Abstract
Tissues of 10 psittacines from aviary 1 (“case birds”) and 5 psittacines from different aviaries were investigated for the presence of Avian bornavirus (ABV) antigen by immunohistochemistry using a polyclonal serum specific for the viral nucleocapsid (N) protein. Seven of 10 case birds had clinical signs, and necropsy findings consistent with proventricular dilatation disease (PDD) while 3 case birds and the 5 birds from other aviaries did not exhibit signs and lesions of this disease. In birds with clinical signs of PDD, ABV antigen was largely limited to neuroectodermal cells including neurons, astroglia, and ependymal cells of the central nervous system, neurons of the peripheral nervous system, and adrenal cells. ABV antigen was present in the nuclei and cytoplasm of infected cells. In 2 case birds that lacked signs and lesions of PDD, viral antigen had a more widespread distribution and was present in nuclei and cytoplasm of epithelial cells of the alimentary and urogenital tract, retina, heart, skeletal muscle, and skin in addition to the mentioned neuroectodermal cells. ABV RNA was identified by reverse transcription polymerase chain reaction (RT-PCR) in tissues of all 7 case birds available for testing from aviary 1, including 4 birds with PDD lesions and the 3 birds without PDD lesions. Sequencing and phylogenetic analysis indicated the presence of ABV genotype 1 in all cases. Findings further substantiate a role of ABV in PDD of psittacine bird species.
Keywords: Avian bornavirus, immunohistochemistry, proventricular dilatation disease, psittacines
Introduction
Proventricular dilatation disease (PDD) has been recognized in more than 50 species of psittacine birds for approximately 30 years but its cause was elusive until recently.3,10 Lesions compatible with PDD have also been described in a variety of non-psittacine birds.4,13,21 In 2008, a novel Avian bornavirus (ABV) was implicated as the possible cause of the disease based on the presence of viral RNA in naturally occurring cases.12,16 ABV was also isolated from brain tissue of psittacines with PDD.9 Avian bornavirus is phylogenetically related to Borna disease virus (BDV), the sole species in the genus Bornavirus of the family Bornaviridae in the order Mononegavirales.20 In contrast to BDV, the genetic variability of ABV is significant; to date, at least 6 genotypes (ABV1–6) have been identified in birds from Europe, the Middle East, Australia, and the United States. Five of these genotypes have been detected in psittacine birds while the sixth genotype was found in a canary bird, the only non-psittacine bird known to be infected with ABV.27, 32 The genetic variability between genotypes is quite considerable, with a homology range of 50–90%, and differentiation of genotypes is currently based on sequence analysis of partial nucleoprotein (NP) and matrix protein (M) genes.
In its classic form, PDD is a wasting syndrome characterized by lymphoplasmacytic inflammation of the autonomous nervous system of the alimentary tract (e.g., ganglionitis) leading to dilatation of crop and proventriculus.10 Although the inflammation of the autonomous nervous system of the alimentary tract is the hallmark lesion of the disease, inflammatory lesions may be more widespread and involve the central nervous system, kidney, adrenal gland, heart, and lungs.13,27 Recently, more pronounced neurologic signs and central nervous system involvement (e.g., encephalitis) have been described more frequently in association with this disease.1,13,15 There is mounting evidence that ABV infection is widespread in birds in North America, Australia, and Europe and can also be seen in apparently healthy animals.5,19,25,32 A recent study demonstrated the presence of ABV antigen in a wide variety of cells in psittacine birds with natural ABV genotypes 2 and 4 infection.22 Experimental infections with genotype 4 virus demonstrated a causative role in PDD.7,9 In the current retrospective study, spontaneous cases of PDD are described, using histopathology, immunohistochemistry (IHC), and quantitative polymerase chain reaction (qPCR). The virus involved in these cases is characterized as a genotype 1 virus.
Materials and methods
Animals
The case birds (birds 1–10) comprise 10 birds from a single aviary (aviary 1) in Minnesota including 5 cockatiels (Nymphicus hollandicus), 1 blue-crowned parakeet (Aratinga acuticaudata), 1 Nanday parakeet (Aratinga nenday), 1 white cockatoo (Cacatua alba), and 2 salmon-crested cockatoos (Cacatua moluccensis; Table 1). The animals from aviary 1 were submitted over the course of 1 year.
Table 1.
Signalment of 10 Avian bornaviras (ABV) RNA and/or ABV antigen–positive psittacine birds with (birds 1–3, 6–9) and without (birds 4, 5, 10) clinicopathologic evidence of proventricular dilatation disease (PDD) from 1 aviary in comparison to 5 ABV RNA–negative psittacine birds without signs of PDD from other aviaries (birds 11–15).*
| Case no. | Aviary | Age (years) | Sex | Species | Specimen | Lesions | PCR | IHC |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 5 | M | Nymphicus hollandicus | Fresh carcass | Yes | ND | + |
| 2 | 1 | ~20 | F | Nymphicus hollandicus | Fixed tissues and frozen remains of carcass | Yes | + | + |
| 3 | 1 | Adult | M | Nymphicus hollandicus | Fresh carcass | Yes | + | + |
| 4 | 1 | Adult | M | Nymphicus hollandicus | Fixed tissues and frozen remains of carcass | No | + | Equivocal |
| 5 | 1 | Adult | F | Nymphicus hollandicus | Fresh carcass | No | + | + |
| 6 | 1 | ~23 | M | Aratinga acuticaudata | Fixed tissues and frozen remains of carcass | Yes | + | + |
| 7 | 1 | Adult | M | Aratinga nenday | Fixed tissues | Yes | ND | + |
| 8 | 1 | 9 | M | Cacatua alba | Fresh carcass | Yes | + | + |
| 9 | 1 | Adult | M | Cacatua moluccensis | Fresh carcass | Yes | ND | + |
| 10 | 1 | >40 | F | Cacatua moluccensis | Fresh carcass | No | + | + |
| 11 | 2 | 25 | M | Eclectus roratus | Fresh carcass | No | – | Equivocal |
| 12 | 3 | 25 | F | Ara ambigus | Fresh carcass | No | – | Equivocal |
| 13 | 4 | 12 | F | Psittacus erithacus | Fresh carcass | No | – | – |
| 14 | 5 | 22 | M | Psittacus erithacus | Fresh carcass | No | – | – |
| 15 | 6 | 6 | F | Nymphicus hollandicus | Fresh carcass | No | – | – |
PCR = polymerase chain reaction; IHC = immunohistochemistry; ND = not available for testing; + = positive; – = negative.
