Abstract
OBJECTIVES
To assess the effects of long-term variations in ambient air pollutants on longitudinal changes in exhaled nitric oxide (FeNO), a potentially useful biomarker of eosinophilic airway inflammation, based on data from the southern California Children’s Health Study.
METHODS
Based on a cohort of 1,211 schoolchildren from 8 Southern California communities with FeNO measurements in 2006/07 and 2007/08, regression models adjusted for short-term effects of air pollution were fitted to assess the association between changes in annual long-term exposures and changes in FeNO.
RESULTS
Increases in annual average concentrations of 24-hr average NO2 and PM2.5 (scaled to the interquartile range (IQR) of 1.8 ppb and 2.4 μg/m3, respectively) were associated with a 2.29 ppb (CI=[0.36,4.21]; p =0.02) and a 4.94 ppb (CI=[1.44,8.47]; p = 0.005) increase in FeNO, respectively, after adjustments for short term effects of the respective pollutants. In contrast, changes in annual averages of PM10 and O3 were not significantly associated with changes in FeNO. These findings did not differ significantly by asthma status.
CONCLUSIONS
Changes in annual average exposure to current levels of ambient air pollutants are significantly associated with changes in FeNO levels in children, independent of short-term exposures and asthma status. Use of this biomarker in population-based epidemiologic research has great potential for assessing the impact of changing real world mixtures of ambient air pollutants on children’s respiratory health.
Keywords: Air pollution, chronic exposures, Children’s respiratory health, Environmental epidemiology, Exhaled nitric oxide, Airway inflammation
INTRODUCTION
There is mounting evidence showing that exposure to ambient air pollution at concentrations below the limits set by current regulatory standards are associated with adverse effects on children’s respiratory health. Several studies have shown that ambient air pollution is associated with deficits in lung function (1–6), increases in bronchitic symptoms (7–8) and development and/or exacerbation of asthma (9–13). These results demonstrated that long-term exposure to ambient air pollution significantly impairs children’s respiratory health, potentially leading to premature disability and mortality.(14–15)
Based on this evidence, there is an urgent need for improved approaches for early detection of these effects that can be used to develop the scientific evidence base to support effective public health, regulatory and clinical interventions to protect children. Studies have reported that exhaled nitric oxide (FeNO) is an indirect biomarker of eosinophilic airway inflammation (16–17) and could be useful for assessing the respiratory effects of short-term air pollution exposures, independent of asthma and allergy status.
It has been previously reported that short term levels of air pollution and traffic related exposures are associated with significant increases in FeNO levels.(16, 18–19) However, although exposures over longer periods have important additional effects(18), it has yet to be firmly established if FeNO is modulated by longer-term exposures to real world ambient air pollution mixtures. To assess the utility of FeNO in detecting the effects of such chronic exposures, we examined data from the southern California Children’s Health Study, an ongoing prospective population-based cohort study of environmental determinants of respiratory health (12, 20–21), to test the hypothesis that long-term temporal changes in ambient air pollutants (including NO2, PM2.5, PM10, and O3) are associated with changes in FeNO.
METHODS
Study design and subjects
Details on the design, site selection, subject recruitment and assessment of health effects are reported elsewhere (12). Briefly, a cohort of 5,093 children aged 5–7 years from 13 communities in Southern California was enrolled in 2002–2003 from kindergarten and first grade classrooms. At baseline and each subsequent year of follow-up, parents completed a written questionnaire. Informed assent was obtained from each child, and informed consent from a parent or guardian. The analyses in this paper are based on data from eight of the 13 communities where FeNO (50 ml/sec flow) was performed at schools following the ATS guidelines using an online technique (22–23) during Year 5 (2006–2007) and Year 6 (2007–2008) of the study on 2673 and 1541 children, respectively. A total of 1,475 children had FeNO measurements in both years. To minimize potential confounding by location and seasonal effects, each community was visited at least twice in different seasons during both study years. Details of the FeNO collection and quality control approaches have been reported earlier (24–25). We excluded data from 264 children because they used inhaled corticosteroids during the 12 months prior to FeNO testing (n=61) or they gave no information on their use of medication (n=203), in order to avoid potential confounding by medication use prior to FeNO testing. Therefore, the final data set included 1,211 children. The protocol was approved by the University of Southern California Institutional Review Board.
Air pollution data were obtained from central monitoring sites in each community. Twelve month averages of each air pollution concentration and temperature level before the test dates were obtained for both study years. In addition, hourly data on the day of the test, data on daily 24-hour averages of PM2.5, PM10, and NO2, temperature; and daily 10AM–6PM averages of O3 were obtained for the test date and each of the 60 days prior to FeNO testing. Interpolation of air pollution data were used as necessary to fill in data gaps missing days of exposure with modeled predictions using data from nearby monitors. While such processes could be potential sources of bias, previous sensitivity analyses on CHS data that limited use to complete data have not altered main study findings.(16)
Questionnaire information was obtained from parental reports on race/ethnicity, physician diagnosis of asthma, history of respiratory allergy (allergic rhinitis and/or hay fever), asthma medication use during the previous 12 months and exposure to secondhand tobacco smoke (SHS). Height and weight were measured on the days of test. Age- and sex-specific percentiles based on the Centers for Disease Control and Prevention body mass index (BMI) growth charts (http://www.cdc.gov/NCCDPHP/dnpa/growthcharts/resources/sas.htm) were used to determine normal, overweight and obesity status of each child during both study periods.
