Abstract
Genome organization and gene expression of Borna disease virus (BDV) are remarkable for the overlap of open reading frames, transcription units and transcription signals, read through of transcription termination signals, differential use of translation initiation codons, and exploitation of the cellular splicing machinery. Here we report an additional control of gene expression at the level of mRNA stability. Levels of BDV proteins in infected cells do not correspond to the transcriptional gradient typically observed in nonsegmented negative-sense RNA viruses. The third transcription unit of BDV’s negative-sense RNA genome encodes viral proteins M, G and L. Analysis of the third transcription unit identified RNA-destabilizing domains with the most pronounced activity located in regions spanning nucleotides 2818–2918 (instability domain-1) and 4022–4071 (instability domain-2). Given that one domain maps to intron-2 and is thereby eliminated upon splicing, this represents an intriguing mechanism for regulating transcript levels independent of a transcriptional gradient. The presence of instability domains in introns offers a mechanism to create the observed discontinuous gradient M>L>G, compatible with the non-cytopathic, persistent infection that is characteristic for BDV, and provides a rationale for the use of alternative splicing by this unusual virus.
Keywords: Borna disease virus, RNA instability, Gene expression, Transcription
1. Introduction
Borna disease virus (BDV), a nonsegmented, negative strand RNA virus, establishes persistent central nervous system (CNS) infection and causes behavioral disturbances in warm-blooded animals (Ludwig et al., 1988; Rott and Becht, 1995). Notable features of its molecular biology include replication and transcription in the nucleus (Briese et al., 1992; Carbone et al., 1991), overlap of open reading frames (ORFs) and transcription units (Briese et al., 1994; Cubitt et al., 1994a; Schneemann et al., 1994), RNA splicing and differential use of transcription termination sites and translation initiation codons (Cubitt et al., 1994b; Schneemann et al., 1994; Schneider et al., 1997a, 1994), and the requirement for phosphorylation by kinases with limited distribution within the CNS (Schwemmle et al., 1997).
The BDV genome is organized into three transcription units. The first transcription unit codes for the viral nucleoprotein (N, p38/40). The second unit contains in overlapping reading frames coding sequences for proteins X (p10) and P (phosphoprotein, p23). The matrix protein (M, p16), the type I membrane glycoprotein (G, p57, gp94) and the RNA-dependent RNA polymerase (L, p190) are encoded by the third transcription unit (Schneemann et al., 1994; Walker et al., 2000). The first two transcripts are found at similar levels in infected cells and tissues, whereas the third transcript is expressed at lower levels (Briese et al., 1994; Walker et al., 2000). Thus, the 5′ to 3′ transcriptional gradient observed in other nonsegmented, negative strand RNA viruses (Abraham and Banerjee, 1976) is modified in BDV.
A potential mechanism to explain the marked reduction in levels of RNA transcripts originating from the third transcription unit may be the presence of negative regulatory elements. RNA instability elements play crucial roles in the regulation of eukaryotic gene expression (Tourriere et al., 2002), and have been demonstrated in several viral systems (Maldarelli et al., 1991; Nasioulas et al., 1994; Saiga et al., 1997; Schneider et al., 1997c; Schwartz et al., 1992; Sokolowski et al., 1999; Sokolowski and Schwartz, 2001). Although such sequences are frequently located in untranslated regions (UTRs) and comprise AU-rich elements (AREs), destabilizing domains are also found in coding sequences and may not involve AREs (Sokolowski et al., 1998).
In previous work we noted that only low levels of BDV G or L protein were obtained with eukaryotic expression plasmids (Walker et al., 2000). These results, together with the observation that low levels of mRNAs derived from the third transcription unit and their cognate proteins are present during BDV infection in vitro and in vivo, led us to speculate that BDV might regulate gene expression through RNA-destabilizing sequences not related to AREs.
2. Methods
2.1. Plasmid constructs
All vectors used in this study were generated from pcDNA3 (Invitrogen, Carlsbad, CA, USA). The firefly luciferase gene was inserted downstream of the CMV promoter between restriction sites BamHI and EcoRV. The downstream NotI and XhoI sites were used to accommodate constructs representing the BDV third transcription unit, or non-specific DNA obtained from West Nile virus (nt 8870–11027; Genbank accession number AF196835), respectively. As an internal control for transfection efficiency we used the pRL-TK Renilla luciferase vector (Promega, Madison, WI, USA). The vector contains Renilla luciferase cDNA under the control of the herpes simplex virus thymidine kinase promoter to provide low to moderate levels of Renilla luciferase expression in co-transfected mammalian cells.