In addition, tissues of 5 psittacine birds without clinical signs of PDD and originating from different aviaries (2–6) in Minnesota were used for IHC and PCR (birds 11–15; Table 1). These birds included 1 eclectus parrot (Eclectus roratus; bird 11), 1 great green macaw (Ara ambiguus; bird 12), 2 grey parrots (Psittacus erithacus; birds 13 and 14), and 1 cockatiel (Nymphicus hollandicus; bird 15). The fresh carcasses of birds 1, 3, 4, 8–15, formalin-fixed tissue of bird 7, and formalin-fixed tissues with the frozen remainder of the carcass of birds 2, 5, and 6 were available for investigation (Table 1).
Polymerase chain reaction
Unfixed tissues, suitable for comparative analysis by quantitative RT-PCR for ABV, were only available from 7 of the 10 case birds and all control birds of the study (Table 2). RNA was extracted, DNase I treated, reverse transcribed using random hexamer primers, and tested by PCR using degenerate primers developed for detection of described ABV genotypes that target the 3’-portion of the NP gene and the 5’-portion of the phosphoprotein (P) gene,12,19 as well as an overlapping segment within the N protein (forward: 5’-GCBCARCCATGGGTYGGHTC -3’; reverse: 5’-GCTCCAGTAAAAAGCGGCCGATGCC-3’). Sequence of the covered fragment (approximately 800 bp) was determined by automated dideoxy-sequencing, and partial nucleoprotein sequence (323 bp) was used for phylogenetic analysis.
Table 2.
Results of reverse transcription polymerase chain reaction testing for nucleocapsid protein gene in 4 case birds with (birds 2, 3, 6, 8) and 3 case birds without (birds 4, 5, 10) clinicopathologic evidence of proventricular dilatation disease (PDD) from 1 aviary in comparison to 5 psittacine birds without signs of PDD from other aviaries (birds 11–15).*
| Case no. | Brainstem | Cerebellum | Cerebrum | Spinal cord | Spinal ganglion | Crop | Proventriculus | Ventriculus | Duodenum | Pancreas | Heart | Adrenal gland | Kidney |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 3 | ND | ND | + | ND | ND | – | – | ND | ND | ND | + | ND | – |
| 4 | – | ND | ND | ND | ND | – | – | + | – | – | ND | ND | ND |
| 5 | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | + | ND | ND | ND | ND | ND | + | + | ND | ND | ND | ND | + |
| 10 | ND | ND | ND | + | + | ND | ND | + | ND | ND | ND | ND | ND |
| 11 | – | ND | ND | ND | ND | ND | ND | – | ND | ND | ND | ND | – |
| 12 | – | ND | ND | ND | ND | ND | – | – | ND | – | – | – | – |
| 13 | – | – | – | ND | ND | – | – | – | ND | – | – | ND | – |
| 14 | ND | – | – | ND | ND | – | – | – | ND | – | – | – | – |
| 15 | – | ND | ND | ND | ND | – | – | – | – | – | – | – | – |
ND = not available for testing; – = negative; + = positive.
Immunohistochemistry
All birds of the study were subjected to IHC for ABV antigen (Table 3). A peroxidase-based polymer system was used for immunohistochemical demonstration of ABV antigen.a The IHC was performed using an automated staining process.b A polyclonal antiserum against N protein of ABV (strain 1367; GenBank accession no. FJ169440) was used as specific primary antibody. The 49-kDa histidine-tagged N protein was expressed in Escherichia coli,c purified on Ni2+-nitrilotriacetic acid agarosed and used for immunization of rabbits.e The specificity of the antibody was confirmed by enzyme-linked immunosorbent assay and Western blot analysis, using purified protein, as well as antigen extract from ABV-infected parrots.19,31 Four-micron thickness sections were mounted on glass slides, deparaffinized with clearing agent,f and rehydrated in a graded alcohol series (100%, 95%, 70%, and 50% distilled H2O). The sections were incubated with 3% H202 for 15 min in order to block the endogenous peroxidase activity. Sections were rinsed in Tris-buffered saline with 0.05% Tween 20g (TBS; 0.05 M, pH 7.6). To block unspecific binding sites, the sections were incubated with normal goat serum that was diluted 1:10 in TBS. After draining excess blocking serum off the sections, the anti-ABV antiserum was applied for 45 min at room temperature. The antibody was diluted 1:1,250 in antibody diluent solution.h The sections were rinsed with TBS-Tween 20 and subsequently incubated with the secondary antibody for 45 min at room temperature (polymer-labeled goat anti-rabbit immunoglobulin Gi; with 2% normal chicken serum added per volume). After rinsing in TBS-Tween 20, positive antigen–antibody reactions were visualized by incubating the slides with 3-amino-9-ethylcarbazolej for 10 min; slides were briefly counterstained with Mayer hematoxylin. Controls included substitution of the primary antiserum with diluted normal serum from nonimmunized rabbits and inclusion of brain tissue of a known ABV RNA–positive bird with every badge. The amount of ABV antigen expression in different organs was graded subjectively into mild, moderate, and marked based on the estimated number of ABV antigen–positive cells.
Table 3.
Distribution of Avian bornavirus (ABV) antigen in tissues of 10 ABV RNA–positive psittacine birds with (birds 1–3, 6–9) and without (birds 4, 5, 10) clinicopathologic evidence of proventricular dilatation disease (PDD) from 1 aviary in comparison to 5 ABV RNA–negative psittacine birds without signs of PDD from other aviaries (birds 11–15).*
| Case no. | Brainstem | Cerebellum | Cerebrum | Spinal cord | Spinal ganglion | Crop | Proventriculus | Ventriculus | Duodenum | Pancreas | Heart | Adrenal gland | Eye | Kidney |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ++ | – | + | ND | ND | + | + | + | – | – | – | ND | + | – |
| 2 | ++ | + | ++ | ND | ND | ND | + | + | ND | ND | – | ND | ND | – |
| 3 | ++ | – | ++ | ++ | + | – | – | – | – | – | – | ND | – | + |
| 4 | – | – | – | ND | ND | – | – | – | – | +cyto | – | ND | – | – |
| 5 | +++ | +++ | +++ | ND | ND | +++ | +++ | +++ | ++ | ++ | ++ | ND | ND | +++ |
| 6 | ++ | ++ | ++ | ND | ND | ++ | + | + | ND | ND | – | ND | – | – |
| 7 | +++ | +++ | +++ | ND | ND | + | ++ | + | ++ | +cyto | + | + | ND | – |
| 8 | ++ | + | +++ | + | + | + | +cyto | + | – | – | – | ND | – | – |
| 9 | +++ | + | ++ | + | + | ND | ++ | +++ | ++ | +cyto | – | ND | ND | – |
| 10 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 11 | – | – | – | ND | ND | – | +cyto | – | – | +cyto | – | – | – | – |
| 12 | – | – | – | ND | ND | – | +cyto | – | – | +cyto | – | – | – | – |
| 13 | – | – | – | ND | ND | – | – | – | – | – | – | – | – | – |
| 14 | – | – | – | ND | ND | – | – | – | – | – | – | – | – | – |
| 15 | – | – | – | ND | ND | – | – | – | – | – | – | – | – | – |
Subjective grading scheme: – = no immunoreactivity; + = mild nuclear and cytoplasmic immunoreactivity; ++ = moderate nuclear and cytoplasmic immunoreactivity; +++ = marked nuclear and cytoplasmic immunoreactivity; +cyto = cytoplasmic immunoreactivity only; ND = not done.