Statistical analysis
Descriptive analyses were conducted to examine the characteristics of the study population during Year 5, to characterize the distribution of the FeNO measurements during Year 5 as well as changes between the two years of study; and to assess the temporal trends of air pollution levels during both study periods. Descriptive analyses were also conducted to compare baseline demographic characteristics between subjects used in the data analysis and those that were excluded from the analysis due to incompleteness of information (e.g., not having FeNO measures in both Years 5 and 6 of the study).
Multiple linear regression was used to determine the relationship between changes in levels of FeNO (ΔFeNO) between the two study periods and corresponding changes in “long term” and “short term” air pollution levels. Effects of changes in long term pollution levels were based on the 12 month period prior to the day of FeNO test, while adjustments for short term levels were assessed using lags of up to 60 days prior to the day of FeNO test. The models assumed the general form provided in equation (1) below.
| (1) |
where ΔAge, ΔAPLT, ΔAPST and ΔTempST denote time elapsed between the two tests, changes in long term pollution levels and short term pollution and temperature levels, respectively. Note that our change-on-change modeling approach (26–28) enables us to investigate determinants of change in FeNO rather than determinants of level of FeNO since these have already been investigated in this cohort and in other studies. (16) The long term effects of air pollution in models also adjusted for short-term effects of air pollution with proper attention to the lag structure at each study period as well as potential confounders and effect modifiers. The potential confounders included age, sex, race/ethnicity, asthma, asthma medication use, history of respiratory allergy, hour and day (of the week) of FeNO collection, BMI percentiles, SHS, parental education (a proxy for socio-economic status), language of the questionnaire (English/Spanish), season, temperature, and baseline levels of FeNO. Seasonal effects were assessed by dividing the study period into “cold” and “warm” seasons. Here, the warm season included March 16 – June 30 while the cold season was defined as the period October 1 – March 15, based on Southern California climatic conditions. For time-independent covariates (ΔZ). e.g., race/ethnicity, effects were assessed in conjunction with time elapsed between the two child-specific yearly test dates (ΔAge). For time-varying covariates (ΔW)., effects were assessed by considering changes over time or considering all possible transitions, for continuous and categorical covariates, respectively.
After consideration of several types of lag-based models for short-term effects of pollution (see the online data supplement for details), linear distributed lag models were found to be the most appropriate. (29) Model selection was based on the Akaike Information Criterion (AIC) (30). Confounding by effects of ambient temperature was tested using the lag structure selected for the air pollutant effects.
After choosing the final models for each pollutant, potential effect modification by sex, asthma, respiratory allergy, baseline FeNO levels, and season was examined. Sensitivity analyses were conducted to assess robustness of findings to factors such as length of time elapsed between FeNO measurements or acculturation issues such as using the Spanish language questionnaire. To test the robustness of the findings and check for consistency with overall findings with prior publications, sensitivity analysis was conducted by fitting mixed effects equivalents of the change-on-change model. All models were fitted using the SAS Version 9.1 statistical package (SAS Institute, Cary, NC) and the R statistical software. Statistical significance was assessed assuming a 0.05 significance level and a two-sided alternative hypothesis.
RESULTS
The study population was well balanced between boys (47.1%) and girls (52.9%) with the majority reporting their ethnicity as Hispanic (56.2%) or non-Hispanic White (37.2%) (Table 1). Between Years 5 and 6, changes in FeNO levels in by ethnic categories were small with the exception of some decrease in African Americans. In fact, the differences between the ethnicity groups was not significant anymore (p=0.67) after combining the “African American”, “Asian” and “Other” groups. In Year 5, FeNO levels did not differ significantly by sex, ethnicity, BMI, parental education, exposure to SHS, and those completing Spanish language questionnaires. See Table E1 in the online data supplement for descriptive data on an extended list of variables. In Year 5, FeNO levels were higher in children with asthma or respiratory allergy as reported previously. (16, 25) Additionally, FeNO levels in Year 5 did not differ significantly by BMI, and those filling in Spanish language questionnaires (Table E1 in the online supplement). Figure E1 in the online data supplement provides the distribution of changes in FeNO levels between Years 5 and 6, showing substantial variability in ΔFeNO with the majority of subjects showing changes within 20 ppb. The levels of eNO in Year 5 were the lowest in those children that did not report physician diagnosis in both Years 5 and 6 (Table 2). Children who reported having allergy in both Years 5 and 6 also had significantly higher eNO levels in Year 5 compared to those without allergy (Table 2). Comparisons of socio demographic characteristics were made between the 1211 subjects that were included in the analysis and those excluded because they did not have FeNO measurements in either Year 5 or Year 6 of the study. As reported in Table E2 (see online supplement), the two groups were generally comparable by sex, age, asthma status, history of respiratory allergy and second hand tobacco smoke exposure. However, the excluded subjects were significantly more likely to fill out Spanish language questionnaires, be more obese and have parents with less than high school education.