2.2. Cell transfection and Luciferase assay
Oligodendrocytes (OL) were cultured in Dulbecco’s modified Eagles Medium (DMEM) with 10% fetal calf serum, 5mM l-glutamine, 10,000 units/ml penicillin G and 10 mg/ml streptomycin at 37 °C and 5% CO2 and seeded in 24-well plates at a density of 1 × 105 cells/well for transfection. Transient transfection of the cells was performed using 1 μg total DNA consisting of 200 ng of the respective plasmid DNA construct, 50 ng Renilla luciferase plasmid DNA and 750 ng pBluescript II SK(+) (Stratagene, La Jolla, CA, USA) carrier DNA to adjust for total DNA amount. Transfection was carried out in 700 µl Opti-MEM with 3 µl Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA). Cells were incubated for 12 h before the medium was replaced. After another 8 h the cells were harvested for luciferase or RNA analyses. Luciferase activity in cell extracts was determined using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA).
2.3. RNA extraction, reverse transcription (RT) and real-time polymerase chain reaction (PCR)
Total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA). RNA pellets were resuspended in 40 µl H2O and treated with DNase I (Ambion, Austin, TX, USA) at 37 °C for 2 h. Reverse transcription was carried out using 200 ng total RNA with random hexamers in a total volume of 22 µl using Taq-Man Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA). Real-time PCR was performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems) using 5 µl cDNA in a reaction volume of 25 µl containing 300 nM of each primer, 200 nM of probe and 1 × real-time PCR mix (TaqMan Universal PCR Master Mix; Applied Biosystems). Activation of UNG (2min at 50°C) and AmpliTaq (10 min at 95 °C) was followed by 45 cycles of PCR (15 s at 95 °C and 1 min at 60 °C). Each cDNA sample was analyzed in duplicate. Primers used for detection of luciferase transcripts were Luc-1582F (5’-TCG AAG TAT TCC GCG TAC GTG) and Luc-1655R (5’-GCC CTG GTT CCT GGA ACA A) and the probe Luc-1604T (5’-FAM-TGT TCA CCT CGA TAT GTG CAT CTG TAA AAG CA-TAMRA). For the amplification of porphobilinogen sequence as an internal standard, primers were PD10–76F (5’-ATT CGG GGA AAC CTC AAC ACC) and PD11–229R (5’-GGC CCA CAG CAT ACATGC AT) and the probe was PDT-174T (5’-TET-AGG ATC TGC CCA ACC CGG TTG TGC-TAMRA).
2.4. Northern Blot analysis
To generate a probe for Northern Blot analysis a 370 nucleotide (nt) fragment of the luciferase gene was amplified with the primers luc1051fwd (5’-TCTGACGCAGGCAGTTCTATG-3’) and luc1421rev (5′-GCGTTATTTATCGGAGTTG-3′). The amplicon was cloned into TOPO TA (Invitrogen, Carlsbad, CA, USA) and the plasmid linearized by restriction digest at the 5′-end of the luciferase fragment. The linearized plasmid was then transcribed in vitro with the MAXIscript® SP6/T7 Kit (Ambion, Austin, TX, USA) including 10 mM biotinylated UTP (Ambion).
RNA from transfected cells was size-fractionated by electrophoresis in a 1% formaldehyde gel and transferred to a positively charged nylon membrane (Ambion, BrightStar®-Plus Positively Charged Nylon Membrane; Ambion, NorthernMax® One-Hour Transfer Buffer). The RNA was cross-linked and prehybridized for 2h at 65°C (5.85ml H2O, 500µl 100 × Denhardt′s solution, 250 µl 20% SDS, 100 µl salmon sperm DNA (10 mg/ml), 100 µl yeast t-RNA (10 mg/ml), 200 µl 0.5 M EDTA, and 3.0 ml 20× SSC). Thereafter, 100 ng of the in vitro transcribed RNA probe was added and hybridized overnight at 65 °C. Subsequent wash steps included 2 washes with 2 × SSC and 0.1% SDS for 5 min each and 2 washes with 0.1 × SSC and 0.1% SDS for 15 min each. Hybridization signal was detected using the BrightStar® BioDetect™ Kit (Ambion). Membranes were exposed to film (biomax light film; Kodak, Rochester, NY, USA) and using Quantity One software (Bio Rad, Hercules, CA, USA).
3. Results
3.1. The third transcription unit of BDV contains sequences that modulate RNA expression
BDV uses alternative splicing to generate a series of transcripts from its third transcription unit. The presence or absence of destabilizing sequences in these transcripts may contribute to the regulation of their relative abundance. To investigate the presence of sequences that potentially destabilize transcripts of the third transcription unit, we initially analyzed a construct representing the primary 2.8 kb transcript. A series of 5′ to 3′, and 3′ to 5′ deletion constructs were created between nt positions 1885 and 4154 of BDV strain V (Genbank accession number NC_001607) and cloned downstream of the firefly luciferase gene in pcDNA Luc (Fig. 1A). Individual constructs were transfected into oligodendrocytes (OL cells) along with a Renilla luciferase expressing plasmid to control for transfection efficiency.
