Summary
Otitis media (OM) is a public health problem in both developed and developing countries. It is the leading cause of hearing loss and represents a significant healthcare burden. In some cases, acute OM progresses to chronic suppurative OM (CSOM), characterized by effusion and discharge, despite antimicrobial therapy. The emergence of antibiotic resistance and potential ototoxicity of antibiotics has created an urgent need to design non-conventional therapeutic strategies against OM based on modern insights into its pathophysiology. In this article, we review the role of innate immunity as it pertains to OM and discuss recent advances in understanding the role of innate immune cells in protecting the middle ear. We also discuss the mechanisms utilized by pathogens to subvert innate immunity and thereby overcome defensive responses. A better knowledge about bacterial virulence and host resistance promises to reveal novel targets to design effective treatment strategies against OM. The identification and characterization of small natural compounds that can boost innate immunity may provide new avenues for the treatment of OM. There is also a need to design novel methods for targeted delivery of these compounds into the middle ear, allowing higher therapeutic doses and minimizing systemic side effects.
Keywords: Otitis media, Innate immunity, Mucin, Epithelial cells
Introduction
Otitis media (OM) is one of the most frequent diseases afflicting humans and is prevalent in both developed and developing countries.1 It represents a significant healthcare burden, with over 5 billion dollars spent every year in the world on this disease.2 The term ‘otitis media’ covers a wide spectrum of disease, and is used to describe illnesses with predominantly middle ear symptoms. With its diverse clinical syndromes and affected host groups, OM remains one of the challenging diseases encountered in clinical practice.3 It is the leading cause of hearing loss and is associated with significant morbidity.4–7 Children are at greater risk and suffer most frequently from OM. This can cause serious deterioration in the quality of life.8 Studies show that 80% of children will have experienced at least one episode of OM by their third birthday and 40% will have six or more recurrences by the age of 7 years.9 OM is also the predominant reason for antibiotic prescription.10 It is the primary indication for tympanostomy tube insertion, which is the most commonly performed operation on children.11
The pathogenesis of OM is thought to be multifactorial and includes Eustachian tube dysfunction, allergy, viral and bacterial invasion, reduced ciliary function of both the middle ear and Eustachian tube mucosa, smoke exposure, gastro-esophageal reflux, and autoimmune and many other etiologies not yet fully understood.12 OM can lead to life-threatening extracranial and intracranial complications.13 Every year 28 000 deaths are attributable to OM complications, mainly through meningitis and brain abscess.14,15
There are two main entities of OM: acute otitis media (AOM) and chronic suppurative otitis media (CSOM).16 AOM is defined as the presence of inflammation in the middle ear accompanied by the rapid onset of signs and symptoms of an ear infection. Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the most common causative agents of AOM.
Despite antibiotic therapy, AOM can progress to CSOM, characterized by the persistent infection and inflammation of the middle ear and mastoid air cells. This condition typically involves a perforation of the tympanic membrane, with intermittent or continuous otorrhea.17 As chronic otomastoiditis and Eustachian tube dysfunction persist, the tympanic membrane is weakened, which increases the likelihood of an atelectatic ear or cholesteatoma formation. The presence of mucin prevents the transmission of sound waves from the middle ear to the inner ear, leading to conductive hearing loss. Pseudomonas aeruginosa and Staphylococcus aureus are the most common pathogens implicated in CSOM.18–21
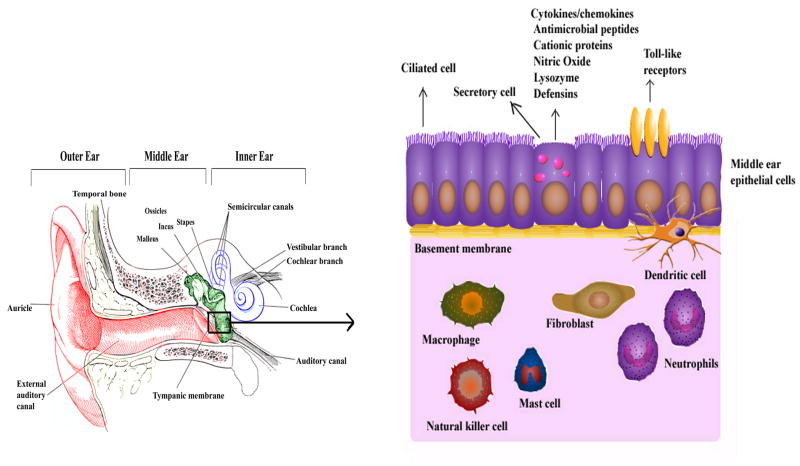
Host resistance against pathogens depends on a complex interplay of innate and adaptive immune mechanisms. The innate immune system provides a first-line, non-specific defense mechanism, in contrast to the adaptive immune process, which is pathogen-specific and requires sensitization. Innate immunity also stimulates and modulates adaptive immune responses. The present review is focused on the role of the innate immune system in OM, specifically the role of middle ear epithelial cells, neutrophils, macrophages, fibroblasts, mast cells, and natural killer cells in protecting the middle ear against pathogens (Figure 1). The innate immune system detects microbial infection and uses pattern recognition receptors (PRRs) to recognize the molecular signature of pathogens, known as pathogen-associated molecular patterns (PAMPs).22 PRRs include toll-like receptors (TLRs), cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene (RIG-I), and C-type lectin receptors (CLRs). The role of these PRRs in OM is also discussed.
Figure 1.
Middle ear innate immunity. The middle ear is lined by epithelial cells, which can provide protection by secreting antimicrobial molecules, or through toll-like receptors (TLRs). The middle ear also possesses innate immune cells such as neutrophils, macrophages, dendritic cells, mast cells, and natural killer cells providing defense against intruding pathogens.
Epithelial cells
The epithelial lining of the middle ear contains several key defense mechanisms including the presence of the mucociliary apparatus, trapping function of mucous glycoproteins and surfactants, ability to secrete innate defense molecules such as defensins, interferons, lactoferrin, and nitric oxide, and antibody production through the adaptive immune response. Middle ear epithelial cells also express PRRs like TLRs, which help in sensing pathogens through PAMPs.22
Defensins
The middle ear epithelial cells primarily release beta defensins, which are cationic proteins with antimicrobial function against a wide range of viruses, bacteria, fungi, and protozoa.23 Their major antimicrobial mechanism is thought to be through the formation of a pore into the microbial membrane. However, some defensins are known to stimulate pro-inflammatory cytokines/chemokines, to act as chemoattractants for neutrophils, mast cells, T cells, and dendritic cells, and to directly inhibit bacterial toxins.23 The up-regulated expression of mouse β-defensins 2, 3, and 4 has been demonstrated in the tubotympanums in experimental OM, while no such up-regulation was seen in the middle ears of healthy controls.24 Human β-defensin 2 (HBD2) is seen to be up-regulated in the middle ear response to bacteria and cytokines like non-typeable Haemophilus influenzae (NTHi) and interleukin (IL)-1α.25,26 HBD2 expression occurs in response to NTHi binding to toll-like receptor 2 (TLR2) and subsequent activation of a toll/IL-1 receptor MyD88-IRAK1-TRAF6-MKK3/6-p38 MAP kinase signal transduction pathway.25,26 Beta-defensin 2 production has been demonstrated to be greatest when the p38 MAP kinase pathway acts synergistically with the MyD88-independent Raf-MEK1/2-ERK MAP kinase pathway stimulated by IL-1α.25,26 In addition, the β-defensin 2 production in epithelial cells is up-regulated with exposure to tumor necrosis factor alpha (TNF-α) and lipopolysaccharide (LPS).27 The recombinant human β-defensin 3 (rhBD-3) plays a critical role in eliminating NTHi, and its function has been shown to be inhibited by biofilms.28
SPLUNC1
Another important molecule that has been demonstrated to play a role in OM is SPLUNC1. It is a predominant component of the surface liquid that covers the mucosal surfaces of the human respiratory tract. SPLUNC1 has broad-spectrum antimicrobial activity and reduces biofilm formation by P. aeruginosa, supporting the assertion that this molecule functions in host defense.29–31 SPLUNC1 can also act as a chemoattractant that recruits macrophages and neutrophils to the site of infection.32 It functions as a surfactant to reduce surface tension at an air–liquid interface in the upper airway, including the Eustachian tube, and acts as a liquid-volume sensor to regulate membrane ion channels. Although the loss of SPLUNC1 has been shown not to impact the survival of NTHi, it has been found to be essential in the maintenance of middle ear fluid pressure and efficient mucociliary clearance. This finding has also been demonstrated in a chinchilla model that lacks the expression of SPLUNC1 ortholog.33
Other antimicrobial molecules
Several other antimicrobial molecules from epithelial cell secretions in OM have been investigated. The administration of apolactoferrin, the iron-free form of lactoferrin, was shown to significantly reduce bacterial counts and the number of inflammatory cells in a chinchilla model of S. pneumoniae-induced OM.34 It has also been shown that mammalian chitinase and chitotriosidase are overexpressed in adenoidal histiocytes and vascular endothelial cells in OM.35 This suggests that dysregulation of chitin-containing pathogens may play a role in OM.