Table 1.
Characteristics of Study Population and Comparisons of FeNO Levels
| Variable | Category | N (%) | GM of Year 5 FeNO (GSD)* | P-Value† | Mean of FeNO Change (S.D.) | P-Value† |
|---|---|---|---|---|---|---|
| Sex | Female | 640 (52.8) | 14.8 (15.9) | 0.10 | 1.3 (12.5) | 0.72 |
| Male | 571 (47.2) | 16.6 (18.7) | 1.0 (13.7) | |||
| Age (yrs) | <10 | 420 (34.7) | 13.7 (13.9) | <0.01 | 1.5 (11.4) | 0.37 |
| 10–11 | 611 (50.3) | 16.1 (18.4) | 0.7 (13.3) | |||
| >11 | 180 (14.9) | 18.5 (19.8) | 2.1 (15.8) | |||
| Race/Ethnicity | Non-Hispanic White | 451 (37.2) | 14.9 (14.9) | 0.07 | 0.7 (12.1) | 0.01 |
| Hispanic | 680 (56.2) | 15.5 (17.1) | 1.4 (12.8) | |||
| African American | 17 (1.4) | 25.7 (45.8) | −7.5 (27.9) | |||
| Asian | 54 (4.5) | 20.2 (20.6) | 3.6 (15.9) | |||
| Other | 9 (0.7) | 15.4 (16.5) | 8.2 (14.3) | |||
| Asthma | Yes | 136 (11.2) | 22.3 (24.9) | <0.01 | 0.01 (14.6) | 0.32 |
| No | 1075 (88.8) | 14.8 (15.8) | 1.3 (12.9) | |||
| History of Respiratory Allergy | Yes | 726 (59.9) | 17.3 (19.7) | <0.01 | 1.3 (14.5) | 0.78 |
| No | 485 (40.1) | 13.04 (12.4) | 1.1 (10.6) | |||
| Exposure to Second Hand Smoke | Yes | 42 (3.5) | 17.8 (18.2) | 0.25 | −4.3 (16.2) | 0.03 |
| No | 1163 (96.5) | 15.5 (17.2) | 1.3 (12.9) | |||
| Medication Use for Asthma | Yes | 91 (7.5) | 26.0 (31.1) | 0.0002 | −0.09 (21.7) | 0.55 |
| No | 1120 (92.5) | 14.8 (15.3) | 1.27 (12.1) | |||
| Community | Long Beach | 67 (5.53) | 14.6 (16.4) | 0.08 | 1.9 (10.4) | <0.01 |
| Mira Loma | 170 (14.04) | 13.9 (13.5) | 2.8 (10.3) | |||
| Riverside | 111 (9.17) | 16.5 (16.6) | −0.5 (11.1) | |||
| San Dimas | 144 (11.89) | 20.8 (26.5) | −2.9 (19.2) | |||
| Upland | 175 (14.45) | 14.8 (16.8) | 0.7 (13.6) | |||
| Glendora | 228 (18.83) | 14.4 (14.2) | 2.5 (11.4) | |||
| Anaheim | 115 (9.50) | 15.2 (13.7) | −0.6 (13.2) | |||
| Santa Barbara | 201 (16.60) | 15.6 (17.3) | 3.4 (12.0) |
Results based on geometric means (GM) and geometric standard deviations (GSD) due to skewness of Year 5 FeNO data.
p-values are based on test for comparisons of FeNO levels across categories.
Table 2.
Temporal Transitions in Selected Study Population characteristics and FeNO Levels
| Variable | Year5– Year6 Transition Categories | N (%) | GM of Yr5 FeNO (G.S.D.)* | P-Value | Mean of FeNO Change (S.D.) | P-Value |
|---|---|---|---|---|---|---|
| Season | Warm-Warm | 226 (18.7) | 15.9 (16.6) | 0.16 | 2.5 (11.7) | 0.33 |
| Cold-Warm | 368 (30.4) | 14.1 (15.9) | 1.1 (12.0) | |||
| Warm-Cold | 213 (17.6) | 15.6 (16.9) | 1.1 (12.9) | |||
| Cold-Cold | 404 (33.4) | 16.9 (18.8) | 0.5 (14.7) | |||
| Asthma | No Asthma-No Asthma | 1052 (86.9) | 14.5 (14.8) | <0.01 | 1.4 (12.4) | 0.07 |
| No Asthma-Asthma | 23 (1.9) | 27.7 (40.3) | −4.2 (27.6) | |||
| Asthma-Asthma | 136 (11.2) | 22.3 (25.0) | 0.01 (14.6) | |||
| Allergy | No Allergy-No Allergy | 441 (36.4) | 13.0 (12.7) | <0.01 | 0.8 (10.7) | 0.54 |
| No Allergy-Allergy | 44 (3.6) | 13.3 (8.6) | 3.1 (9.6) | |||
| Allergy-Allergy | 726 (59.9) | 17.3 (19.7) | 1.2 (14.5) | |||
| Second Hand Smoking | No SHS-No SHS | 1128 (93.15) | 15.4 (16.5) | 0.11 | 1.4 (12.5) | 0.04 |
| (SHS) | No SHS-SHS | 20 (1.65) | 24.6 (43.2) | −3.2 (28.6) | ||
| SHS-No SHS | 26 (2.15) | 15.7 (11.7) | −2.9 (6.8) | |||
| SHS-SHS | 13 (1.07) | 19.2 (27.4) | −5.5 (27.8) |
Results based on Geometric means (GM) and standard deviations (GSD) due to skewness of Year 5 FeNO data.