Fig. 1.
(A) BDV genome organization and schematic of deletion constructs of the third transcription unit that were cloned downstream of the firefly luciferase gene under the control of a CMV promoter. (B) Luciferase assays of oligodendrocytes transfected with pLucB2 (B2), pLucB3 (B3), pLucB4 (B4), pLucB5 (B5) and pLucB6 (B6) (left panel) and with pLucBR2 (BR2), pLucBR3 (BR3), pLucBR4 (BR4), pLucBR5 (BR5) and pLucBR6 (BR6) (right panel). The value of firefly luciferase expression with the empty vector pLuc (Luc) was set at 100% (7689±34 light units). Values in arbitrary light units were standardized with Renilla luciferase as internal control.
Constructs spanning the entire region from nt 1888 to 4154 and 1885 to 4152 resulted in a significant reduction of reporter signal when compared to the empty vector (B6, 22%; BR2, 15%; Fig. 1B). Analysis of the 5′ and 3′ deletion construct series indicated several critical domains: effects on reporter signal was observed for nt 3871–4154, and nt 2263–2918 (3′ deletion series); and for nt 2913–3650 and nt 3650–3855 (5′ deletion series). These findings suggested that the function of some of these domains is modulated by the presence or absence of neighboring sequences.
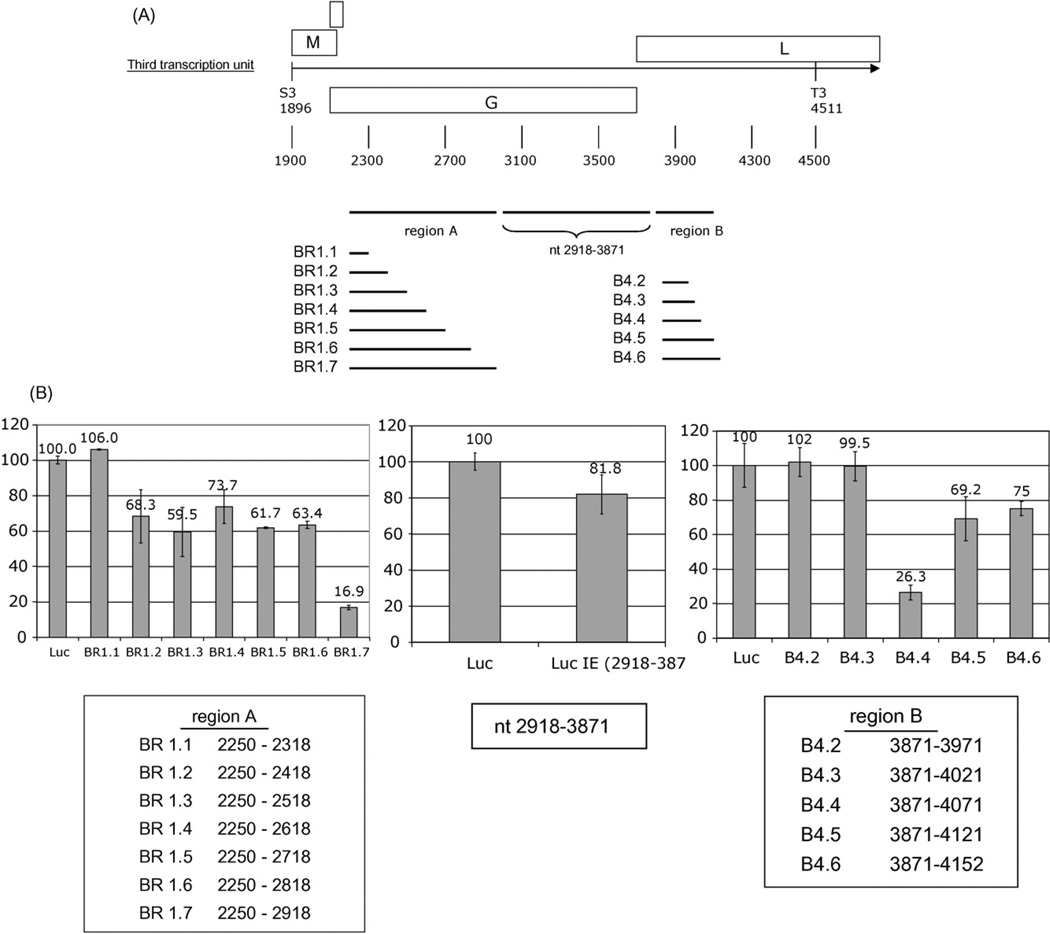
3.2. Mapping of destabilizing regions
The region spanning nt 2263–2918 that resulted in a reduction of reporter signal was further mapped using an additional set of deletion constructs (BR1.1 through BR1.7; Fig. 2A). Comparison of constructs BR1.1 and BR1.2 implicated a region between nt 2318 and 2418. Comparison of constructs BR1.6 and BR 1.7 implicated a region between nt 2818 and 2918. Comparison of the empty vector (Luc) and a construct comprising nt 2918–3871 revealed no dramatic effect. However, analyses of a series of constructs spanning the region between nt 3871 and 4152 (B4.2 through B4.6; Fig. 2A) indicated a decrease in reporter signal when nt 4022–4071 were present (B4.4); although the decrease was partially attenuated by downstream sequences between nt 4072 and 4121 (B4.5; Fig. 2B).