Transmission electron microscopy
Based on their widespread and abundant ABV antigen presence (see below), 2 birds (birds 5 and 10) were selected for electron microscopy to identify viral particles. Mesencephalon and kidney of a cockatiel (bird 5), and cerebellum, retina, kidney, and small intestine of a salmon-crested cockatoo (bird 10) were first post-fixed in 2.5% glutaraldehydek and then postfixed in 1% osmium tetroxidel 0.1 M sodium cacodylate bufferm (pH 7.4). Tissues were embedded in resinn and polymerized at 60°C for 48 hr. Ultrathin sections at silver interference (70–90 nm thickness) were stained with uranyl acetate and lead citrate.o Three serial sections of all tissues were examined with an electron microscope.p
Results
Seven of the 10 case birds from aviary 1 were clinically suspected of having PDD due to emaciation, regurgitation, and passage of undigested seeds (birds 1–3, 6–9). One salmon-crested cockatoo (bird 10) was submitted for necropsy after it had been attacked and fatally wounded by a white cockatoo. One cockatiel (bird 4) died after having dyspnea for several days. Another cockatiel (bird 5) died during what appeared to be a seizure without previous signs of illness. Birds 11, 13, and 14 were submitted for sudden death, while bird 12 was submitted for a rapidly progressive abdominal distension. Bird 15 had likely died from exsanguination after chronic recurrent feather picking with formation of granulation tissue.
Necropsy and histopathology
Seven case birds (birds 1–3, 6–9) had dilatation of crop, proventriculus, and ventriculus and/or duodenum suggestive of PDD (Table 1). The degree of dilatation varied from mild to marked. The 7 case birds had lymphoplasmacytic brainstem encephalitis varying from mild to marked. Five of the 7 case birds with gross lesions of PDD (birds 1, 6–9) had a subtle lymphoplasmacytic ganglionitis of the crop, proventriculus, and/or ventriculus. Two cockatiels (birds 2, 3) did not have any significant inflammatory changes in these compartments of the alimentary tract despite having gross evidence of proventricular dilatation. Spinal cord and spinal ganglia were only available for examination from 3 case birds with gross PDD lesions. All 3 animals had mild to moderate lymphoplasmacytic spinal ganglionitis and lymphoplasmacytic myelitis ranging from mild to marked (birds 3, 8, 9). Mild lymphoplasmacytic endocarditis was present in 1 bird (bird 9). The adrenal gland was only sampled in 2 of the affected birds; lymphoplasmacytic adrenalitis was detected in both birds (birds 7, 10).
Gross and histologic lesions suggestive of PDD, including lymphoplasmacytic ganglionitis and encephalitis, were absent in 3 case birds from aviary 1, including one of the salmon-crested cockatoos (bird 10) and 2 cockatiels (birds 4, 5). The salmon-crested cockatoo (bird 10) had skull and cervical spine fractures consistent with the history of interspecies aggression. One cockatiel had a large gonadal neoplasm (seminoma; bird 4) but no significant histologic inflammatory lesions of the peripheral or central nervous system. The other cockatiel (bird 5) had moderate multifocal lymphofollicular nephritis, pancreatitis, and salpingitis that were considered to be atypical of PDD.
Gross and histologic lesions of PDD were not present in any of the birds from aviaries 2–6 (birds 11–15). Gross and histologic lesions of these birds included fungal aortitis (bird 11), cloacal adenocarcinoma (bird 12), marked aortic atherosclerosis (bird 14), and dermal ulceration with granulation tissue (bird 15). In one of these birds, significant lesions were not detected and the cause of death remained uncertain (bird 13).
Polymerase chain reaction
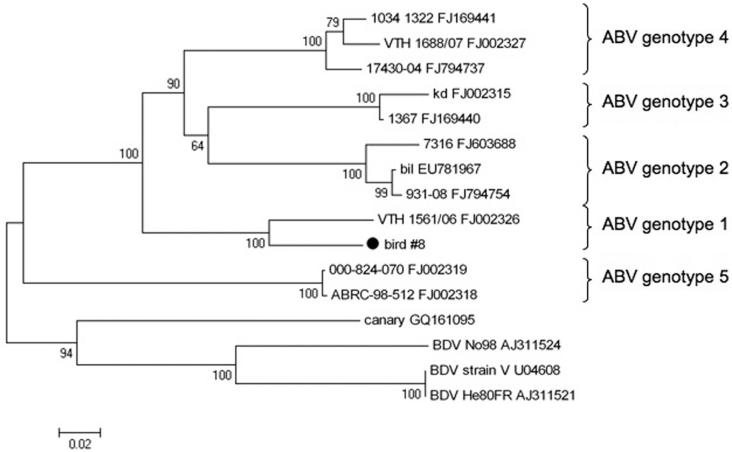
Frozen tissues of 7 case birds were tested for ABV nucleic acids by PCR (Table 2). Analysis of the generated approximately 800-bp amplification products revealed identical sequence for birds from aviary 1 including the 3 birds without gross and histologic PDD lesions. In 5 birds, all examined samples were positive for ABV RNA. In 1 bird with PDD lesions, heart and cerebrum were positive, while crop, proventriculus, and kidney were negative for ABV RNA (bird 3). In 1 bird without PDD lesions, only the ventriculus was positive for ABV RNA, while crop, proventriculus, duodenum, pancreas, and brainstem were negative (bird 4). Phylogenetic analysis indicated the sequences to be most closely related to ABV genotype 1 (Fig. 1); this assignment was also supported by analysis of partial M gene sequence (not shown). All frozen tissues of the birds from aviaries 2–6 were negative for ABV RNA (Table 2).