Mucin
Mucins are high molecular weight glycoproteins (2–30 MDa) that are implicated in a variety of pathological conditions.36,37 Variation in the quantity and character of middle ear secretions, and specifically mucin secretion, is known to be important in the pathophysiological mechanisms of OM. Mucins are the only component of middle ear effusions responsible for its viscous properties, and an over-production of these viscous mucins can prevent normal mucociliary clearance.
The persistent accumulation of mucin in the middle ear cavity is likely to involve extensive proliferation of mucous cells and associated mucosal metaplasia. Mice middle ear infected with NTHi resulted in mucosal metaplasia and overexpression of Cxcl2, a major inflammatory cell recruiter, thus delaying resolution of infection.38 P. aeruginosa exotoxin A promotes metaplasia, necrosis, and apoptosis of epithelial cells of the middle ear, thus exposing the submucosal and lamina propria layers, perhaps leading to inner ear infection and damage.39 Molecular analyses have shown that TNF-α and retinoic acid act synergistically with Math1, a transcription factor essential for differentiation of epithelial cells into mucosal cells, to transform mouse middle ear epithelial cells into metaplastic mucous-like cells.40
The overproduction of mucin by epithelial cells leads to CSOM. In humans, 19 mucin genes have been identified. Among these, MUC2, MUC5AC, and MUC5B have been shown to play an important role in the pathogenesis of OM.41–43 Abnormally high levels of mucin proteins have been demonstrated in middle ear effusions of CSOM patients.44 This presence of mucin prevents the transmission of sound waves from the middle ear to the inner ear, hence leading to conductive hearing loss. However, the molecular mechanisms underlying mucin overproduction by human middle ear epithelial cells (HMEECs) during CSOM are still unknown. We have observed, using scanning electron microscopy, that P. aeruginosa binds and adheres to HMEECs (Figure 2).45 Transmission electron microscopy demonstrated P. aeruginosa internalization inside HMEECs enclosed in membrane-bound vacuoles (Figure 3).45 However, how this adhesion and subsequent internalization leads to mucin up-regulation needs to be explored in future studies.
Figure 2.
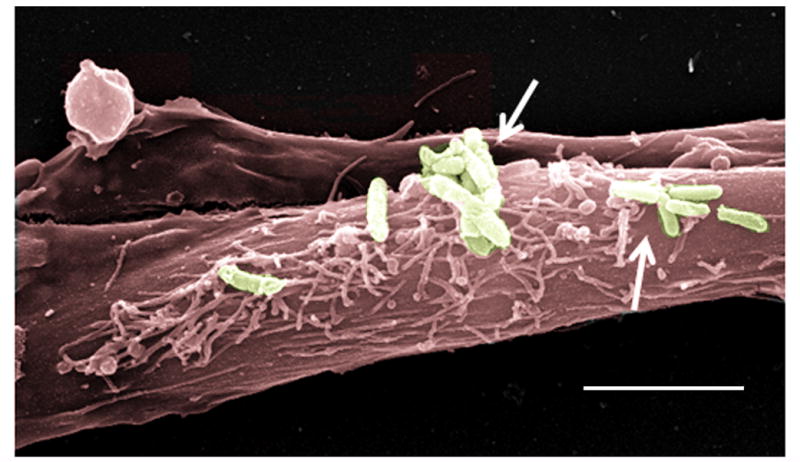
Pseudomonas aeruginosa binds to human middle ear epithelial cells (HMEECs). Scanning electron micrograph showing adhesion of P. aeruginosa to HMEECs. The micrograph was pseudo-colored using Adobe photoshop software. Arrows indicate bacteria. Scale bar 5 μm.
Figure 3.
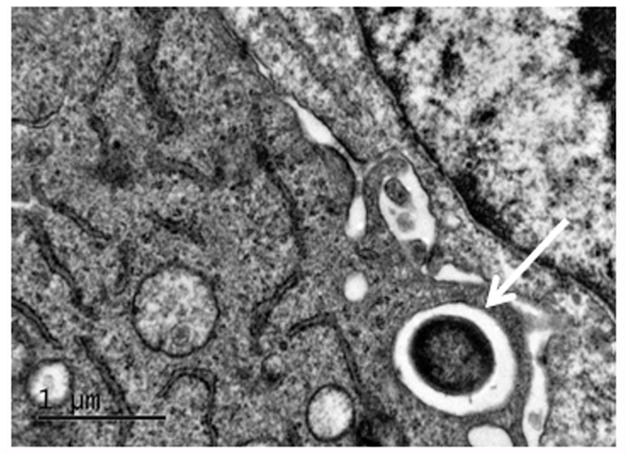
Pseudomonas aeruginosa invades human middle ear epithelial cells (HMEECs). Transmission electron micrograph demonstrating internalization of P. aeruginosa inside HMEECs. Arrow indicates bacteria. Scale bar 1 μm.
Lysozyme
Various cells of the human airway, including surface epithelial cells, have been shown to produce lysozyme, an antimicrobial innate immune molecule that degrades the peptidoglycan of the bacterial cell wall.46 It is particularly found in the tubotympanum of mammals and plays a significant role in OM.47 Lysozyme and HBD2 have synergistic killing effects against S. pneumoniae.48 Lysozyme M knock-out mice are more susceptible to S. pneumoniae infection, thus suggesting the role of lysozyme in host defense.49
Toll-like receptors (TLRs)
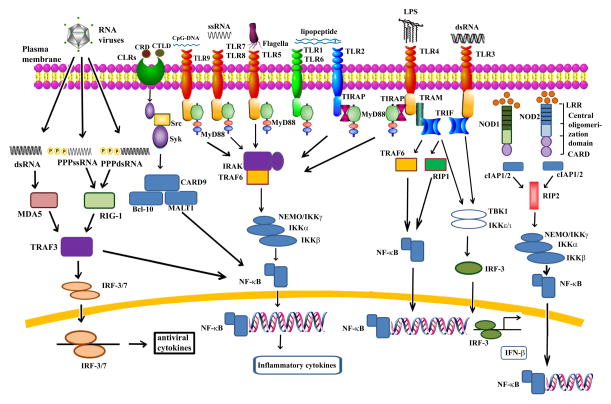
There are at least 10 different TLRs found on the surface of epithelial cells of the human middle ear. TLRs contain an N-terminal extracellular leucine-rich repeat domain and a cytoplasmic toll/IL-1 receptor domain. TLR molecules provide protection against infection by recognizing intruding pathogens through their invariant PAMPS and then mobilizing appropriate immune defenses.50,51 The activation of most TLRs results in downstream activation of the MAPK or the nuclear factor kappa B (NF-κB)-dependent cell signaling cascades, thus leading to further activation of immune responses (Figure 4).52
Figure 4.