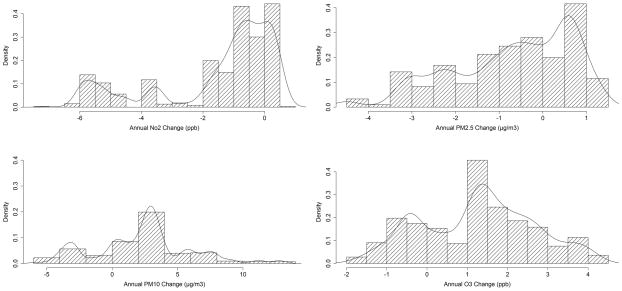
Figure 1 depicts density curves for long term changes in air pollution between Years 5 and 6. For NO2 and PM2.5, the annual averages were lower in year 6 than in year 5 for most children, while for PM10 and O3, the annual averages tended to be surprisingly higher in year 6 than in year 5 for most children. See Figure E2 and Figure E3 in the online data supplement for corresponding density curves for short term changes in air pollution and temperature between Years 5 and 6, respectively, over the selected lag structures for all four pollutants. Findings from models assessing effects of long term annual levels of air pollution on changes in FeNO are presented in Table 3. We found that changes in long-term levels of NO2, and PM2.5 were significantly associated with changes in FeNO. Increases in annual averages of 24-hr NO2 were significantly associated with 2.29 ppb (p = 0.02) higher levels of FeNO over the inter-quartile range (IQR) of 1.8 ppb in annual changes in NO2 concentration. Similarly, increases in annual averages of PM2.5 concentration were significantly associated with 4.94 ppb (p = 0.005) higher levels of FeNO over the IQR of 2.4 μg/m3 in annual changes in PM2.5 concentration. In contrast, changes in annual average levels of PM10 and O3 were not significantly associated with changes in FeNO. The models also adjusted for changes (or interactions with changes in age for time-independent covariates) in study community, race/ethnicity, sex, asthma, asthma medication use, history of respiratory allergy, age, day of FeNO collection, season and baseline levels of FeNO. Note that these long-term effects of air pollution were adjusted for analogous short term effects in both study years, based on linear distributed lag models. However, none of these short term effect terms were found to be significantly associated with changes in FeNO levels (data not shown). Based on our AIC criterion, the adjustments for short term air pollution effect were based on (3,4), (1,4), (1,4) and (1,5) day lags for the (year 5, year 6) combinations for NO2, PM2.5, PM10, and O3, respectively.
Figure 1.
Histograms and Density Curves of Changes in Annual Air Pollution Levels over the Study Period. The four panels depict distributions of changes in levels of NO2, PM2.5, PM10 and O3 between Years 5 and 6.
Table 3.
Effects of Changes in Long Term Air Pollution (annual average) Concentrations on Changes in FeNO Levels Adjusted for Changes in Short-term Concentrations
| Pollutant | Difference* (95% C.I.) |
|---|---|
| NO2 | 2.29 (0.36, 4.21)† |
| PM2.5 | 4.94 (1.44, 8.47)‡ |
| PM10 | 0.14 (−2.01, 2.28) |
| O3 | 0.74 (−4.47, 3.01) |
Results are scaled to the IQR values of 1.8 ppb (NO2), 2.4 μg/m3 (PM2.5), 3.4 μg/m3 (PM10), and 2.1 ppb (O3) of changes in annual average pollution levels between the two years of study. Estimates are adjusted for short-term effects of the same air pollutant, for Year 5 and Year 6 of the study. Models are also adjusted for changes (or interactions with changes in age for time-independent covariates) in community, race/ethnicity, sex, asthma, asthma medication use, history of respiratory allergy, age, day of FeNO collection, and season.
P<0.05
P<0.01
The effects of changes in long term air pollution did not significantly vary by temporal transitions in asthma status, respiratory allergy status, season and gender (Table 4). We found that long term changes in NO2 and PM2.5 had relatively larger effects in the Warm-Cold season transition between Years 5 and 6, but the overall differences in the season-transition specific effects were not statistically significant. In sensitivity analysis, the main findings in Table 3 were found to be robust to variability in the time elapsed between the two FeNO measurements or whether participants chose to fill out the Spanish language questionnaire (data not shown). In sensitivity analysis, mixed effects models analogues of the change-on-change models were fitted, focusing on main effects of air pollution, and the results were found to be similar to those reported in Table 3 (data not shown). Models that had group specific effects stratified by Year 5 asthma status or Year 5 levels of FeNO (categorized at the median level of 9.5 ppb) did not show significant heterogeneity by these factors (data not shown).