Figure 1.
Phylogenetic tree showing the relationship of the Avian bornavirus (ABV) strain identified in the current study (bird 8) to previously published ABV sequences (with their respective GenBank accession numbers). Partial nucleoprotein sequence was aligned using ClustalW, and phylogenetic relationships were deduced by neighbor joining analysis using MEGA version 4.0.2,30 applying a Jukes-Cantor model, and performing 1,000 pseudo-replicate analyses. Bootstrap values are given at the respective nodes; scale bar indicates number of nucleotide substitutions per side.
Immunohistochemistry
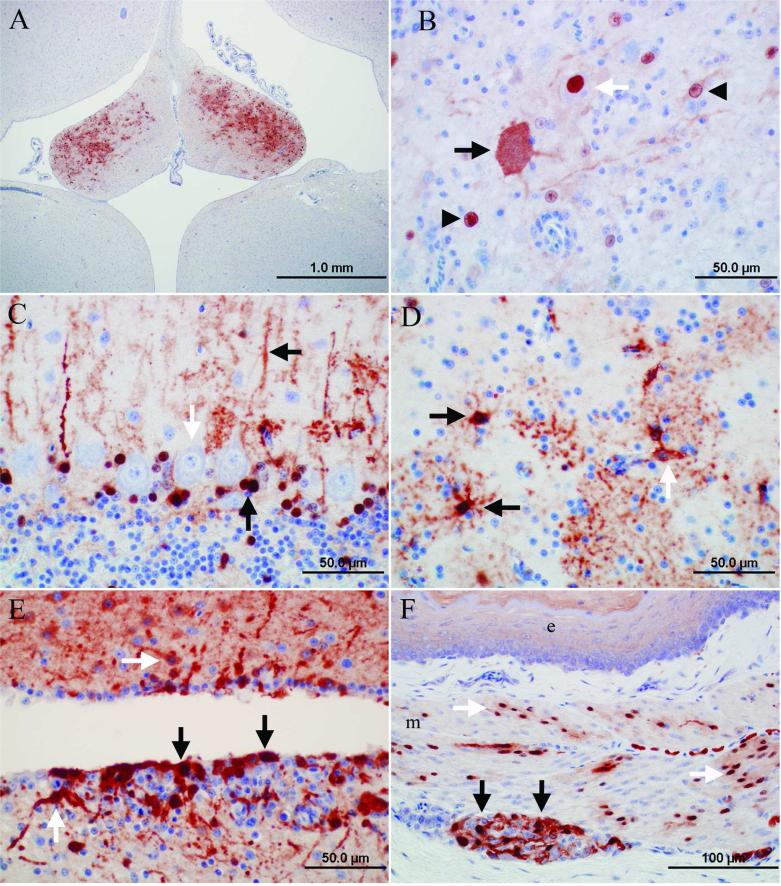
Avian bornavirus antigen was detected in the brainstem and cerebrum of all 7 birds with gross and histologic PDD lesions (Table 3). Antigen was present in the cerebellum of 5 of these birds (Table 3). Occasionally, the distribution of ABV antigen–positive cells was bilateral symmetrical in cerebrum and brainstem (e.g., nucleus septalis lateralis and medialis; Fig. 2A). In the brain, the gray matter was the site of the predominant ABV antigen immunoreactivity, while fewer cells (presumably glial cells) were positive in the white matter. Affected cells included neurons, astroglial cells, and ependymal cells (Fig. 2B–E). Usually, a large number of cells and cell processes of neurons and glial cells were ABV antigen–positive in the brainstem and cerebrum and to a lesser extent in the cerebellum. In the cerebellum, frequently, the Purkinje cells were negative, while Bergmann glia was strongly positive (Fig. 2C). In addition, astroglial cells bordering capillaries were frequently positive for ABV antigen (Fig. 2D). Ependymal cells were ABV antigen positive in 2 birds (cases 7, 8; Fig. 2E). Avian bornavirus antigen–positive neurons and glial cells were also detected in the spinal cord (birds 3, 8, 9), spinal ganglia (birds 8, 9), and ganglia of the myenteric plexus in crop (birds 1, 6, 7, 8; Fig. 2F), proventriculus (birds 1, 6–9), ventriculus (birds 1, 2, 6–9), and duodenum (birds 7, 9). Positive neurons had a strong brownish precipitate in the nucleus with or without weaker immunoreactivity in the cytoplasm. Cytoplasmic staining, only, was detected in the pancreatic islet cells of 2 birds (birds 7, 9; Table 3). Avian bornavirus antigen was also present in smooth muscle cells of the muscularis of crop and proventriculus (birds 6, 7, 9; Fig. 2F). Smooth muscle cells of arteries in the alimentary tract and heart (birds 1, 2, 7) and axons and nuclei of presumably Schwann cells of unmyelinated visceral nerves were positive for ABV antigen in few cases (birds 2, 3, 9). In 1 case each, a low amount of ABV antigen was present in few cardiomyocytes (bird 7), tubular epithelial cells of the kidney (bird 3), epithelial cells of the proventriculus (bird 6), epithelial cells of the duodenum (bird 7), photoreceptors and nuclei of the outer and inner nuclear layers of retina of the eye (bird 1), and adrenal cells of the adrenal gland (bird 7).
Figure 2.
Photomicrographs of the immunoreactivity for Avian bornavirus (ABV) antigen in psittacine birds with clinical signs and lesions of proventricular dilatation disease. Envision system horseradish peroxidase: nucleocapsid protein of ABV; polyclonal antiserum. A, cerebrum, cockatiel, bird 3: bilateral symmetrical immunoreactivity in the nuclei septalis. Bar = 1.0 mm. B, brainstem, cockatiel, bird 1: immunoreactive neurons. Note that the nucleus of some neurons is strongly immunoreactive (white arrow) while in another neuron the cytoplasm is immunoreactive (black arrow). In addition, the nuclei of astrocytes are immunoreactive (arrowheads). Bar = 50.0 μm. C, cerebellum, Nanday parakeet, bird 7: immunoreactive Bergmann glia. Note that the nuclei and processes of Bergman glia are immunoreactive (black arrows) but the Purkinje cells are negative for ABV antigen in this case (white arrow). Bar = 50.0 μm. D, cerebrum, Nanday parakeet, bird 7: immunoreactive glial cells. The processes and nuclei of glial cells in the neuropil are immunoreactive (black arrows). The cell process of 1 glial cell extends to a capillary (white arrow). Bar = 50.0 μm. E, cerebrum, cockatiel, bird 2: ependymal cells (black arrows) and subependymal glial cells (white arrows) are immunoreactive. Bar = 50.0 μm. F, crop, blue-crowned parakeet, bird 6: nuclei and cytoplasm of neurons of a subserosal ganglion (black arrows) and smooth muscle cells of the crop muscularis layer (m, white arrows) are strongly immunoreactive. The epithelial lining (e) of the crop is on the top of the picture. Bar = 100 μm.
