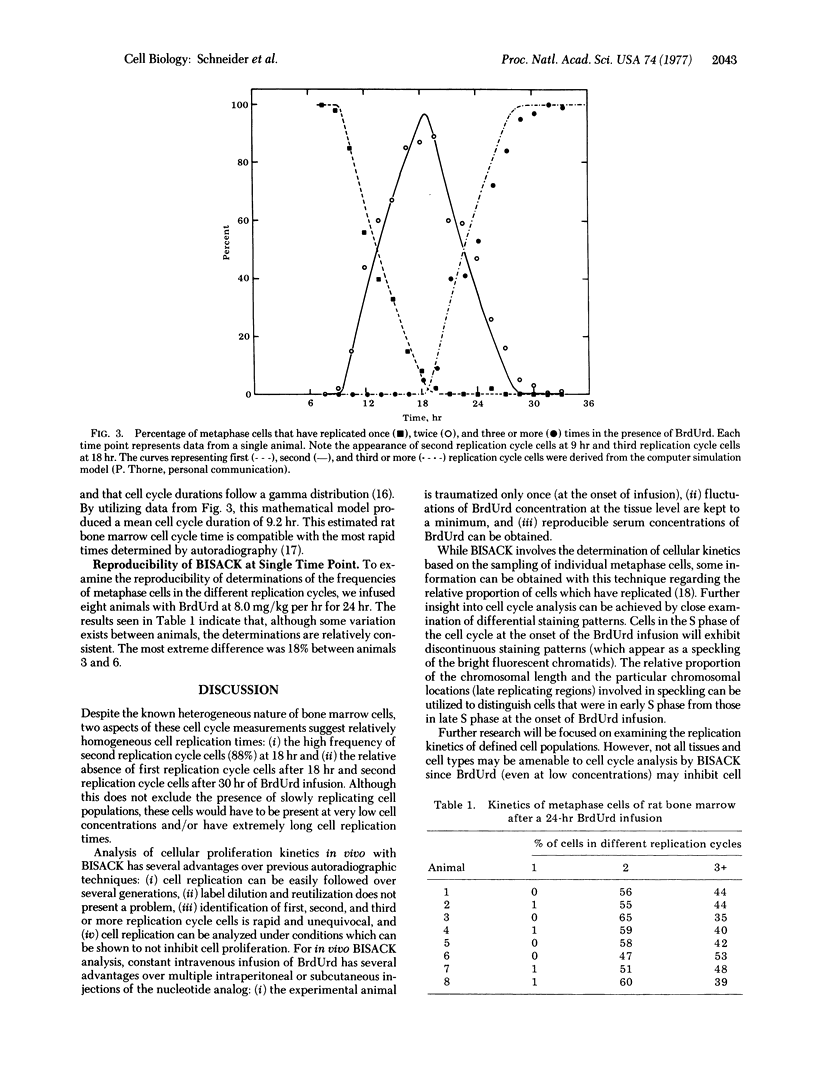
The number of previous cell replications that a metaphase cell has undergone in the presence of BrdUrd can be determined by the differential fluorescent patterns of metaphse chromosomes stained with Hoechst dye 33258. To examine if this technique could be applied to analyzing cell cycle kinetics in vivo, we infused Wistar rats with BrdUrd for 7.5-33 hr at concentrations of the nucleotide analog that did not inhibit cellular replication. Examination of the frequency of one, two, and three or more replication cycle cells as a function of BrdUrd infusion time indicates that cell replication times for rat bone marrow cells are relatively homogeneous. Analysis of this data with a computer simulation model produced a mean cell cycle duration of 9.2 hr, which is compatible with the fastest times obtained with radioisotope studies. These results support the potential of nonradioisotope analysis of cell replication in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. W., Latt S. A. Analysis of sister chromatid exchange formation in vivo in mouse spermatogonia as a new test system for environmental mutagens. Nature. 1976 Apr 1;260(5550):449–451. doi: 10.1038/260449a0. [DOI] [PubMed] [Google Scholar]
- Allen J. W., Latt S. A. In vivo BrdU-33258 Hoechst analysis of DNA replication kinetics and sister chromatid exchange formation in mouse somatic and meiotic cells. Chromosoma. 1976 Nov 29;58(4):325–340. doi: 10.1007/BF00292841. [DOI] [PubMed] [Google Scholar]
- Bloom S. E., Hsu T. C. Differential fluorescence of sister chromatids in chicken embryos exposed to 5-bromodeoxyuridine. Chromosoma. 1975 Jul 21;51(3):261–267. doi: 10.1007/BF00284819. [DOI] [PubMed] [Google Scholar]
- Cairnie A. B., Lamerton L. F., Steel G. G. Cell proliferation studies in the intestinal epithelium of the rat. I. Determination of the kinetic parameters. Exp Cell Res. 1965 Sep;39(2):528–538. doi: 10.1016/0014-4827(65)90055-8. [DOI] [PubMed] [Google Scholar]
- Frindel E., Tubiana M., Vassort F. Generation cycle of mouse bone marrow. Nature. 1967 Jun 3;214(5092):1017–1018. doi: 10.1038/2141017a0. [DOI] [PubMed] [Google Scholar]