Fig. 2.
(A) Map of deletion constructs in identified destabilizing regions. BDV sequences were cloned downstream of the firefly luciferase gene in pcDNALuc generating plasmids for region A: pLucBR1.1, pLucBR1.2, pLucBR1.3, pLucBR1.4, pLucBR1.5, pLucBR1.6, pLucBR1.7, and for region B: pLucB4.2, pLucB4.3, pLucB4.4, pLucB4.5, pLucB4.6. (B) Luciferase assay of oligodendrocytes transfected with constructs shown in panel A. The value of the luciferase expression of the unmodified clone was set to 100% (6978±28 light units). The middle panel shows the luciferase assay of cells transfected with pLuc2918–3871 containing BDV nt 2918–3871. Values in arbitrary light units were standardized with Renilla luciferase as internal control.
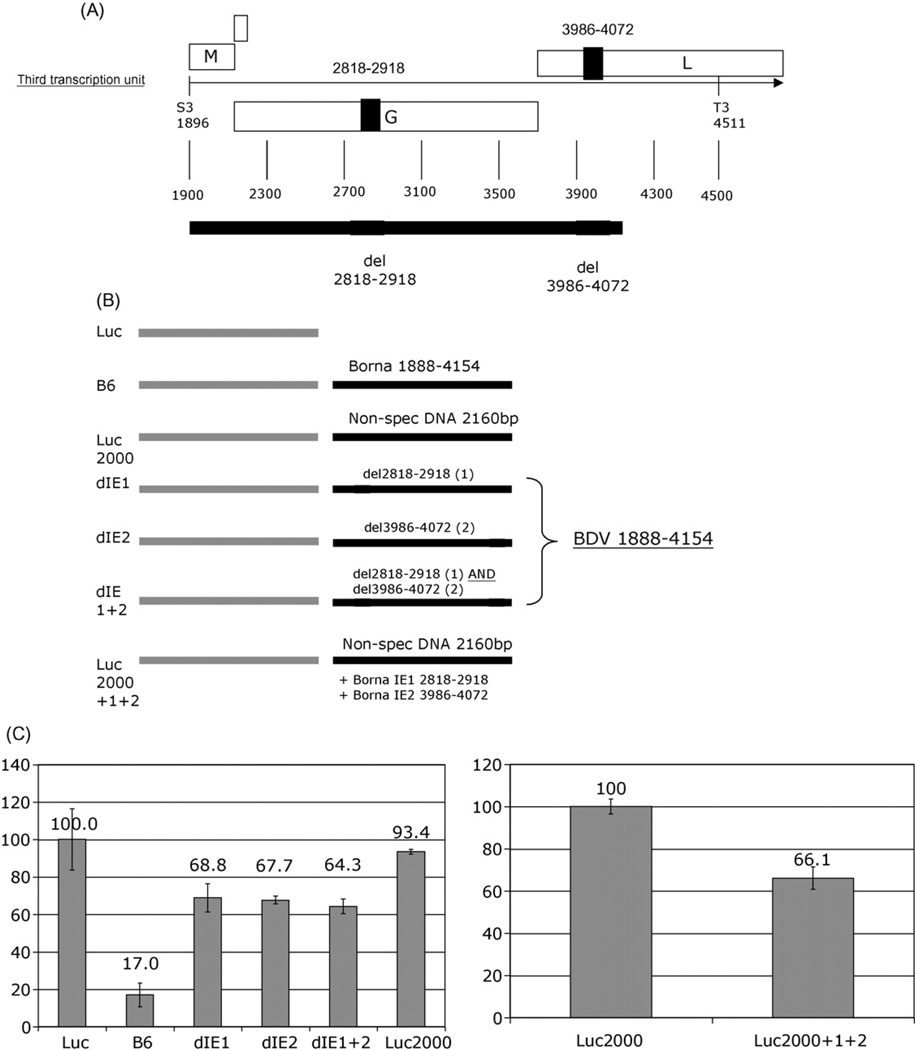
3.3. Analysis of instability domain activity
The two regions containing sequences for which the most pronounced reduction in reporter activity was observed (nt 2818–2918 and nt 4022–4071) were either deleted from the original BDV construct B6 (Fig. 3A), or cloned into an unrelated West Nile virus (WNV) sequence background (Luc2000) (Fig. 3B). To eliminate potential for differences in stability due to variations in length of BDV sequence, nt 3986–4072 (containing nt 4022–4071) and nt 2818–2918 were cloned into the WNV sequence background at a distance from one another similar to that in BDV. These insertions resulted in a 34% reduction in reporter signal (pLuc2000+1+2; Fig. 3C). Similarly, deletion of nt 2818–2918 and nt 3986–4072 from the BDV pLucB6 construct resulted in an increase in reporter signal from 17% to 64% (construct dIE1+2; Fig. 3C). Individual deletion of either domain alone from the BDV construct pLucB6 restored the reporter signal to an approximately similar level (dEI1 and dEI2; Fig. 3C). This result confirmed a synergistic interaction of the two domains, nt 2818–2918 (IE1) and nt 3986–4072 (IE2).