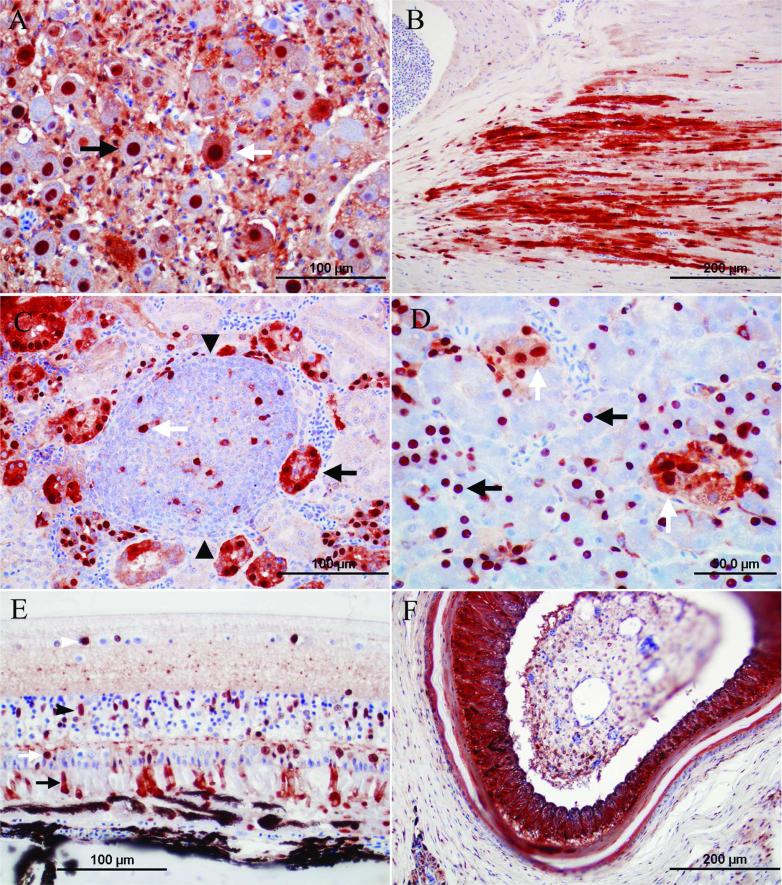
In one of the salmon-crested cockatoos (bird 10) and 1 cockatiel without PDD lesions (bird 5) from aviary 1, ABV antigen was widespread with respect to organ distribution and cell type (Table 3), and abundant based on the intensity of the immunoreaction. The immunohistochemical findings in the central and peripheral nervous system resembled the findings described above in terms of cell tropism and distribution although the reactivity was more intense (Fig. 3A). However, in addition to the reactivity in the central and peripheral nervous system, an intensive immunoreactivity was present in nuclei and cytoplasm of smooth muscle cells of arteries and alimentary tract muscularis and in visceral nerves, cardiomyocytes (Fig. 3B), epithelial cells of the proximal tubuli of the kidney (Fig. 3C), islet cells, and acinar cells of the pancreas (Fig. 3D), as well as epithelial cells of the crop, proventriculus, ventriculus, and duodenum. In 1 of these 2 cases (bird 10, photoreceptors, nuclei of the outer and inner nuclear layers, and Müller cells of the retina (Fig. 3E), adrenal cells, Purkinje fibers of the heart, keratinocytes of epidermis and feather follicles (Fig. 3F), biliary epithelial cells of the liver, epithelial cells of the lung, skeletal muscle fibers, macrophages of the spleen, and follicular cells of the ovary were ABV antigen positive. In the bird without PDD signs (bird 5) but that had significant lymphofollicular inflammatory lesions in kidney, salpinx, and pancreas, individual immunoreactive cells were present among the inflammatory infiltrate (possibly macrophages; Fig. 3C). In another bird without PDD lesions (bird 4), ABV antigen was only detected in the cytoplasm of pancreatic islet cells.
Figure 3.
Photomicrographs of the immunoreactivity for Avian bornavirus (ABV) antigen in 2 psittacine birds without clinical signs and lesions of proventricular dilatation disease. Envision system horseradish peroxidase: nucleocapsid protein of ABV; polyclonal antiserum. A, mesentericoceliac ganglion, salmon-crested cockatoo, bird 10: nuclei of numerous neurons (black arrow), cytoplasm of occasional neurons (white arrow), and cytoplasm and nuclei of numerous satellite cells are immunoreactive. Bar = 100 μm. B, heart, cockatiel, bird 5: cytoplasm and nuclei of cardiomyocytes is immunoreactive. Bar = 200 μm. C, kidney, cockatiel, bird 5: cytoplasm and nuclei of epithelial cells of proximal tubuli is immunoreactive (black arrow). Note an inflammatory “lymphofollicular” aggregate in the center of the pictures (arrowheads). Multiple cells within the aggregate (presumably macrophages) are immunoreactive (white arrow). Bar = 100 μm. D, pancreas, salmon-crested cockatoo, bird 10: nuclei of numerous acinar pancreatic cells (black arrows) and cytoplasm and nuclei of islet cells are immunoreactive (white arrows). Bar = 50 μm. E, retina, salmon-crested cockatoo, bird 10: photoreceptors (black arrow), nuclei of outer nuclear layer neurons (white arrow), inner nuclei layer neurons, neurons of the ganglion cell layer (white arrowhead), and nuclei of Müller cells are immunoreactive (black arrowhead). In addition, multiple photoreceptors are immunoreactive (black arrow). Bar = 100 μm. F, skin, salmon-crested cockatoo, bird 10: numerous epithelial cells of a feather follicle are immunoreactive. Bar = 200 μm.