Table 4.
Subgroup Analysis on Effects of Changes in Long Term Air Pollution (annual average) Concentrations on Changes in FeNO Levels Adjusted for Changes in Short-term Concentrations*
| Modifying Factor (N) | NO2 | PM2.5 | PM10 | O3 |
|---|---|---|---|---|
| Asthma | ||||
| No Asthma-No Asthma (1052) | 2.2 (0.22,4.12) † | 5.3 (1.68,8.83) ‡ | 0.1 (−2.07,2.24) | −0.5 (−4.24,3.30) |
| No Asthma-Asthma (23) | 3.1 (−2.38,8.64) | 1.8 (−9.1,12.65) | −4.6 (−9.76,0.65) | −2.7 (−10.79,5.33) |
| Asthma-Asthma (136) | 2.8 (0.32,5.31) † | 3.5 (−1.272,8.28) | 0.6 (−2.14,3.264) | −2.31 (−7.31,2.71) |
| Interaction p-value | 0.75 | 0.55 | 0.14 | 0.53 |
|
| ||||
| Allergy | ||||
| No Allergy-No Allergy (441) | 2.3 (0.32,4.46) † | 5.0 (1.20,8.78) ‡ | 0.7 (−1.60,3.09) | −1.0 (−5.04,2.98) |
| No Allergy-Allergy (44) | 1.1 (−2.83,5.02) | 4.7 (−2.54,12.02) | 1.2 (−3.60,5.98) | 0.9 (−5.06,6.78) |
| Allergy-Allergy (726) | 2.2 (0.23,4.28) † | 4.9 (1.25,8.62) ‡ | −0.1 (−2.35,2.04) | −0.756 (−4.60,3.09) |
| Interaction p-value | 0.78 | 0.99 | 0.41 | 0.77 |
|
| ||||
| Season | ||||
| Warm-Warm (226) | 2.0 (−0.50,4.50) | 6.9 (1.90,11.86) ‡ | −0.2 (−2.79,2.38) | 1.1 (−4.03,6.22) |
| Cold-Warm (368) | 2.4 (−0.90,5.74) | 3.4 (−0.77,7.66) | 0.5 (−2.7,3.74) | −0.7 (−5.92,5.59) |
| Warm-Cold (213) | 3.1 (0.36,5.81) † | 11.8 (3.22,20.30) ‡ | 0.4 (−2.55,3.30) | 3.3 (−3.80,10.58) |
| Cold-Cold (404) | 2.8 (−1.15,6.71) | 4.5 (−0.48,9.41) | 0.6 (−3.03,4.15) | −0.6 (−5.59,4.41) |
| Interaction p-value | 0.82 | 0.08 | 0.90 | 0.63 |
|
| ||||
| Gender | ||||
| Boys (571) | 2.5 (0.41,4.67) † | 4.2 (0.46,7.94) ‡ | 0.1 (−2.14,2.28) | −1.6 (−5.52,2.40) |
| Girls (640) | 2.2 (0.22,4.14) † | 5.7 (1.94,9.41) ‡ | 0.2 (−2.07,2.45) | −0.1 (−3.99,3.72) |
| Interaction p-value | 0.59 | 0.24 | 0.87 | 0.22 |
Results are scaled to the IQR values of 1.8 ppb (NO2), 2.4 μg/m3 (PM2.5), 3.4 μg/m3 (PM10), and 2.1 ppb (O3) of changes in annual average pollution levels between the two years of study. Estimates are adjusted for short-term effects of the same air pollutant. Models are also adjusted, as appropriate, for changes (or interactions with changes in age for time-independent covariates) in community, race/ethnicity, sex, asthma, asthma medication use, history of respiratory allergy, age, day of FeNO collection and season.
P<0.05
P<0.01
DISCUSSION
We have shown that changes in long-term averages of NO2 and PM2.5 are associated with significant longitudinal changes in FeNO in children, independent of asthma and allergy status. These findings were observed in models that adjusted for short term effects of air pollution that we have shown previously to be important in cross-sectional analysis of data from the Children’s Health Study.(16)
A large body of evidence from epidemiological studies indicates that ambient air pollutants have acute effects (i.e., for exposures over few hours to few weeks) on FeNO (16, 31–38); however, the impact of longer-term trend (months to years) in air pollution on FeNO has not been extensively investigated (17). In a panel study conducted among medical residents during the 2008 Beijing Olympics, levels of FeNO and other oxidative markers in airways (e.g., exhaled breath condensate 8-hydroxy-2-deoxyguanosine, nitrate+ nitrite, and hydrogen ion concentrations) were significantly decreased during the month of Olympic Games due to strict pollution control measures (e.g., mean NO2 level decreased from 25.6ppb to 14.61ppb) compared to the pre-Olympic measurements. There was dramatic increase in pollution levels during the post-Olympic period (e.g., mean post-Olympic NO2 level was 41.39ppb) that resulted in significant increase in these inflammatory biomarkers(17) While this study shows that difference in pollution levels over months could influence FeNO levels in young adults, our findings demonstrate that even longer-term (i.e., annual) changes in PM2.5 and NO2 affect FeNO levels in children. Because earlier studies have documented that FeNO levels predict asthma risk in children (39–41), one could hypothesize that sustained or increasing levels of air pollution may further influence the risk in non-asthmatic children with higher baseline FeNO levels. Future studies are warranted to test this hypothesis.