Toll-like receptor (TLR), nucleotide-binding oligomerization domain (NOD)-like receptor (NLR), and retinoic acid-inducible gene (RIG-I)-like receptor (RLR) signaling. TLR1 or TLR2 or TLR6 recognizes lipoproteins, TLR3 recognizes dsRNA, TLR4 recognizes bacterial lipopolysaccharide (LPS), TLR5 recognizes bacterial flagellin, TLR7/8 mediates recognition of ssRNA. TLR9 acts as a DNA sensor and recognizes CpG DNA of bacteria and viruses. The NLR proteins NOD1 and NOD2 are cytosolic pattern recognition receptors (PRRs). NOD1 senses intracellular meso-diaminopimelic acid (DAP), whereas NOD2 recognizes muramyl dipeptide (MDP). C-type lectin receptor (CLR) signaling senses carbohydrate recognition domains (CRDs), or structurally similar C-type lectin-like domains (CTLDs). The RLR pathway is involved in the recognition of viral dsRNA. TLR, NLR, CLR, and RLR signaling causes activation of NF-κB, leading to the production of proinflammatory cytokines and the stimulation of immune responses.
In OM, NTHi is associated with several molecular patterns that function as TLR ligands. NTHi cell surface peptidoglycans and the associated proteins such as outer membrane protein (OMP) P6 serves as a ligand for TLR2.53 Another notable example is lipooligosaccharide (LOS), which serves as a ligand for TLR2 and TLR4.54 Not surprisingly, polymorphisms in the gene encoding for TLR4 have been associated with recurrent acute OM.55 When infected with NTHi, TLR4 knock-out mice had a worse mucosal immune response as compared to wild-type mice.56 These TLR4 knock-out mice had inferior immune responses with regards to mucosal IgA, systemic IgG, and Th1 cells.56 NTHi and its various immunogenic molecules have been shown not only to directly activate TLR, but to up-regulate TLR2 gene expression in middle ear epithelial cell lines.57 Patients with chronic middle ear disease like CSOM have been shown to exhibit lower mRNA levels of TLR4, TLR5, and TLR7 than a control group.58 A recent report has further confirmed these findings, demonstrating lower mRNA and protein levels of TLR2, TLR4, and TLR5 in middle ear mucosa of CSOM patients.59 The down-regulation of TLR expression during OM can lead to inefficient host defense in the middle ear. This can cause repeated infections and persistent inflammations, eventually leading to recurrent, persistent suppurative chronic middle ear diseases.
DNA sensors
Microbial and other forms of non-self DNA can also serve as PAMPs.60 DNA sensors like TLR9 recognize unmethylated cytidine–phosphate–guanosine (CpG) motifs that are common in bacterial, but not mammalian, DNA.61 TLR9 access to bacterial DNA is further enhanced by the nuclear high-mobility group box 1 protein (HMGB1).62 Like other TLRs, TLR9 utilizes the TLR adaptor molecule MyD88, which leads to the production of proinflammatory cytokines via the activation of NF-κB (Figure 4).61 Besides TLR9, there are six intracellular receptors that have also been implicated in cytosolic DNA sensing in the innate immune response. These include DNA-dependent activator of interferon (IFN) regulatory factors (DAI) (also called Z-DNA binding protein 1, ZBP1), absent in melanoma 2 (AIM2), RNA polymerase III (Pol III), leucine-rich repeat (in Flightless I) interacting protein-1 (Lrrfip1), DExD/H box helicases (DHX9 and DHX36), and most recently, the IFN inducible protein IFI16.63–70 These DNA sensors play a crucial role in the pathogenesis of OM. OM-prone children show lower TLR9 mRNA levels as compared to the non-prone group.71 Decreased TLR9 mRNA expression has also been demonstrated in patients with OM with effusion (OME) with confirmed bacteria in the middle ear like S. aureus, S. pneumoniae, Bacillus spp, and NTHi.72 TLR9 knock-out mice (TLR9−/−) demonstrated prolonged and enhanced morphological signs of mucosal hyperplasia and inflammation in the middle ear, as well as a delay in bacterial clearance and OM recovery compared to wild-type animals in a murine model of OM induced by NTHi.73 There was modest up-regulation of TLR9 signaling genes in this OM mouse model, which corroborated with the protein levels in both middle ear mucosal cells and infiltrating leukocytes. However, there was dramatic up-regulation in genes known to be regulated by CpG DNA, as well as those involved in DNA sensing by DAI, Pol-III, and AIM2.73 These results suggest that bacterial DNA sensing by the innate immune system plays an important role in OM resolution and recovery. However, further studies are warranted to explore the role of bacterial DNA in the pathogenesis and resolution of OM. The availability of gene deletion models for other DNA receptors will help in characterizing the role of DNA sensors in OM.
NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs)
When pathogens bypass the membrane-associated PRRs like TLRs, they encounter cytoplasmic PRRs such as caspase activation and recruitment domain (CARD) helicases like RLRs and NLRs.74,75 A wide variety of cells such as macrophages and dendritic cells, as well as middle ear epithelial cells, express these PRRs.76 RLRs are expressed ubiquitously at low levels, and their expression is induced by treatment with type I IFN or a viral infection.77 RLRs like melanoma differentiation-associated 5 (MDA5) and RIG-1 can recognize dsRNA or ssRNA with 5′ triphosphate ends (ppp-ssRNA). They contain an RNA helicase domain critical for RNA recognition and two CARDs critical for initiating downstream signaling pathways.78 NLRs are a group of evolutionarily conserved intracellular PRRs that play a vital role in innate immunity.76 There are 23 members of NLRs out of which NOD1 and NOD2 have gained a lot of attention in the pathogenesis of OM.74 RLRs and NLRs like NOD1 and NOD2 have the ability to initiate and support robust immune responses through the formation of inflammasomes and the activation of NF-κB, leading to the production of inflammatory cytokines (Figure 4).76 NLRs and RIG-1 play an important role during OM. Patients with OME show significantly lower levels of NOD1 and NOD2, as well as RIG-1.79 These decreased PPR expression levels may be associated with the development of recurrent OME, demonstrating the protective role of NOD1, NOD2, and RIG-1 in OM.
C-type lectin receptors (CLRs)
CLRs are a diverse family of soluble and transmembrane proteins that bind carbohydrates through one or more carbohydrate recognition domains, or which possess structurally similar C-type lectin-like domains that may not necessarily recognize carbohydrate ligands.80,81 CLRs are divided into 17 groups based on features including phylogeny and structure.82 Multiple members of the CLR family are considered to be PRRs due to their ability to recognize pathogen-associated molecules and induce intracellular signaling pathways that regulate the immune response.83 The adaptor molecules in CLR signaling like Bcl-10 are crucial in innate resistance to bacterial infection and LPS signaling.84 Bcl-10 is an intracellular NF-κB activator with a CARD that can form homo-oligomers through CARD–CARD interactions under appropriate conditions.85 The other CLR members such as Dec-205 are involved in endocytosis and antigen presentation.86 Triggering receptor expressed on myeloid cells 1 (Trem-1) CLR is up-regulated by bacterial LPS and helps in the activation of neutrophils and monocytes.87 It plays a crucial role in the production of cytokines and chemokines as well as up-regulating adhesion molecules.88 The role of these CLRs in the pathogenesis of OM has only begun to be elucidated. Patients with OME demonstrate higher mRNA levels of CLRs or CLR adaptor molecules such as dectin-1, MR1, MR2, DC-SIGN, Syk, Card-9, Bcl-10, Malt-1, Src, Dec-205, galectin-1, Tim-3, Trem-1, and DAP-12 in middle ear effusions.89 The increased expression of CLRs and their adaptor molecules like Dec-205, Bcl-10, Tim-3, and Trem-1 mRNA have also been observed in chronic OM with cholesteatoma.90 However, further studies are warranted to assess the protein expression of CLRs and their association with the pathogenesis of OM.