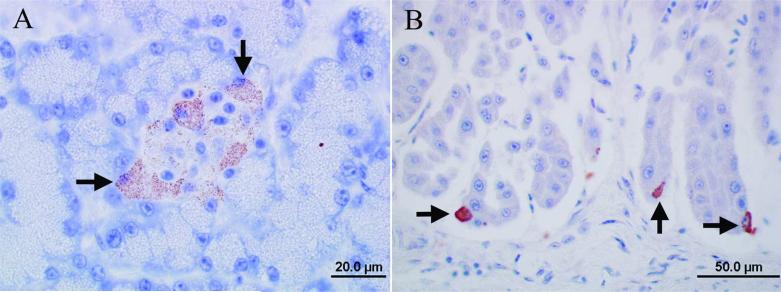
Although all tested organs of the 5 birds from aviaries 2–6 (birds 11–15) were negative for ABV RNA, 2 of them had sporadic immunoreactivity in individual tissues (birds 11, 12; Table 3). The immunoreactivity in these cases was limited to the cytoplasm similar to the findings in case bird 4 of aviary 1. Both birds had immunoreactive islet cells of the pancreas (Fig. 4A) and a few individual immunoreactive cells among the epithelial cells of the proventriculus (Fig. 4B). Three ABV RNA–negative birds (birds 13–15) also tested consistently negative for ABV antigen by IHC in all submitted tissues (cerebrum, cerebellum, and brainstem, crop, proventriculus, ventriculus, duodenum, adrenal gland, heart, and kidney), thus serving as negative controls for the study (Table 3).
Figure 4.
Photomicrographs of the immunoreactivity for Avian bornavirus (ABV) antigen in an ABV RNA–negative eclectus parrot (case 11) without clinical signs and lesions of proventricular dilatation disease. Envision system horseradish peroxidase: nucleocapsid protein of ABV; polyclonal antiserum. A, pancreas: the cytoplasm of islet cells has a fine granular immunoreactivity (black arrows). Bar = 20.0 μm. B, proventriculus, the cytoplasm of few individual epithelial cells has immunoreactivity (black arrows). Bar = 50.0 μm.
Transmission electron microscopy
Few round virus-like particles, 84–104 nm in diameter, were present in the cytoplasm of neurons in the mesencephalon of 1 cockatiel (bird 5), while virus-like particles were not detected in the brain of the salmon-crested cockatoo. Few virus-like particles were present in unmyelinated nerve fibers of small intestine in the salmon-crested cockatoo (bird 10). Virus-like particles were not detected in the other examined organs of either bird. Ultrastructural cellular degenerative lesions were not detected in either one of the animals but mild artifacts due to postmortem tissue autolysis were present.
Discussion
The current study describes the organ distribution and cell tropism of ABV genotype 1 in naturally infected psittacine birds based on immunohistochemical detection of viral antigen and PCR amplification of nucleic acids. A polyclonal ABV N protein–specific antiserum demonstrated strong nuclear immunoreactivity in neurons, epithelial cells, and smooth muscle cells, in a pattern consistent with the nuclear replication and the pathognomonic intranuclear inclusion bodies previously described for BDV.2,14,26
Three of the ABV RNA–positive birds did not have gross or histologic lesions of PDD. Birds without PDD signs or lesions, but infected with ABV, have been reported previously although the antigen distribution in these cases has not been reported.5,19 A 2010 study demonstrated a sensitivity and specificity of 100% for both ABV-specific PCR and IHC in birds of a flock infected with ABV genotypes 2 and 4.22 In the 2010 study, only birds with gross and/or histologic lesions of PDD were positive for ABV RNA and antigen, while all birds without these lesions were negative for viral N protein antigen and M protein antigen. In 1 case of the present study (bird 4) that was positive for ABV RNA solely in the ventriculus, ABV antigen was present only in the cytoplasm of pancreatic islet cells. However, in 2 of the case birds in the present study, large quantities of viral antigen were detected in all examined organs and numerous cell types in the absence of gross or histologic PDD lesions (bird 10), or in the presence of an unusual disseminated lymphofollicular pattern of inflammation (bird 5). Tissue distribution and cell tropism in these birds were pantropic, infecting epithelial, mesenchymal, and neuroectodermal cells. A widespread cell tropism of ABV has been previously described in symptomatic, PDD-affected birds infected with ABV genotypes 2 and 4.19,22
The reasons for the remarkably widespread viral antigen distribution in 2 of the birds in the current study (birds 5, 10) without signs and lesions of PDD remain unclear. It may reflect early stages of a viremic infection that possibly manifests itself in clinical disease only during later stages when virus is beginning to be cleared from tissues by the immune response. However, this differs from the neurotropic infection and the immunopathological disease described in BDV-infected animals.23,24,28 Alternatively, and comparable to the widespread peripheral distribution of BDV observed in immunosuppressed or neonatally infected rats the 2 animals may be incapable of mounting an efficient ABV-specific immune response, possibly due to neonatal or in ovo exposure to ABV or a decreased immune competence in aged animals.11,29 The presence of viral antigen in the retina in 1 of the 2 birds is in agreement with observations in naturally BDV-infected horses and experimentally infected rats that also express viral antigen in retinal neurons and Müller cells.6 It is unclear whether the disseminated lymphofollicular inflammation in one of the birds (bird 5) was directly associated with the ABV infection or may reflect a concurrent disease problem of unknown etiology. The lymphofollicular pattern of the inflammation in this bird was considered to be atypical of PDD.
In the present study, electron microscopy for detection of viral particles was conducted in the 2 birds without PDD lesions (birds 5, 10) because IHC suggested the presence of a significant amount of viral antigen. Interestingly, electron microscopy demonstrated only a few virus-like particles in the cytoplasm of nerve fibers in the intestine of 1 animal (bird 10), and the mesencephalic neurons of the second animal (bird 5). Of note is also the absence of virus-like particles in the strongly ABV N antigen–positive cerebellum of the salmon-crested cockatoo (bird 10). The lack of virus-like particles in some tissues or only low numbers of particles in other tissues in conjunction with the high viral N protein antigen load of these tissues may suggest that virus maturation may be limited in some tissues despite successful and copious synthesis of N protein antigen. The observation of virus-like particles in the cytoplasm that could reflect maturation of ABV on intracellular membranes after export of viral genomes from the nucleus by a mechanism different from messenger RNA export is difficult to interpret in view of the reported budding of the virus on the cytoplasm membrane in an experimental model of BDV.18 Since bornaviral particles have never before been detected in tissues from naturally infected birds or mammals, verification of the authenticity of the detected particles will require the use of immunogold labeling techniques in future studies.