The long-term effects were robust to adjustment for short-term exposures suggesting that the previously reported associations reflected a mix of both short-term and their correlated long-term exposures. A longer longitudinal follow-up of this cohort may provide further clarification about the inter-relationship of short and long-term effects on FeNO and how they related to adverse health outcomes. It would also be of interest to interpret findings from longer follow-up data in light of air pollution levels that have been declining over the past several years in Southern California.
We found little evidence for significant influences of a number of potential susceptibility factors (i.e., asthma and allergy, sociodemographic factors) on the associations between changes in long-term ambient air pollutant concentrations and changes in FeNO suggesting that both healthy children and those with allergic airways disease are susceptible. Thus, changes in FeNO may be detecting chronic effects mediated by non-allergic processes. We have also reported that chronic effects of air pollutants on lung development did not differ among children with and without asthma (1). Taken together, these results support the hypothesis that, in contrast to the corresponding acute effects, chronic effects of air pollution do not depend on the presence of allergic airway disease. However, our findings should be interpreted with caution since the sample sizes were small for the categories with prevalent asthma cases and those with new physician diagnosis of asthma over the one year follow-up period.
This study has several strengths, including prospective longitudinal evaluation of effects of temporal variations in air pollution on changes in FeNO using a large, ethnically diverse population of children, substantial range in exposures to the spectrum of complex multi-pollutant mixtures available in Southern California representing the full national range in the USA, determination of independent effects of short and long term variations in air pollutants, and testing whether the associations varied by patterns in susceptibility factors (e.g., asthma and allergy) and season.
There are some concerns in the scientific community regarding use of FeNO as a marker of airway inflammation (42–43). However, our data along with a large body of evidence from earlier work indicate that FeNO is influenced significantly by ambient air pollution and is correlated with atopic conditions (asthma, allergy, sputum or blood eosinophil, and airway hyperresponsiveness). Therefore, it can be argued that FeNO reflects air pollution responsive domains of airway inflammation and more research is needed to understand its correlates.
Our results should be interpreted in light of some limitations. There is potential for misclassification of exposure assignments because we do not have information on time-activity patterns for the study subjects for the duration of the evaluated lags. However, the regional nature of NO2, PM and O3 reduces any potential misclassification. Further research should incorporate time-activity pattern to improve exposure assignment.
Our air pollution measures were obtained across both study years from central site monitors placed in each study community. To minimize potential confounding by location and seasonal effects, each community was visited at least twice in different seasons during both study years. However, there is still potential for residual spatio-temporal confounding since budgetary/logistical limitations did not allow measurement of FeNO levels across all space-time grids. To the extent possible, our models included seasonal and other temporal factors as adjustment factors and models with temporal season-specific patterns were considered. Missing pollution data were imputed by model predictions employing interpolation of data from nearby monitors, and are potential sources of bias. However, the main findings of this study focused on effects of changes in pollution levels over 12 month averaging periods and are unlikely to be affected by this data replacement.
Our ability to thoroughly assess the effects on FeNO of medication use for asthma or allergy is limited. We used parent-reported questionnaire data on history of respiratory allergy, physician diagnosis of asthma, and medication use for asthma to assess their effects in confounding and/or modifying the association between longitudinal changes in air pollution and FeNO. Our findings are internally consistent showing higher FeNO among children with asthma or respiratory allergy for each of the two study years. Recently reported results suggest that FeNO might be associated with wheezing phenotypes in atopic, but not in non-atopic children (44). However, the lack of IgE data in the CHS cohort prevents us from assessing this phenomenon in our multi-ethnic study population.
This study was made possible by the ability to measure fractional concentration of exhaled nitric oxide (FeNO), a validated measure of important aspects of airway inflammation (22–23), in large population-based studies. Measurement of FeNO allows the assessment of biomarkers of inflammation to detect early chronic effect of air pollution exposures and to identify populations that may be at increased risk for adverse health outcomes (40, 45). Thus, assessment of FeNO may contribute to innovative approaches to optimize interventions and regulatory policies designed to protect children.
To conclude, our data showed that changes in longer-term exposures to current levels of ambient air pollutants are significantly associated with longitudinal changes in FeNO levels in children independent of short-term levels and asthma status. Based on our prior findings that elevated FeNO is associated with increased risk of new onset asthma (39), a finding supported by a recent study that found FeNO could predict wheeze onset in adults (40), the effects of long-term exposure to air pollution on longitudinal FeNO suggest that airway inflammation as measured by FeNO may mediate the effects of air pollution on asthma pathogenesis. Further research is warranted to examine effects of air pollution on FeNO with longer follow-up and also to examine whether FeNO could predict asthma/wheeze incidence in children longitudinally exposed to different levels of ambient air pollution. Because FeNO measurement is an indirect biomarker of eosinophilic airway inflammation that can be measured non-invasively, use of this biomarker in population-based epidemiologic research has great potential as a useful intermediate marker to detect susceptible children who are at high risk of adverse respiratory health outcomes from air pollution, and for understanding the effects of long-term air pollution exposures on children’s respiratory health.