Neutrophils
Neutrophil involvement in OM has been a topic of increased interest over the past few years. Neutrophils, or polymorphonuclear granulocytes (PMNs), are the most abundant leukocytes and form the first line of defense against invading pathogens.91 Recent research focusing on acute OM caused by NTHi demonstrated the formation of neutrophil extracellular traps (NETs) by PMNs in response to bacterial infection.92 NETs are characterized by a double-stranded DNA lattice decorated with histones and elastase.93–95 NETs are hypothesized to determine bacterial persistence (rather than clearance) by providing a niche within the middle ear chamber. NETs were found to be positively correlated with higher bacterial loads within middle ear fluids and surface-attached communities in the chinchilla infection model.96 The inability of NETs to clear otopathogens may contribute to establishing stable bacterial communities in the middle ear. Bacteria entangled in DNA stranding from NETs can contribute to effusion viscosity, one of the hallmarks of CSOM. This may also lead to insufficient penetration of antibiotics and hence failure to clear infection.
TLRs are also seen to be important in bacterial clearance by neutrophils during OM. TLR 2, 4, 5, and 9 mRNA expression is up-regulated in neutrophils infected with M. catarrhalis with and without pili.97 When TLR4 is non-functional in C3H/HeJ mice, NTHi infections are prolonged and phagocytosis as well as phagosome maturation in PMNs are impaired.98 Thus, TLR4 appears to be intimately involved in the bactericidal function of PMNs and plays a vital role in eradicating NTHi infection in the middle ear.
Macrophages
Macrophages play a central role in innate immunity of the middle ear.99 Macrophages have been shown to be a major cellular component of middle ear effusions present in humans.100 Middle ear macrophages harvested from human effusions produce suppressive factors that lead to a hyporesponsiveness of lymphocytes to several mitogens.101 This may contribute to the chronicity of OM by promoting ineffective elimination of bacteria. It has also been shown that macrophages recruited to the middle ear are functional and capable of discriminate phagocytosis and intracellular killing of bacteria.102 However, S. pneumoniae types 14 and 19F, which are associated with the highest relapse frequency in cases of acute OM, were found to be the most resistant to phagocytosis. The clinical isolates of NTHi obtained from children with OM have also been demonstrated to survive in macrophages.103 This ability may enable NTHi to gain an intracellular niche within its host and contribute to the observed frequency of recurrent infection. The relative importance of macrophage function in defense of the middle ear, therefore, may be dependent on the causative agent. Inefficient phagocytosis or intracellular killing and/or incomplete digestion of bacteria by middle ear phagocytes could lead to bacterial antigens being trapped in the middle ear. This could promote middle ear effusions by a sustained release of inflammatory mediators or immune injury. Further studies are warranted to characterize the role of bacterial and host factors leading to the survival of OM pathogens inside macrophages.
Dendritic cells/Langerhans cells
Dendritic cells (DCs) are antigen-presenting cells (APCs) and play an important role in the orchestration of immune responses.104,105 The epithelial residents of DCs are Langerhans cells (LCs), which serve as the ‘sentinels’ of the mucosa.106 LCs are capable of engaging and internalizing a wide variety of pathogens. The normal tympanic membrane in the ear contains abundant DCs/LCs.107 These tympanic membrane DCs/LCs have also been shown to have the potential to migrate and to efficiently sensitize T cells.107 A significant increase in the number of DCs/LCs in CSOM as compared with that in the normal tympanic membrane has been reported in animal models and human patients.108,109 An increase in the size and more branching of LCs has also been observed in OM.110 However the exact function of DCs/LCs in the middle ear under normal and OM conditions has yet to be elucidated.
Fibroblasts
Fibroblasts are the most common cells of connective tissue and synthesize the extracellular matrix and collagen. They are ‘sentinel cells’ capable of producing various immune modulators such as peptide growth factors, cytokines, chemokines, and low molecular weight inflammatory mediators. In OM, fibroblasts are largely responsible for the architectural changes found in the middle ear. These changes include hyperplasia of the mucosal epithelium as well as subepithelial connective tissue.111–114 During OM, the middle ear mucosa proliferates into a pseudostratified ciliated secretory columnar epithelium. There is significant neovascularization, which not only allows for increased thickness but also provides a means for leukocyte infiltration. This process is a potential factor in middle ear effusion and subsequent tissue edema formation. During NTHi-induced OM, it appears that the up-regulation of fibroblast and vascular endothelial growth factors (FGFs and VEGFs) is responsible for the angiogenesis associated with these changes.115 It has also been observed that VEGF is a significant mediator of vascular permeability and therefore middle ear effusion. Further studies are warranted to understand the role of fibroblasts and underlying signaling mechanisms responsible for angiogenesis during OM.
Mast cells
Mast cells are an integral part of innate immunity and are deeply involved in the pathogenesis of OM.116–120 Mast cells are predominantly found in the pars flaccida compared to the pars tensa of the tympanic membrane in the middle ear.121,122 They have also been shown to be distributed throughout the tubotympanum in guinea pigs, in a decreasing gradient from the pharyngeal to the tympanic orifices, and in the ciliated epithelium and highly vascularized parts of the middle ear.123 High densities of mast cells have also been found in the floor of the rat tympanic bulla.124
Mast cell involvement in OM has been strongly linked to histamine release. High levels of histamine in middle ear effusions in various clinical studies and animal models have been correlated with mast cell proliferation.125–128 In fact, increased histamine release and thus mast cell proliferation, has been linked to mast cells originating from the adenoids, as described in the adenoid mediator release theory.129–131 One study found that the mast cell count is higher in combined OME and adenoiditis than adenoiditis alone. A greater hearing loss has been correlated with mast cell density, thus revealing the complex nature of mast cell involvement in OME.132
Mast cells appear to be involved in the longer clinical courses of CSOM.133 But the relationship between mast cells and bacterial infection in CSOM has not been entirely conclusive. Increased mast cell activity has been shown in CSOM in which S. aureus and P. aeruginosa are implicated, and in acute otitis media.134–136 Yet Pajor et al. failed to show an increased density of mast cells in CSOM due to bacterial infection.133 Ebmeyer et al. demonstrated that NTHi infection causes middle ear mucosal inflammatory changes in both wild-type and mast cell-deficient mice.137 However the early response was significantly blunted in mast cell-deficient mice. It has been suggested that mast cells may be involved in the bone resorption observed in CSOM.138,139 Bone resorption is the process by which osteoclasts break down bone and release minerals, resulting in a transfer of calcium from bone fluid to the blood. It is an important aspect of CSOM contributing to many complications of this disease such as ossicular erosion.
Natural killer cells
Natural killer (NK) cells are effector lymphocytes of the innate immune system.140 While the importance of NK cells in innate immune protection against tumors or viral infections is well documented, their role in providing protection against bacteria is still emerging. Jecker et al. demonstrated that although in healthy rat middle ears there was no local proliferation of immune-competent cells, approximately 15% of NK cells at day 5 of acute OM were newly formed and likely to have been due to local proliferation, while the remaining portion represented those migrated from the outside mucosa.141 An increase in the number of NK cells in the serum of CSOM children suggests a possible role of these lymphocytes in protecting the middle ear against infection.142 However, further studies are warranted to explore the role of NK cells in the pathogenesis of acute and chronic OM.