Fig. 3.
(A) Map of the BDV third transcription unit with nt regions 2818–2918 and 3986–4072 indicated. (B) Both or individual regions were deleted from pLucB6 and constructs cloned downstream of the firefly luciferase gene, resulting in pLuc-dIE1 (BDV 1888–4154 with deletion of nt 2818–2918), pLuc-dIE2 (BDV 1888–4154 with deletion of nt 3986–4072) and pLuc-dIE1+2 (BDV 1888–4154 with deletion of nt 2818–2918 and 3986–4072). A non-specific sequence derived from West Nile virus was cloned downstream of the luciferase gene (pLuc2000) and the BDV regions nt 2818–2918 and nt 3986–4072 were introduced at a similar distance from one another as found in BDV resulting in pLuc2000+1+2. (C) The left panel shows Luciferase assay of oligodendrocytes transfected with pLuc, pLucB6, pLuc-dIE1, pLuc-dIE2, pLuc-dIE1+2 and pLuc2000. The right panel shows the Luciferase assay of cells transfected with pLuc2000 and pLuc2000+1+2. In both panels the expression of the unmodified luciferase clone (6785±52 light units) or the luciferase clone with the non-specific sequence (6337 ±41) was set to 100%, respectively. Values in arbitrary light units were standardized with Renilla luciferase as internal control.
3.4. Reduced reporter gene expression reflects decreased mRNA levels
The capacity of IE1 and IE2 to influence RNA transcript levels was further tested through quantitation of the luciferase-containing transcripts by real-time PCR. Cells transfected with pLucB6 showed only 26% of the luciferase-containing RNA compared to cells transfected with the empty pLuc construct, whereas deletion of IE1 and IE2 from the pLucB6 construct (dIE1 +2) resulted in an increase of luciferase-containing RNA to 73% in comparison to the pLuc control (Table 1). Similar results were obtained with IE1 and IE2 cloned into the unrelated WNV background. Analysis of cells transfected with pLuc2000+1+2 revealed a 60% decrease in luciferase-containing mRNA expression compared to cells transfected with the pLuc2000 control (Table 1). The correlation between the total RNA copy number and observed luciferase activity confirms that reduction in the luciferase reporter signal indeed reflects reduced mRNA levels rather than RNA retention in the nucleus. Northern Blot analyses further confirmed this observation; hybridization signal of the luciferase RNA probe showed that band intensity was reduced to 27% in B6 transfected cells compared to cells that were transfected with pLuc control plasmid (Fig. 4). The deletion of the destabilizing domains IE1 and IE2 from the B6 construct resulted in an intensity of 57%.
Table 1.
Quantitative real-time PCR analysis of transcript levels after transfection with construct containing RNA-destabilizing domains.
| Plasmid | Copy numbera | %b |
|---|---|---|
| pLuc | 2359.6 | 100.0 |
| pLucB6 | 617.9 | 26.1 |
| pLucdIE1+2 | 1733.2 | 73.5 |
| pLuc2000 | 9037.3 | 100.0 |
| pLuc2000+1+2 | 3620.4 | 40.1 |
Quantitated by real-time PCR and normalized to cellular porphobilinogen mRNA.
Relative level in reference to empty vector plasmid pLuc (100%).
Fig. 4.