Supplementary Material
What this paper adds.
Exhaled nitric oxide (FeNO) is an air-pollution-responsive biomarker of aspects of airway inflammation that is associated with asthma.
The effects of temporal changes in long-term exposure to ambient air pollution on airway inflammation as measured by FeNO have yet to be established.
Long-term exposures to PM2.5 and NO2 are associated with increases in FeNO level in children.
FeNO is a useful indirect biomarker of eosinophilic airway inflammation for understanding the effects of long-term air pollution exposures on children’s respiratory health.
Acknowledgments
We are indebted to the school principals, teachers, students and parents in each of the 13 study communities for their cooperation and especially to the members of the health testing field team for their efforts. This work was supported by the National Heart, Lung and Blood Institute (grants 5R01HL61768 and 5R01HL76647); the Southern California Environmental Health Sciences Center (grant 5P30ES007048) funded by the National Institute of Environmental Health Sciences; the Children’s Environmental Health Center (grants 5P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grant 5P01ES011627); and the Hastings Foundation.
Footnotes
Declaration of competing interests: None.
Contributorship: All authors have contributed to this work and fulfill the criteria of authorship.
Licence statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Occupational and Environmental Medicine and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms) and the Corresponding Author accepts and understands that any supply made under these terms is made by BMJPGL to the Corresponding Author.
References
- 1.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 2.Gauderman WJ, Vora H, McConnell R, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–7. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 3.Neas LM, Dockery DW, Koutrakis P, et al. The association of ambient air pollution with twice daily peak expiratory flow rate measurements in children. Am J Epidemiol. 1995;141(2):111–22. doi: 10.1093/oxfordjournals.aje.a117399. [DOI] [PubMed] [Google Scholar]
- 4.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176(4):377–84. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 5.Braun-Fahrlander C, Ackermann-Liebrich U, Schwartz J, et al. Air pollution and respiratory symptoms in preschool children. Am Rev Respir Dis. 1992;145(1):42–7. doi: 10.1164/ajrccm/145.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Barraza-Villarreal A, Escamilla-Nunez MC, Hernandez-Cadena L, et al. Elemental carbon exposure and lung function in school children from Mexico City. Eur Respir J. 2011;38(3):548–52. doi: 10.1183/09031936.00111410. [DOI] [PubMed] [Google Scholar]
- 7.McConnell R, Berhane K, Gilliland F, et al. Air pollution and bronchitic symptoms in Southern California children with asthma. Environ Health Perspect. 1999;107(9):757–60. doi: 10.1289/ehp.99107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WC, Hartley WR, Myers L, et al. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ Res. 2007;104(3):402–9. doi: 10.1016/j.envres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.McConnell R, Berhane K, Gilliland FD, et al. Sports and asthma in children exposed to ozone. Am J Respir Crit Care Med. 2001;163(5):A174. [Google Scholar]
- 10.McConnell R, Berhane K, Gilliland FD, et al. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am J Respir Crit Care Med. 2003;168(7):790–7. doi: 10.1164/rccm.200304-466OC. [DOI] [PubMed] [Google Scholar]
- 11.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: A cohort study. Lancet. 2002;359(9304):386–91. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 12.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766–72. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig JQ, Mar TF, Allen RW, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113(4):499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roemer W, Hoek G, Brunekreef B. Effect of ambient winter air pollution on respiratory health of children with chronic respiratory symptoms. Am Rev Respir Dis. 1993;147(1):118–24. doi: 10.1164/ajrccm/147.1.118. [DOI] [PubMed] [Google Scholar]
- 15.Holguin F, Flores S, Ross Z, et al. Traffic-related exposures, airway function, inflammation, and respiratory symptoms in children. Am J Respir Crit Care Med. 2007;176(12):1236–42. doi: 10.1164/rccm.200611-1616OC. [DOI] [PubMed] [Google Scholar]
- 16.Berhane K, Zhang Y, Linn WS, et al. The effect of ambient air pollution on exhaled nitric oxide in the Children’s Health Study. Eur Respir J. 2011;37(5):1029–36. doi: 10.1183/09031936.00081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Wang G, Lu S-E, et al. Inflammatory and Oxidative Stress Responses of Healthy Young Adults to Changes in Air Quality during the Beijing Olympics. American Journal of Respiratory and Critical Care Medicine. 2012;186(11):1150–9. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckel SP, Berhane K, Salam MT, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the children’s health study. Environ Health Perspect. 2011;119(10):1472–7. doi: 10.1289/ehp.1103516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Grutta S, Ferrante G, Malizia V, et al. Environmental effects on fractional exhaled nitric oxide in allergic children. J Allergy. 2012;2012:916926. doi: 10.1155/2012/916926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159(3):760–7. doi: 10.1164/ajrccm.159.3.9804143. [DOI] [PubMed] [Google Scholar]