Concluding remarks
OM is an important public health problem with substantial economic and societal costs. It is a major global cause of hearing impairment. Hearing loss caused by OM leads to speech difficulties, attention deficit, language learning problems, and reading problems, as well as cognitive and behavioral disorders in children. In the past several years, considerable advances have been made in understanding the role of innate immunity in the pathogenesis of AOM. However, very few studies are available regarding the role of innate immunity in CSOM. The rise in the incidence of CSOM cases, emergence of antibiotic resistance, and potential ototoxicity of antibiotics has created an immediate incentive to focus research studies in this area so that novel therapeutic strategies may be developed. There is a need to explore the role of PRRs like TLRs, NLRs, RLRs, and CLRs in the pathophysiology of CSOM. Understanding the role of innate immunity in the pathogenesis of OM, especially CSOM, will open up new avenues to design effective non-conventional therapies against the disease. The antimicrobial innate immune molecules (AIIMs) like β-defensins will be of immense use to treat OM caused by antibiotic-resistant strains of otopathogens. Implementing these approaches could decrease physician visits, healthcare costs, antibiotic use, and surgery, and at the same time increase quality of life for many OM patients.
Highlights.
In this review article, we discuss recent advances in understanding the role of innate immunity in otitis media (OM).
We will also discuss the mechanisms utilized by pathogens to subvert innate immunity and thereby overcoming defensive responses.
OM is a serious healthcare problem and a leading cause of hearing loss.
Understanding the role of innate immunity in the pathogenesis of OM will open up new avenues to design effective non-conventional therapies against the disease.
Acknowledgments
The research work in Dr Liu’s laboratory is supported by grants R01 DC05575, R01 DC01246, and R01 DC012115 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders. The research work in Dr Mathee’s laboratory is supported by the National Science Foundation IIP-1237818 (PFI-AIR: CREST-I/UCRC-Industry Ecosystem to Pipeline Research).
Footnotes
Conflict of interest: No conflict of interest to declare.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pichichero ME. Otitis media. Pediatr Clin North Am. 2013;60:391–407. doi: 10.1016/j.pcl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureishi A, Lee Y, Belfield K, Birchall JP, Daniel M. Update on otitis media—prevention and treatment. Infect Drug Resist. 2014;7:15–24. doi: 10.2147/IDR.S39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen RG, Koch A, Homøe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2013;77:1530–5. doi: 10.1016/j.ijporl.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Kolo ES, Salisu AD, Yaro AM, Nwaorgu OG. Sensorineural hearing loss in patients with chronic suppurative otitis media. Indian J Otolaryngol Head Neck Surg. 2012;64:59–62. doi: 10.1007/s12070-011-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp Z, Rezes S, Jokay I, Sziklai I. Sensorineural hearing loss in chronic otitis media. Otol Neurotol. 2003;24:141–4. doi: 10.1097/00129492-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 7.da Costa SS, Rosito LP, Dornelles C. Sensorineural hearing loss in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2009;266:221–4. doi: 10.1007/s00405-008-0739-0. [DOI] [PubMed] [Google Scholar]
- 8.Harmes KM, Blackwood RA, Burrows HL, Cooke JM, Harrison RV, Passamani PP. Otitis media: diagnosis and treatment. Am Fam Physician. 2013;88:435–40. [PubMed] [Google Scholar]
- 9.Grindler DJ, Blank SJ, Schulz KA, Witsell DL, Lieu JE. Impact of otitis media severity on children’s quality of life. Otolaryngol Head Neck Surg. 2014 doi: 10.1177/0194599814525576. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein JO. The burden of otitis media. Vaccine. 2000;19(Suppl):S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- 11.Lambert E, Roy S. Otitis media and ear tubes. Pediatr Clin North Am. 2013;60:809–26. doi: 10.1016/j.pcl.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham M, Guardiani E, Kim HJ, Brook I. Otitis media. Future Microbiol. 2012;7:733–53. doi: 10.2217/fmb.12.38. [DOI] [PubMed] [Google Scholar]
- 13.Mostafa BE, El Fiky LM, ElSharnouby MM. Complications of suppurative otitis media: still a problem in the 21st century. ORL J Otorhinolaryngol Relat Spec. 2009;71:87–92. doi: 10.1159/000191472. [DOI] [PubMed] [Google Scholar]
- 14.Chew YK, Cheong JP, Khir A, Brito-Mutunayagam S, Prepageran N. Complications of chronic suppurative otitis media: a left otogenic brain abscess and a right mastoid fistula. Ear Nose Throat J. 2012;91:428–30. [PubMed] [Google Scholar]
- 15.Ibrahim SI, Cheang PP, Nunez DA. Incidence of meningitis secondary to suppurative otitis media in adults. J Laryngol Otol. 2010;124:1158–61. doi: 10.1017/S0022215110000976. [DOI] [PubMed] [Google Scholar]
- 16.Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet. 2004;363:465–73. doi: 10.1016/S0140-6736(04)15495-0. [DOI] [PubMed] [Google Scholar]
- 17.Morris P. Chronic suppurative otitis media. Am Fam Physician. 2013;88:694–6. [Google Scholar]
- 18.Afolabi OA, Salaudeen AG, Ologe FE, Nwabuisi C, Nwawolo CC. Pattern of bacterial isolates in the middle ear discharge of patients with chronic suppurative otitis media in a tertiary hospital in north central Nigeria. Afr Health Sci. 2012;12:362–7. doi: 10.4314/ahs.v12i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madana J, Yolmo D, Kalaiarasi R, Gopalakrishnan S, Sujatha S. Microbiological profile with antibiotic sensitivity pattern of cholesteatomatous chronic suppurative otitis media among children. Int J Pediatr Otorhinolaryngol. 2011;75:1104–8. doi: 10.1016/j.ijporl.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Dayasena R, Dayasiri M, Jayasuriya C, Perera D. Aetiological agents in chronic suppurative otitis media in Sri Lanka. Australas Med J. 2011;4:101–4. doi: 10.4066/AMJ.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattar A, Alamgir A, Hussain Z, Sarfraz S, Nasir J, Badar-e-Alam Bacterial spectrum and their sensitivity pattern in patients of chronic suppurative otitis media. J Coll Physicians Surg Pak. 2012;22:128–9. [PubMed] [Google Scholar]
- 22.Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by toll-like receptors. Int Rev Immunol. 2013;32:116–33. doi: 10.3109/08830185.2013.774391. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. Defensin participation in innate and adaptive immunity. Curr Pharm Des. 2007;13:3131–9. doi: 10.2174/138161207782110453. [DOI] [PubMed] [Google Scholar]
- 24.Jin Shin D, Gan-Undram S, Jin Kim S, Joon Jun Y, Jung Im G, Hyun Jung H. Expression of beta-defensins in the tubotympanum of experimental otitis media. Acta Otolaryngol. 2006;126:1040–5. doi: 10.1080/00016480600672626. [DOI] [PubMed] [Google Scholar]
- 25.Lee HY, Takeshita T, Shimada J, Akopyan A, Woo JI, Pan H, et al. Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect Dis. 2008;8:87. doi: 10.1186/1471-2334-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon SK, Lee HY, Pan H, Takeshita T, Park R, Cha K, et al. Synergistic effect of interleukin 1 alpha on nontypeable Haemophilus influenzae-induced up-regulation of human beta-defensin 2 in middle ear epithelial cells. BMC Infect Dis. 2006;6:12. doi: 10.1186/1471-2334-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon SK, Lee HY, Li JD, Nagura M, Kang SH, Chun YM, et al. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochim Biophys Acta. 2002;1590:41–51. doi: 10.1016/s0167-4889(02)00196-9. [DOI] [PubMed] [Google Scholar]