Histopathology and electron microscopy failed to demonstrate any significant degenerative lesions of infected cells corroborating observations in naturally and experimentally infected horses and rats that bornavirus is noncytopathic.8 The presence of few individual immunoreactive epithelial cells in the proventriculus and immunoreactive pancreatic islet cells in ABV RNA–negative birds (birds 11, 12) deserves further discussion. This finding may suggest an unspecific signal since the reactivity pattern indeed differed from that observed in PCR-positive cases where predominantly the nuclei of infected cells were immunoreactive. The pancreas and proventriculus of only one of the birds (bird 12) were available for PCR, and were negative for bornaviral RNA. Based on a previous study demonstrating that cerebellum, cerebrum, brainstem, spinal cord, and adrenal gland are consistently positive for ABV RNA in ABV-infected animals,22 the negative ABV PCR results of tissues of birds 11–15 would suggest the absence of ABV infection. Nevertheless, it cannot be ruled out that the immunologic reactivity was specific, since comparable staining was observed also in an ABV RNA–positive animal, which, however, tested negative for ABV RNA in the pancreas (bird 4). In addition, such cytoplasmic immunoreactivity was absent in the proventriculus and pancreas of both ABV RNA–positive and other negative animals indicating that the antiserum is not reacting nonspecifically to cell-specific antigens.
At least 6 genotypes of ABV have thus far been suggested through limited sequence analyses of NP and M gene fragments.13,17,32,33 There has been speculation that ABV genotypes 2 and 4 are predominant in acute symptomatic PDD cases.7,13,17,22,25,32 Brain homogenates of genotype 4–infected birds have been shown to induce PDD after inoculation into naïve birds,7 and a tissue culture isolate of a genotype 4 virus provided proof that ABV can cause PDD after inoculation into a susceptible host.9 In the birds in the current study, ABV genotype 1 was present. Genotype 1 ABV had previously been described in only 2 cases, a galah (Eolophus roseicapillus) from Israel with myenteric ganglioneuritis of the crop and ventriculus and a PDD-affected yellow-collared macaw (Primolius auricollis) from the United States.9,16 In the present study, genotype 1 virus was consistently detected in the birds from the affected aviary, with identical sequence in all cases. This conservation seems noteworthy considering that the first and last infected cases of the series were submitted approximately 1 year apart indicating that the virus may circulate for an extended period of time in a collection or that the disease may have a long subclinical course in some infected animals. It is currently unknown whether reservoir species may be involved in the epidemiology of borna disease.
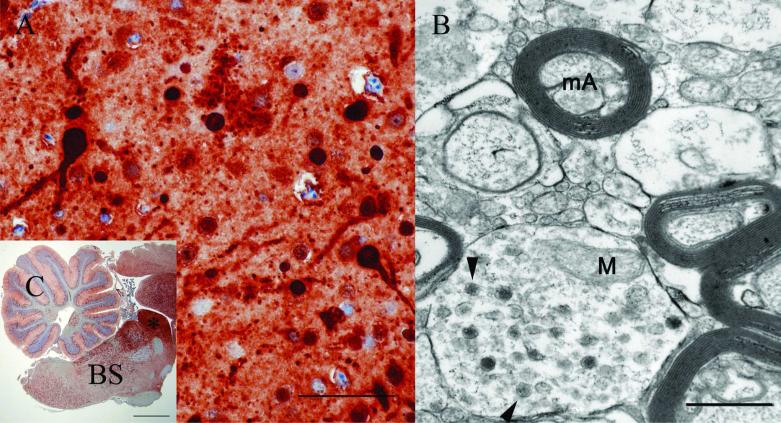
Figure 5.
A, photomicrographs of the immunoreactivity for Avian bornavirus (ABV) antigen in the mesencephalon (colliculus) of a cockatiel (bird 5) that was positive for ABV by polymerase chain reaction. Cytoplasm and nuclei of virtually all cells (neurons and astrocytes) in the photomicrograph are strongly positive. Bar = 50 μm. Inset: low magnification photomicrograph of the immunoreactivity for ABV antigen in the brain of the cockatiel depicting the location of the colliculus (*). C = cerebellum; BS = brainstem. Bar = 2 mm. B, transmission electron microscope micrograph, cockatiel, bird 5 (tissue from the colliculus was postfixed and processed): spherical 84–104 nm virus-like particles (arrowheads) were present in the cytoplasm of a neuronal cell process. mA = myelinated axon; M = mitochondrion. Bar = 500 nm.
Acknowledgments
The authors thank Ronda Aho, Michelle Nelson, Don Ariyakumar, and Dean Muldoon from the histology, immunohistochemistry, and electron microscopy service of the Minnesota Veterinary Diagnostic Laboratory for their excellent technical assistance.
Funding
The work was supported by National Institutes of Health grants AI051292 and AI57158 (Northeast Biodefense Center-Lipkin), and an award from the U.S. Department of Defense.
Footnotes
Declaration of conflicting interests
The authors declare that they had no conflicts of interests in their authorship and publication of this contribution.
Envision™ HRP, Dako North America Inc., Carpinteria, CA.
Dako autostainer plus, Dako North America Inc., Carpinteria, CA.
pDest17 Gateway vector, Invitrogen, Carlsbad, CA.
Qiagen Inc., Valencia, CA.
Lampire Biological Laboratories Inc., Pipersville, PA.
Fisher Scientific, Pittsburgh, PA.
Dako North America Inc., Carpinteria, CA.
Dako North America Inc., Carpinteria, CA.
Dako North America Inc., Carpinteria, CA.
Dako North America Inc., Carpinteria, CA.
Electron Microscopy Sciences, Hatfield, PA.
Electron Microscopy Sciences, Hatfield, PA.
Sigma-Aldrich, St. Louis, MO.
Embed 812, Electron Microscopy Sciences, Hatfield, PA.
Electron Microscopy Sciences, Hatfield, PA.
JEOL 1200EX II, JEOL Ltd., Tokyo, Japan.