Northern Blot analysis of oligodendrocytes transfected with pLuc, pLucB6, pLuc-dIE1+2 and non-transfected oligodendrocytes. The intensity of the luciferase mRNA in cells transfected with pLuc was set to 100% and the relative percentage of other signals calculated using Quantity One software (Bio Rad).
3.5. Differential regulation of RNA stability through alternative splicing of instability domains
Given that IE1 is located in intron-2, we hypothesized that alternative splicing might contribute to the control of BDV gene expression through effects on the interaction between IE1 and IE2. We therefore analyzed the relative stability of alternative splice products generated from the 2.8 kb primary transcript of the BDV third transcription unit (1.4, 1.5, and 2.6 kb).
The primary 2.8 kb transcript and its 2.6, 1.5, 1.4 kb splice products were cloned downstream of the luciferase reporter gene to generate constructs pLuc2.8, pLuc2.6, pLuc1.5, and pLuc1.4, respectively (Fig. 5A). Consistent with the results for pLucB6/pLucBR2 in the previouse xperiments (Fig.1B), the BDV2.8 kb construct pLuc2.8 resulted in a reduction of reporter signal to 15% of the control values (Fig. 5B). The pLuc2.6 construct, which contains both IE1 and IE2 but lacks intron-1 (nt 1932–2025), showed a comparable reduction in reporter signal. In contrast, the pLuc1.5 construct, which lacks IE1 due to splicing and removal of intron-2, showed only a reduction in reporter signal to 60% of the control, which was again consistent with the results in the previous experiment where constructs comprised deletion of IE1 or IE2 from the BDV sequence (Fig. 3C). A higher reduction to 41% of the control was observed with the pLuc1.4 construct, which lacks both introns (Fig. 5B). This suggests that an additional region in intron-1, included in nt 1888–2263 (see B2 in Fig. 1), may participate in modulating RNA expression.
Fig. 5.
(A) Map of the third transcription unit with BDV 1.4, 1.5. 2.6 and 2.8 kb authentic splice products. (B) Luciferase assay of oligodendrocytes transfected with constructs harboring cDNA representing BDV 1.4, 1.5. 2.6 or 2.8 kb RNAs. The expression of the unmodified luciferase clone was set to 100% (7012±46 light units). Values in arbitrary light units were standardized with Renilla luciferase as internal control.
4. Discussion
mRNA turnover plays an important role in the control of eukaryotic gene expression. The process of transcript degradation typically includes a poly(A)-shortening endonucleolytic cleavage that is controlled by trans-acting factors binding to cis-acting elements within the 3′-UTR of mRNAs (Brewer, 1999; Couttet et al., 1997; Korner et al., 1998). However, in the case of mammalian c-fos, c-myc, and (3-tublin, mRNAs encode instability determinants in their coding regions (Bernstein et al., 1992; Gay et al., 1987; Herrick and Ross, 1994; Schiavi et al., 1994; Shyu et al., 1991; Yen et al., 1988). RNA instability elements comprising AU-rich elements (AREs) (Wilson and Brewer, 1999) and other instability determinants have also been described in coding regions of Human immunodeficiency virus type 1 (HIV-1) (Lee and Rossi, 2004; Maldarelli et al., 1991), Human papillomavirus type 16 (HPV-16) (Sokolowski et al., 1998), and Human T-cell leukemia virus type 1 (HTLV-1) (Saiga et al., 1997). In HIV-1, mRNA instability elements are found in Gag/Pol and Env transcripts (Cochrane et al., 1991; Nasioulas et al., 1994; Schneider et al., 1997c; Schwartz et al., 1992), and interactions reported between the AU-rich instability element in the 5′-portion of gag and nuclear factors PSF and p54nrb (Zolotukhin et al., 2003). In HPV-16, instability elements are reported not only in the 3′-UTR (Sokolowski et al., 1999; Sokolowski and Schwartz, 2001; Wiklund et al., 2002), but also in the coding region where cis-acting AREs are located primarily in the 5′-half of the L1 gene (Tan et al., 1995); inhibitory elements lacking AUUUA or UUUUU motifs but displaying a 60% A + U content are located in the 5′-end of the L2 gene (Sokolowski and Schwartz, 2001; Sokolowski et al., 1998). HTLV-1 contains an RNA instability element within the pol region (Saiga et al., 1997), but although the HTLV-1 pol sequence contains a typical AU-motif, mutation of this motif does not affect RNA stability; thus, other, to be defined determinants must be implicated.