- 28.Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun. 2013;5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di YP. Functional roles of SPLUNC1 in the innate immune response against Gram-negative bacteria. Biochem Soc Trans. 2011;39:1051–5. doi: 10.1042/BST0391051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gakhar L, Bartlett JA, Penterman J, Mizrachi D, Singh PK, Mallampalli RK, et al. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS One. 2010;5:e9098. doi: 10.1371/journal.pone.0009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Di ME, Chu HW, Liu X, Wang L, Wenzel S, et al. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol. 2013;191:4259–68. doi: 10.4049/jimmunol.1202340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayeed S, Nistico L, St Croix C, Di YP. Multifunctional role of human SPLUNC1 in Pseudomonas aeruginosa infection. Infect Immun. 2013;81:285–91. doi: 10.1128/IAI.00500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGillivary G, Bakaletz LO. The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One. 2010;5:e13224. doi: 10.1371/journal.pone.0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schachern PA, Tsuprun V, Cureoglu S, Ferrieri PA, Briles DE, Paparella MM, et al. Effect of apolactoferrin on experimental pneumococcal otitis media. Arch Otolaryngol Head Neck Surg. 2010;136:1127–31. doi: 10.1001/archoto.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo KW, Hur DY, Park SK, Yang YI, Kwak HH, Kim TY. Expression of chitinases in hypertrophied adenoids of children. Otolaryngol Head Neck Surg. 2011;145:660–5. doi: 10.1177/0194599811410656. [DOI] [PubMed] [Google Scholar]
- 36.Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117:1666–76. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]
- 37.Argüeso P, Gipson IK. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods Mol Biol. 2012;842:313–25. doi: 10.1007/978-1-61779-513-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preciado D, Burgett K, Ghimbovschi S, Rose M. NTHi induction of Cxcl2 and middle ear mucosal metaplasia in mice. Laryngoscope. 2013;123:E66–71. doi: 10.1002/lary.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenqvist M, Anniko M, Rask-Andersen H. Middle ear mucosa changes following exposure to Pseudomonas aeruginosa exotoxin A. Eur Arch Otorhinolaryngol. 1999;256:484–90. doi: 10.1007/s004050050196. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Komori M, Yamakawa K, Hamajima Y, Suzuki M, Kim Y, et al. Math1, retinoic acid, and TNF-alpha synergistically promote the differentiation of mucous cells in mouse middle ear epithelial cells in vitro. Pediatr Res. 2013;74:259–65. doi: 10.1038/pr.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawano H, Paparella MM, Ho SB, Schachern PA, Morizono N, Le CT, et al. Identification of MUC5B mucin gene in human middle ear with chronic otitis media. Laryngoscope. 2000;110:668–73. doi: 10.1097/00005537-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Ubell M, Kerschner JE, Wackym A, Burrows A. MUC2 expression in human middle ear epithelium of patients with otitis media. Arch Otolaryngol Head Neck Surg. 2008;134:1–6. doi: 10.1001/archoto.2007.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerschner JE, Tripathi S, Khampang P, Papsin BC. MUC5AC expression in human middle ear epithelium of patients with otitis media. Arch Otolaryngol Head Neck Surg. 2010;136:819–24. doi: 10.1001/archoto.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elsheikh MN, Mahfouz ME. Up-regulation of MUC5AC and MUC5B mucin genes in nasopharyngeal respiratory mucosa and selective up-regulation of MUC5B in middle ear in pediatric otitis media with effusion. Laryngoscope. 2006;116:365–9. doi: 10.1097/01.MLG.0000195290.71090.A1. [DOI] [PubMed] [Google Scholar]
- 45.Mittal R, Grati M, Gerring R, Blackwelder P, Yan D, Li JD, et al. In vitro interaction of Pseudomonas aeruginosa with human middle ear epithelial cells. PLoS One. 2014;9:e91885. doi: 10.1371/journal.pone.0091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim DJ, Chun YM, Lee HY, Moon SK, Chang KH, Li JD, et al. Cell biology of tubotympanum in relation to pathogenesis of otitis media—a review. Vaccine. 2000;19:S17–25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 48.Lee HY, Andalibi A, Webster P, Moon SK, Teufert K, Kang SH, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimada J, Moon SK, Lee HY, Takeshita T, Pan H, Woo JI, et al. Lysozyme M deficiency leads to an increased susceptibility to Streptococcus pneumoniae-induced otitis media. BMC Infect Dis. 2008;8:134. doi: 10.1186/1471-2334-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–46. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 52.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250:216–29. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen R, Lim JH, Jono H, Gu XX, Kim YS, Basbaum CB, et al. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324:1087–94. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 54.DeMaria TF, Apicella MA, Nichols WA, Leake ER. Evaluation of the virulence of nontypeable Haemophilus influenzae lipooligosaccharide htrB and rfaD mutants in the chinchilla model of otitis media. Infect Immun. 1997;65:4431–5. doi: 10.1128/iai.65.11.4431-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emonts M, Veenhoven RH, Wiertsema SP, Houwing-Duistermaat JJ, Walraven V, de Groot R, et al. Genetic polymorphisms in Immuno response genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120:814–23. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- 56.Hirano T, Kodama S, Fujita K, Maeda K, Suzuki M. Role of toll like receptor 4 in innate immune responses in a mouse model of acute otitis media. FEMS Immunol Med Microbiol. 2007;49:75–83. doi: 10.1111/j.1574-695X.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 57.Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, et al. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–70. doi: 10.1074/jbc.M112190200. [DOI] [PubMed] [Google Scholar]
- 58.Granath A, Cardell LO, Uddman R, Harder H. Altered toll- and Nod-like receptor expression in human middle ear mucosa from patients with chronic middle ear disease. J Infect. 2011;63:174–6. doi: 10.1016/j.jinf.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Si Y, Zhang ZG, Chen SJ, Zheng YQ, Chen YB, Liu Y, et al. Attenuated TLRs in middle ear mucosa contributes to susceptibility of chronic suppurative otitis media. Hum Immunol. 2014;75:771–6. doi: 10.1016/j.humimm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Häcker G, Redecke V, Häcker H. Activation of the immune system by bacterial CpG-DNA. Immunology. 2002;105:245–51. doi: 10.1046/j.0019-2805.2001.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 62.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–81. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–80. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barber GN. Cytoplasmic DNA innate immune pathways. Immunol Rev. 2011;243:99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- 65.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 66.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 67.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–91. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–94. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 69.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, et al. Aspartate–glutamate–alanine–histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:15181–6. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sirois CM, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim MG, Park DC, Shim JS, Jung H, Park MS, Kim YI, et al. TLR-9, NOD-1, NOD-2, RIG-I and immunoglobulins in recurrent otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2010;74:1425–9. doi: 10.1016/j.ijporl.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 72.Lee HY, Kim YI, Lee JW, Byun JY, Park MS, Yeo SG. Decreased expression of TLR-9 and cytokines in the presence of bacteria in patients with otitis media with effusion. Clin Exp Otorhinolaryngol. 2013;6:195–200. doi: 10.3342/ceo.2013.6.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leichtle A, Hernandez M, Lee J, Pak K, Webster NJ, Wollenberg B, et al. The role of DNA sensing and innate immune receptor TLR9 in otitis media. Innate Immun. 2012;18:3–13. doi: 10.1177/1753425910393539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 75.Dixit E, Kagan JC. Intracellular pathogen detection by RIG-I-like receptors. Adv Immunol. 2013;117:99–125. doi: 10.1016/B978-0-12-410524-9.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olive C. Pattern recognition receptors: sentinels in innate immunity and targets of new vaccine adjuvants. Expert Rev Vaccines. 2012;11:237–56. doi: 10.1586/erv.11.189. [DOI] [PubMed] [Google Scholar]
- 77.Yoo JS, Kato H, Fujita T. Sensing viral invasion by RIG-I like receptors. Curr Opin Microbiol. 2014;20C:131–8. doi: 10.1016/j.mib.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Bruns AM, Horvath CM. Activation of RIG-I-like receptor signal transduction. Crit Rev Biochem Mol Biol. 2012;47:194–206. doi: 10.3109/10409238.2011.630974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YJ, Cha SH, Lee HY, Lee SK, Chung HY, Yeo JH, et al. Decreased pattern-recognition receptor-mediated cytokine mRNA expression in obese children with otitis media with effusion. Clin Exp Otorhinolaryngol. 2014;7:7–12. doi: 10.3342/ceo.2014.7.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan H, Ohno N, Tsuji NM. The role of C-type lectin receptors in immune homeostasis. Int Immunopharmacol. 2013;16:353–7. doi: 10.1016/j.intimp.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 81.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 83.Kingeter LM, Lin X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–12. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao XQ, Zhu LL, Chang Q, Jiang C, You Y, Luo T, et al. C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent NF-κB activation. J Biol Chem. 2014 doi: 10.1074/jbc.M114.588574. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong W, Liu Y, Peng J, Chen L, Zou T, Xiao H, et al. The IRAK-1-BCL10-MALT1-TRAF6-TAK1 cascade mediates signaling to NF-kappaB from toll-like receptor 4. J Biol Chem. 2006;281:26029–40. doi: 10.1074/jbc.M513057200. [DOI] [PubMed] [Google Scholar]
- 86.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46:1229–39. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arts RJ, Joosten LA, vander Meer JW, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93:209–15. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 88.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 89.Lee JH, Park DC, Oh IW, Kim YI, Kim JB, Yeo SG. C-type lectin receptors mRNA expression in patients with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2013;77:1846–51. doi: 10.1016/j.ijporl.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 90.Kim MG, Park DC, Oh IH, Kim YI, Choi SA, Jung SY, et al. Increased expression of Dec-205, Bcl-10, Tim-3, and Trem-1 mRNA in chronic otitis media with cholesteatoma. Acta Otolaryngol. 2014;134:475–80. doi: 10.3109/00016489.2013.878474. [DOI] [PubMed] [Google Scholar]
- 91.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011;79:431–8. doi: 10.1128/IAI.00660-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simon D, Simon HU, Yousefi S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy. 2013;68:409–16. doi: 10.1111/all.12111. [DOI] [PubMed] [Google Scholar]
- 94.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–94. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 95.Magdalena Arazna M, Pruchniak MP, Demkow U. Neutrophil extracellular traps in bacterial infections: strategies for escaping from killing. Respir Physiol Neurobiol. 2013;187:74–7. doi: 10.1016/j.resp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Hong W, Juneau RA, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1:215–24. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawano T, Hirano T, Kodama S, Mitsui MT, Ahmed K, Nishizono A, et al. Pili play an important role in enhancing the bacterial clearance from the middle ear in a mouse model of acute otitis media with Moraxella catarrhalis. Pathog Dis. 2013;67:119–31. doi: 10.1111/2049-632X.12025. [DOI] [PubMed] [Google Scholar]
- 98.Hirano T, Kodama S, Fujita K, Maeda K, Suzuki M. Role of toll-like receptor 4 in innate immune responses in a mouse model of acute otitis media. FEMS Immunol Med Microbiol. 2007;49:75–83. doi: 10.1111/j.1574-695X.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 99.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lim DJ, Lewis DM, Schram JL. Otitis media with effusion: cytological and microbiological correlates. Arch Otolaryngol Head Neck Surg. 1979;105:404–12. doi: 10.1001/archotol.1979.00790190030006. [DOI] [PubMed] [Google Scholar]
- 101.Yamanaka T, Bernstein JM, Cumella J. Lymphocyte–macrophage interaction in otitis media with effusion. In: Lim DJ, Bluestone CD, Klein JO, et al., editors. Recent advances in otitis media with effusion. Philadelphia: BC Decker Inc; 1984. pp. 173–7. [Google Scholar]
- 102.Bakaletz LO, DeMaria TF, Lim DJ. Phagocytosis and killing of bacteria by middle ear macrophages. Arch Otolaryngol Head Neck Surg. 1987;113:138–44. doi: 10.1001/archotol.1987.01860020030007. [DOI] [PubMed] [Google Scholar]
- 103.Craig JE, Cliffe A, Garnett K, High NJ. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett. 2001;203:55–61. doi: 10.1111/j.1574-6968.2001.tb10820.x. [DOI] [PubMed] [Google Scholar]
- 104.Cerboni S, Gentili M, Manel N. Diversity of pathogen sensors in dendritic cells. Adv Immunol. 2013;120:211–37. doi: 10.1016/B978-0-12-417028-5.00008-9. [DOI] [PubMed] [Google Scholar]
- 105.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–42. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 106.Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hussl B, Egg G, Romani N, Kong W, Schrott-Fischer A. Dendritic cells in the normal human tympanic membrane. Ann Otol Rhinol Laryngol. 1995;104:803–7. doi: 10.1177/000348949510401010. [DOI] [PubMed] [Google Scholar]
- 108.Palva T, Taskinen E. Inflammatory cell subpopulations in chronic otitis media. The Langerhans’ cells. Arch Otolaryngol Head Neck Surg. 1987;113:149–54. doi: 10.1001/archotol.1987.01860020041009. [DOI] [PubMed] [Google Scholar]
- 109.Ichimiya I, Kurono Y, Mogi G. Immunological potential of the tympanic membrane. Observation under normal and inflammatory conditions. Am J Otolaryngol. 1997;18:165–72. doi: 10.1016/s0196-0709(97)90077-6. [DOI] [PubMed] [Google Scholar]
- 110.Jacob TM, Indrasingh I, Yadav BK, Rupa V. Langerhans cells in the human tympanic membrane in health and disease: a morphometric analysis. Otol Neurotol. 2013;34:325–30. doi: 10.1097/MAO.0b013e31826dbce5. [DOI] [PubMed] [Google Scholar]
- 111.Ryan AF, Baird A. Growth factors during proliferation of the middle ear mucosa. Acta Otolaryngol. 1993;113:68–74. doi: 10.3109/00016489309135769. [DOI] [PubMed] [Google Scholar]