References
- 1.Berhane Y, Smith DA, Newman S, et al. Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathol. 2001;53:563–570. doi: 10.1080/03079450120078770. [DOI] [PubMed] [Google Scholar]
- 2.Briese T, de la Torre JC, Lewis A, et al. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark FD. Proventricular dilatation syndrome in large psittacine birds. Avian Dis. 1984;28:813–815. [PubMed] [Google Scholar]
- 4.Daoust PY, Jullan RJ, Artsob H. Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese. J Wildl Dis. 1991;27:513–517. doi: 10.7589/0090-3558-27.3.513. [DOI] [PubMed] [Google Scholar]
- 5.De Kloet SR, Dorrenstein GM. Presence of avian bornavirus RNA and anti avian bornavirus antibodies in apparently healthy macaws. Avian Dis. 2009;53:568–573. doi: 10.1637/8828-040209-Reg.1. [DOI] [PubMed] [Google Scholar]
- 6.Dietzel J, Kurth H, Stahl T, et al. Morphometric analysis of the retina from horses infected with the borna disease virus. Vet Pathol. 2007;44:57–63. doi: 10.1354/vp.44-1-57. [DOI] [PubMed] [Google Scholar]
- 7.Gancz AY, Kistler AL, Greninger AL, et al. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenous containing avian bornavirus 4. Virol J. 2009;6:100. doi: 10.1186/1743-422X-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocztonyi G. Natural and experimental Borna disease virus infections—neuropathology and pathogenetic considerations. APMIS Suppl. 2008;124:53–57. doi: 10.1111/j.1600-0463.2008.000m8.x. [DOI] [PubMed] [Google Scholar]
- 9.Gray P, Hoppes S, Suchudolski P, et al. Use of avian bornavirus isolates to induce proventricular dilatation disease in conures. Emerg Infect Dis. 2010;16:473–479. doi: 10.3201/eid1603.091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory CR, Latimer KS, Niagro FD, et al. A review of proventricular dilatation syndrome. J Assoc Avian Vet. 1994;8:69–75. [Google Scholar]
- 11.Herzog S, Kompter C, Frese K, Rott R. Replication of Borna disease virus in rats: age dependent differences in tissue distribution. Med Microbiol Immunol. 1984;173:171–177. doi: 10.1007/BF02122108. [DOI] [PubMed] [Google Scholar]
- 12.Honkavuori KS, Shivaprasad HL, Williams BL, et al. Novel bornavirus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14:1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoppes S, Gray PL, Payne S, et al. The isolation, pathogenesis, diagnosis, transmission, and control of avian bornavirus and proventricular dilatation disease. Vet Clin North Am Exot Anim Pract. 2010;13:495–508. doi: 10.1016/j.cvex.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joest E, Degen K. Untersuchungen über die pathologische Histologie, Pathogenese und postmortale Diagnose der seuchenhaften Gehirn-Rückenmarksentzündung (Bornasche Krankheit) des Pferdes [Investigation of the histopathology, pathogenesis, and postmortem diagnosis of the endemic brain and spinal cord inflammation (Bornasche Krankheit) of the horse]. Z Infkrankh Haustiere. 1911;9:1–98. In German. [Google Scholar]
- 15.Keller DL, Honkavuori KS, Briese T, et al. Proventricular dilatation disease associated with avian bornavirus in a scarlet macaw (Ara macao). J Vet Diagn Invest. 2010;22:961–965. doi: 10.1177/104063871002200619. [DOI] [PubMed] [Google Scholar]
- 16.Kistler AL, Gancz AY, Clubb S, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kistler AL, Smith JM, Greninger AL, et al. Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease in captive psittacine birds. J Virol. 2010;84:2176–2179. doi: 10.1128/JVI.02191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohno T, Goto T, Takasaki T, et al. Fine structure and morphogenesis of Borna disease virus. J Virol. 1999;73:760–766. doi: 10.1128/jvi.73.1.760-766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lierz M, Hafez HM, Honkavuori KS, et al. Anatomical distribution of avian bornavirus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection. Avian Pathol. 2009;38:491–497. doi: 10.1080/03079450903349238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipkin WI, Briese T. Bornaviridae. In: Knipe DM, Howley RM, editors. Virology. 5th ed. Lippincott; Williams & Wilkins; Philadelphia, PA: 2007. pp. 1829–1851. [Google Scholar]
- 21.Perpinan D, Fernadez-Bellon H, Lopez C, Ramis A. Lymphoplasmacytic myenteric, subepicardial, and pulmonary ganglioneuritis in four nonpsittacine birds. J Avian Med Surg. 2007;21:210–214. doi: 10.1647/1082-6742(2007)21[210:LMSAPG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Raghav R, Taylor M, DeLay J, et al. Avian bornavirus is present in many tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease. J Vet Diagn Invest. 2010;22:495–508. doi: 10.1177/104063871002200402. [DOI] [PubMed] [Google Scholar]
- 23.Richt JA, Pfeuffer I, Christ M, et al. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richt JA, Rott R. Borna disease virus: a mystery as an emerging zoonotic pathogen. Vet J. 2001;161:24–40. doi: 10.1053/tvjl.2000.0533. [DOI] [PubMed] [Google Scholar]
- 25.Rinder M, Ackemann A, Kempff H, et al. Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease. J Virol. 2009;83:5401–5407. doi: 10.1128/JVI.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki S, Ludwig H. In borna disease virus infected rabbit neurons 100 nm particle structures accumulate at areas of Joest-Degen inclusion bodies. Zentralbl Veterinarmed B. 1993;40:291–297. doi: 10.1111/j.1439-0450.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 27.Staeheli P, Rinder M, Kaspers B. Avian bornavirus associated with fatal disease in psittacine birds. J Virol. 2010;84:6269–6275. doi: 10.1128/JVI.02567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stitz L, Bilzer T, Richt JA, Rott R. Pathogenesis of Borna disease. Arch Virol Suppl. 1993;7:135–151. doi: 10.1007/978-3-7091-9300-6_11. [DOI] [PubMed] [Google Scholar]
- 29.Stitz L, Nöske K, Planz O, et al. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J Virol. 1998;72:8884–8892. doi: 10.1128/jvi.72.11.8884-8892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 31.Villanueva I, Gray P, Mirhosseini N, et al. The diagnosis of proventricular dilatation disease: use of a Western blot assay to detect antibodies against avian Borna virus. Vet Microbiol. 2010;143:196–201. doi: 10.1016/j.vetmic.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Weissenböck H, Bakonyi T, Sekulin K, et al. Avian bornavirus is psittacines from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis. 2009;15:1453–1459. doi: 10.3201/eid1509.090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weissenböck H, Sekulin K, Bakonyi T, et al. Novel avian bornavirus in a nonpsittacine species (canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J Virol. 2009;83:11367–11371. doi: 10.1128/JVI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]