We have identified at least two RNA instability domains in the coding region of the third transcription unit of BDV. One of the domains is located within nt 2818–2918; the other within nt 4021–4072. These regions do not contain AUUUA or UUUUU motifs and show A + U contents of only 58% (IE1) and 48% (IE2). The activity of the domains is modified by context and modulated through synergistic interaction between the domains. Placement of IE1 and IE2 into a WNV sequence background (Luc2000/Luc2000+1+2) resulted in a reduction in reporter signal by 34% rather than the 83% observed in the BDV sequence background. Likewise, deletion of either domain, as well as both IEs from the BDV construct did not result in the same RNA stability as observed for the control. Potential candidate regions involved in further modulating RNA instability include nt 2318–2418 (see BR1.1/BR1.2; Fig. 2B) and sequences in intron-1 (nt 1932–2025, see B2; Fig. 1 and Luc2.8/Luc2.6 and Luc1.5/Luc1.4; Fig. 5).
Analysis of sequence downstream of nt 3871 located the main activity of IE2 to nt 4021–4072. This portion of the 2.8 kb transcript has been previously implicated in mRNA regulation in experiments with a different strain of BDV, He/80, where 3′ extension of sequence beyond nt 4055 resulted in a reduction of cytoplasmic RNA levels (Schneider et al., 1997b). Strain differences may account for subtle shifts in sequence motifs, nonetheless, regional effects are consistent in strain V reported here and He/80. Integrity of sequence between nt 4021 and 4072 in strain V (nt 4055–4100 in He/80) results in RNA instability, whereas sequence downstream of nt 4072 in strain V (nt 4070–4269 in He/80) partially attenuates this effect (Fig. 2B), presumably through enhanced nucleocytoplasmic transport of the unspliced 2.8 kb transcript (Schneider et al., 1997b).
The mechanism for the observed instability of BDV transcripts remains to be resolved. Recently, specific cytoplasmic foci were found to harbor enzymes for mRNA degradation (Cougot et al., 2004; Eystathioy et al., 2003; Ingelfinger et al., 2002). However, we were not able to implicate any pathway or protein through gel shift assays with the instability of the BDV sequences (data not shown).
Whereas other negative strand RNA viruses have a linear transcription gradient, such that levels of polymerase, needed only in catalytic amounts, are lower than those of structural proteins encoded 3′ on the genome, BDV is unusual in that levels of G are lower than those of the polymerase (L). The locations of the instability domains in the BDV genome (one in intron-2 and the other in the exon portion) correlate with the levels observed for the corresponding transcripts and proteins in vivo. The transcripts encoding M and L (intron-2 spliced transcripts that lack IE1) are present at higher levels than those encoding G (unspliced or intron-1 spliced transcripts containing IE1 and IE2), generating in BDV the discontinuous transcript gradient M>L>G. The restriction in G expression may facilitate persistent, non-cytopathic infection and account at least in part for the observation that the virus disseminates through cell-to-cell contact rather than virion release (Clemente and de la Torre, 2007).
Acknowledgement
We are grateful for support through National Institutes of Health grant NS29425.
References
- Abraham G, Banerjee AK. Sequential transcription of the genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. U.S.A. 1976;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev. 1992;6(4):642–654. doi: 10.1101/gad.6.4.642. [DOI] [PubMed] [Google Scholar]
- Brewer G. Evidence for a 3′-5′ decay pathway for c-myc mRNA in mammalian cells. J. Biol. Chem. 1999;274(23):16174–16179. doi: 10.1074/jbc.274.23.16174. [DOI] [PubMed] [Google Scholar]
- Briese T, de la Torre JC, Lewis A, Ludwig H, Lipkin WI. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89(23):11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Schneemann A, Lewis AJ, Park YS, Kim S, Ludwig H, Lipkin WI. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. U.S.A. 1994;91(10):4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone KM, Moench TR, Lipkin WI. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J. Neuropathol. Exp. Neurol. 1991;50(3):205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- Clemente R, de la Torre JC. Cell-to-cell spread of Borna disease virus proceeds in the absence of the virus primary receptor and furin-mediated processing of the virus surface glycoprotein. J. Virol. 2007;81(11):5968–5977. doi: 10.1128/JVI.02426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane AW, Jones KS, Beidas S, Dillon PJ, Skalka AM, Rosen CA. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J. Virol. 1991;65(10):5305–5313. doi: 10.1128/jvi.65.10.5305-5313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N, Babajko S, Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004;165(1):31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttet P, Fromont-Racine M, Steel D, Pictet R, Grange T. Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94(11):5628–5633. doi: 10.1073/pnas.94.11.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt B, Oldstone C, de la Torre JC. Sequence and genome organization of Borna disease virus. J. Virol. 