- 112.Palacios SD, Pak K, Rivkin AZ, Bennett T, Ryan AF. Growth factors and their receptors in the middle ear mucosa during otitis media. Laryngoscope. 2002;112:420–3. doi: 10.1097/00005537-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 113.Ryan AF, Luo L, Baird A. Implantation of cells transfected with the FGF-1 gene induces ME mucosal proliferation. In: Lim D, et al., editors. Recent advances in otitis media with effusion. Amsterdam: Kugler; 1997. pp. 248–50. [Google Scholar]
- 114.Van Blitterswijk C, Ponec M, Van Muijen G, Wijsman M, Koerten H, Grote J. Culture and characterization of rat middle-ear epithelium. Acta Otolaryngol. 1986;101:453–66. doi: 10.3109/00016488609108632. [DOI] [PubMed] [Google Scholar]
- 115.Husseman J, Palacios SD, Rivkin AZ, Oehl H, Ryan AF. The role of vascular endothelial growth factors and fibroblast growth factors in angiogenesis during otitis media. Audiol Neurootol. 2012;17:148–54. doi: 10.1159/000333805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sankovic S, Dergenc R, Bojic P. Mast cells in chronic inflammation of the middle ear mucosa. Rev Laryngol Otol Rhinol (Bord) 2005;126:15–8. [PubMed] [Google Scholar]
- 117.Hurst DS, Amin K, Seveus L, Venge P. Mast cells and tryptase in the middle ear of children with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 1999;49:S315–9. doi: 10.1016/s0165-5876(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 118.Hurst DS, Amin K, Seveus L, Venge P. Evidence of mast cell activity in the middle ears of children with otitis media with effusion. Laryngoscope. 1999;109:471–7. doi: 10.1097/00005537-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 119.Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000;55:435–41. doi: 10.1034/j.1398-9995.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 120.St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol. 2013;190:4458–63. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Widemar L, Hellstrom S, Stenfors LE, Bloom GD. An overlooked site of tissue mast cells—the human tympanic membrane. Implications for middle ear affections. Acta Otolaryngol. 1986;102:391–5. doi: 10.3109/00016488609119422. [DOI] [PubMed] [Google Scholar]
- 122.Widemar L, Alm PE, Bloom GD, Hellstrom S, Stenfors LE. Membrana shrapnelli of maturing rats. The occurrence of mast cells, and histamine content in relation to otitis media with effusion. Acta Otolaryngol. 1984;98:302–7. doi: 10.3109/00016488409107567. [DOI] [PubMed] [Google Scholar]
- 123.Watanabe T, Kawauchi H, Fujiyoshi T, Mogi G. Distribution of mast cells in the tubotympanum of guinea pigs. Ann Otol Rhinol Laryngol. 1991;100:407–12. doi: 10.1177/000348949110000511. [DOI] [PubMed] [Google Scholar]
- 124.Albiin N, Hellstrom S, Stenfors LE, Cerne A. Middle ear mucosa in rats and humans. Ann Otol Rhinol Laryngol Suppl. 1986;126:2–15. doi: 10.1177/00034894860950s501. [DOI] [PubMed] [Google Scholar]
- 125.Palva T, Taskinen E, Lehtinen T, Ramsay H, Bjorksten F, Hackman P. Mast cells and histamine in adenoid tissue and middle ear. Acta Otolaryngol. 1991;111:349–53. doi: 10.3109/00016489109137399. [DOI] [PubMed] [Google Scholar]
- 126.Boisvert P, Wasserman SI, Schiff M, Ryan AF. Histamine-induced middle ear effusion and mucosal histopathology in the guinea pig. Ann Otol Rhinol Laryngol. 1985;94:212–6. doi: 10.1177/000348948509400224. [DOI] [PubMed] [Google Scholar]
- 127.Berger G, Hawke M, Proops DW, Ranadive NS, Wong D. Histamine levels in middle ear effusions. Acta Otolaryngol. 1984;98:385–90. doi: 10.3109/00016488409107578. [DOI] [PubMed] [Google Scholar]
- 128.Eriksson PO, Hellstrom S. Degranulation of mast cells provokes a massive inflammatory reaction in the tympanic membrane. Laryngoscope. 2001;111:1264–70. doi: 10.1097/00005537-200107000-00023. [DOI] [PubMed] [Google Scholar]
- 129.Marseglia GL, Poddighe D, Caimmi D, Marseglia A, Caimmi S, Ciprandi G, et al. Role of adenoids and adenoiditis in children with allergy and otitis media. Curr Allergy Asthma Rep. 2009;9:460–4. doi: 10.1007/s11882-009-0068-4. [DOI] [PubMed] [Google Scholar]
- 130.Berger G, Ophir D. Possible role of adenoid mast cells in the pathogenesis of secretory otitis media. Ann Otol Rhinol Laryngol. 1994;103:632–5. doi: 10.1177/000348949410300810. [DOI] [PubMed] [Google Scholar]
- 131.Abdullah B, Hassan S, Sidek D, Jaafar H. Adenoid mast cells and their role in the pathogenesis of otitis media with effusion. J Laryngol Otol. 2006;120:556–60. doi: 10.1017/S0022215106000818. [DOI] [PubMed] [Google Scholar]
- 132.Ulualp SO, Sahin D, Yilmaz N, Anadol V, Peker O, Gursan O. Increased adenoid mast cells in patients with otitis media with effusion. Int J Pediatr Otorhinolaryngol. 1999;49:107–14. doi: 10.1016/s0165-5876(99)00043-9. [DOI] [PubMed] [Google Scholar]
- 133.Pajor A, Danilewicz M, Jankowski A, Durko T. Participation of mast cells in chronic otitis media. Folia Histochem Cytobiol. 2011;49:479–85. doi: 10.5603/fhc.2011.0068. [DOI] [PubMed] [Google Scholar]
- 134.Lin TJ, Garduno R, Boudreau RT, Issekutz AC. Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 alpha and beta. J Immunol. 2002;169:4522–30. doi: 10.4049/jimmunol.169.8.4522. [DOI] [PubMed] [Google Scholar]
- 135.Boudreau RT, Garduno R, Lin TJ. Protein phosphatase 2A and protein kinase C alpha are physically associated and are involved in Pseudomonas aeruginosa-induced interleukin 6 production by mast cells. J Biol Chem. 2002;277:5322–9. doi: 10.1074/jbc.M108623200. [DOI] [PubMed] [Google Scholar]
- 136.Arock M, Ross E, Lai-Kuen R, Averlant G, Gao Z, Abraham SN. Phagocytic and tumor necrosis factor alpha response of human mast cells following exposure to Gram-negative and Gram-positive bacteria. Infect Immun. 1998;66:6030–4. doi: 10.1128/iai.66.12.6030-6034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ophir D, Hahn T, Schattner A, Wallach D, Aviel A. Tumor necrosis factor in middle ear effusions. Arch Otolaryngol Head Neck Surg. 1988;114:1256–8. doi: 10.1001/archotol.1988.01860230050021. [DOI] [PubMed] [Google Scholar]
- 138.Kuczkowski J, Sakowicz-Burkiewicz M, Izycka-Swieszewska E, Mikaszewski B, Pawelczyk T. Expression of tumor necrosis factor-alpha, interleukin-1alpha, interleukin-6 and interleukin-10 in chronic otitis media with bone osteolysis. ORL J Otorhinolaryngol Relat Spec. 2011;73:93–9. doi: 10.1159/000323831. [DOI] [PubMed] [Google Scholar]
- 139.Berger G, Hawke M, Ekem JK. Bone resorption in chronic otitis media. The role of mast cells. Acta Otolaryngol. 1985;100:72–80. doi: 10.3109/00016488509108590. [DOI] [PubMed] [Google Scholar]
- 140.Jecker P, Pabst R, Westermann J. The mucosa of the middle ear and Eustachian tube in the young rat: number of granulocytes, macrophages, dendritic cells, NK cells and T and B lymphocytes in healthy animals and during otitis media. Acta Otolaryngol. 1996;116:443–50. doi: 10.3109/00016489609137871. [DOI] [PubMed] [Google Scholar]
- 141.Jecker P, Pabst R, Westermann J. Proliferating macrophages, dendritic cells, natural killer cells, T and B lymphocytes in the middle ear and Eustachian tube mucosa during experimental acute otitis media in the rat. Clin Exp Immunol. 2001;126:421–5. doi: 10.1046/j.1365-2249.2001.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wojdas A, Stankiewicz W, Zielnik-Jurkiewicz B, Sobiczewska E, Stasiak-Barmuta A. Early and late activation markers on thymus-dependent lymphocytes and natural killer cells in the blood of children with adenoid hypertrophy and concomitant otitis media with effusion. Centr Eur J Immunol. 2011;36:262–6. [Google Scholar]