- 21.Peters JM, Avol E, Gauderman WJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–75. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 22.Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 1999;160(6):2104–17. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 23.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 24.Linn WS, Berhane KT, Rappaport EB, et al. Relationships of online exhaled, offline exhaled, and ambient nitric oxide in an epidemiologic survey of schoolchildren. J Expo Sci Environ Epidemiol. 2009;19(7):674–81. doi: 10.1038/jes.2008.64. [DOI] [PubMed] [Google Scholar]
- 25.Linn WS, Rappaport EB, Berhane KT, et al. Exhaled nitric oxide in a population-based study of southern California schoolchildren. Respir Res. 2009;10:28. doi: 10.1186/1465-9921-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel SP, Louis TA, Chaves P, et al. Modification by frailty status of the association between ambient air pollution and lung function in older adults in the Cardiovascular Health Study. American Journal of Epidemiology. 2012 doi: 10.1093/aje/kws001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DC. Statistical methods in environmental epidemiology. Oxford University Press; USA: 2009. [Google Scholar]
- 28.Downs SH, Schindler C, Liu LJ, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–47. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 29.Berhane K, Thomas DC. A two-stage model for multiple time series data of counts. Biostatistics. 2002;3(1):21–32. doi: 10.1093/biostatistics/3.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Akaike H. In: Petrov BN, Csaki F, editors. Information theory and an extension of the maximum likelihood principle; 2nd International Symposium on Information Theory; Budapest: Akademia Kiado; 1973. pp. 267–81. [Google Scholar]
- 31.Sarnat SE, Raysoni AU, Li WW, et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environ Health Perspect. 2012;120(3):437–44. doi: 10.1289/ehp.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuurbier M, Hoek G, Oldenwening M, et al. Respiratory effects of commuters’ exposure to air pollution in traffic. Epidemiology. 2011;22(2):219–27. doi: 10.1097/EDE.0b013e3182093693. [DOI] [PubMed] [Google Scholar]
- 33.Graveland H, Van Roosbroeck SA, Rensen WM, et al. Air pollution and exhaled nitric oxide in Dutch schoolchildren. Occup Environ Med. 2011;68(8):551–6. doi: 10.1136/oem.2010.056812. [DOI] [PubMed] [Google Scholar]
- 34.Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, et al. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008;116(6):832–8. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickmilder M, Carbonnelle S, de Burbure C, et al. Relationship between ambient ozone and exhaled nitric oxide in children. JAMA. 2003;290(19):2546–7. doi: 10.1001/jama.290.19.2546-b. [DOI] [PubMed] [Google Scholar]
- 36.Fischer PH, Steerenberg PA, Snelder JD, et al. Association between exhaled nitric oxide, ambient air pollution and respiratory health in school children. Int Arch Occup Environ Health. 2002;75(5):348–53. doi: 10.1007/s00420-002-0320-x. [DOI] [PubMed] [Google Scholar]
- 37.Van Amsterdam JG, Verlaan BP, Van Loveren H, et al. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch Environ Health. 1999;54(5):331–5. doi: 10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- 38.Steerenberg PA, Snelder JB, Fischer PH, et al. Increased exhaled nitric oxide on days with high outdoor air pollution is of endogenous origin. Eur Respir J. 1999;13(2):334–7. doi: 10.1034/j.1399-3003.1999.13b19.x. [DOI] [PubMed] [Google Scholar]
- 39.Bastain TM, Islam T, Berhane KT, et al. Exhaled nitric oxide, susceptibility and new-onset asthma in the Children’s Health Study. Eur Respir J. 2011;37(3):523–31. doi: 10.1183/09031936.00021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olin AC, Rosengren A, Thelle DS, et al. Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population. Am J Respir Crit Care Med. 2010;181(4):324–7. doi: 10.1164/rccm.200907-1079OC. [DOI] [PubMed] [Google Scholar]
- 41.Singer F, Luchsinger I, Inci D, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy. 2013 doi: 10.1111/all.12127. [DOI] [PubMed] [Google Scholar]
- 42.Taylor D, Pijnenburg M, Smith A, et al. Exhaled nitric oxide measurements: Clinical application and interpretation. Thorax. 2006;61:817–27. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming L, Wilson N, Bush A. No the evidence: what have measurements of exhaled nitric oxide got to offer? J Pediatr. 2006;149(2):156–8. doi: 10.1016/j.jpeds.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 44.van der Valk RJP, Caudri D, Savenije O, et al. Childhood wheezing phenotypes and FeNO in atopic children at age 8. Clinical & Experimental Allergy. 2012;42(9):1329–36. doi: 10.1111/j.1365-2222.2012.04010.x. [DOI] [PubMed] [Google Scholar]
- 45.Kharitonov S, Barnes P. Exhaled biomarkers. Chest. 2006;130:1541–6. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.