- Kligerman A. D., Bloom S. E. Sister chromatid differentiation and exchanges in adult mudminnows (Umbra limi) after in vivo exposure to 5-bromodeoxyuridine. Chromosoma. 1976 Jun 30;56(2):101–109. doi: 10.1007/BF00293110. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Localization of sister chromatid exchanges in human chromosomes. Science. 1974 Jul 5;185(4145):74–76. doi: 10.1126/science.185.4145.74. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Stetten G., Juergens L. A., Willard H. F., Scher C. D. Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J Histochem Cytochem. 1975 Jul;23(7):493–505. doi: 10.1177/23.7.1095650. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Osmond D. G. Lymphocyte populations in mouse bone marrow: quantitative kinetic studies in young, pubertal and adult C3H mice. Cell Tissue Kinet. 1975 Mar;8(2):97–110. doi: 10.1111/j.1365-2184.1975.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Pera F., Mattias P. Labelling of DNA and differential sister chromatid staining after BrdU treatment in vivo. Chromosoma. 1976 Aug 4;57(1):13–18. doi: 10.1007/BF00292946. [DOI] [PubMed] [Google Scholar]
- Rosse C. 2 morphologically and kinetically distinct populations of lymphoid cells in the bone marrow. Nature. 1970 Jul 4;227(5253):73–75. doi: 10.1038/227073a0. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Chaillet J. R., Tice R. R. In vivo BUdR labeling of mammalian chromosomes. Exp Cell Res. 1976 Jul;100(2):396–399. doi: 10.1016/0014-4827(76)90165-8. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice R., Chaillet J., Schneider E. L. Demonstration of spontaneous sister chromatid exchanges in vivo. Exp Cell Res. 1976 Oct 15;102(2):426–429. doi: 10.1016/0014-4827(76)90062-8. [DOI] [PubMed] [Google Scholar]
- Tice R., Schneider E. L., Rary J. M. The utilization of bromodeoxyuridine incorporation into DNA for the analysis of cellular kinetics. Exp Cell Res. 1976 Oct 15;102(2):232–236. doi: 10.1016/0014-4827(76)90037-9. [DOI] [PubMed] [Google Scholar]
- Vogel W., Bauknecht T. Differential chromatid staining by in vivo treatment as a mutagenicity test system. Nature. 1976 Apr 1;260(5550):448–449. doi: 10.1038/260448a0. [DOI] [PubMed] [Google Scholar]
- Wolff S., Perry P. Differential Giemsa staining of sister chromatids and the study of chromatid exchanges without autoradiography. Chromosoma. 1974;48(4):341–353. doi: 10.1007/BF00290991. [DOI] [PubMed] [Google Scholar]