1994a;68(3):1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt B, Oldstone C, Valcarcel J, Carlos de la Torre J. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a nonsegmented negative strand RNA virus. Virus Res. 1994b;34(1):69–79. doi: 10.1016/0168-1702(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Eystathioy T, Jakymiw A, Chan EK, Seraphin B, Cougot N, Fritzler MJ. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9(10):1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay DA, Yen TJ, Lau JT, Cleveland DW. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Herrick DJ, Ross J. The half-life of c-myc mRNA in growing and serum-stimulated cells: influence of the coding and 3′ untranslated regions and role of ribosome translocation. Mol. Cell. Biol. 1994;14(3):2119–2128. doi: 10.1128/mcb.14.3.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelfinger D, Arndt-Jovin DJ, Luhrmann R, Achsel T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA. 2002;8(12):1489–1501. [PMC free article] [PubMed] [Google Scholar]
- Korner CG, Wormington M, Muckenthaler M, Schneider S, Dehlin E, Wahle E. The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBOJ. 1998;17(18):5427–5437. doi: 10.1093/emboj/17.18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Rossi JJ. Control of HIV-1 replication by RNA interference. Virus Res. 2004;102(1):53–58. doi: 10.1016/j.virusres.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Maldarelli F, Martin MA, Strebel K. Identification of posttranscriptionally active inhibitory sequences in human immunodeficiency virus type 1 RNA: novel level of gene regulation. J. Virol. 1991;65(11):5732–5743. doi: 10.1128/jvi.65.11.5732-5743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, Felber BK. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J. Virol. 1994;68(5):2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R, Becht H. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- Saiga A, Orita S, Minoura-Tada N, Maeda M, Aono Y, Asakawa M, Nakahara K, Kubota R, Osame M, Igarashi H. cis-Acting inhibitory elements within the pol-env region of human T-cell leukemia virus type 1 possibly involved in viral persistence. J. Virol. 1997;71(6):4485–4494. doi: 10.1128/jvi.71.6.4485-4494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi SC, Wellington CL, Shyu AB, Chen CY, Greenberg ME, Belasco JG. Multiple elements in the c-fos protein-coding region facilitate mRNA deadenylation and decay by a mechanism coupled to translation. J. Biol. Chem. 1994;269(5):3441–3448. [PubMed] [Google Scholar]
- Schneemann A, Schneider PA, Kim S, Lipkin WI. Identification of signal sequences that control transcription of borna disease virus, a nonsegmented, negative-strand RNA virus. J. Virol. 1994;68(10):6514–6522. doi: 10.1128/jvi.68.10.6514-6522.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PA, Kim R, Lipkin WI. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J. Virol. 1997a;71(7):5614–5619. doi: 10.1128/jvi.71.7.5614-5619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PA, Schneemann A, Lipkin WI. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J. Virol. 1994;68(8):5007–5012. doi: 10.1128/jvi.68.8.5007-5012.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PA, Schwemmle M, Lipkin WI. Implication of a cis-acting element in the cytoplasmic accumulation of unspliced Borna disease virus RNAs. J. Virol. 1997b;71(11):8940–8945. doi: 10.1128/jvi.71.11.8940-8945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 1997c;71(7):4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J. Virol. 1992;66(1):150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle M, De B, Shi L, Banerjee A, Lipkin WI. Borna disease virus P-protein is phosphorylated by protein kinase Cepsilon and casein kinase II. J. Biol. Chem. 1997;272(35):21818–21823. doi: 10.1074/jbc.272.35.21818. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5(2):221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Sokolowski M, Furneaux H, Schwartz S. The inhibitory activity of the AU-rich RNA element in the human papillomavirus type 1 late 3′ untranslated region correlates with its affinity for the elav-like HuR protein. J. Virol. 1999;73(2):1080–1091. doi: 10.1128/jvi.73.2.1080-1091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M, Schwartz S. Heterogeneous nuclear ribonucleoprotein C binds exclusively to the functionally important UUUUU-motifs in the human papillomavirus type-1 AU-rich inhibitory element. Virus Res. 2001;73(2):163–175. doi: 10.1016/s0168-1702(00)00238-0. [DOI] [PubMed] [Google Scholar]
- Sokolowski M, Tan W, Jellne M, Schwartz S. mRNA instability elements in the human papillomavirus type 16 L2 coding region. J. Virol. 1998;72(2):1504–1515. doi: 10.1128/jvi.72.2.1504-1515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Felber BK, Zolotukhin AS, Pavlakis GN, Schwartz S. Efficient expression of the human papillomavirus type 16 L1 protein in epithelial cells by using Rev and the Rev-responsive element of human immunodeficiency virus or the cis-acting transactivation element of simian retrovirus type 1. J. Virol. 1995;69(9):5607–5620. doi: 10.1128/jvi.69.9.5607-5620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Tazi J. mRNA degradation machines in eukaryotic cells. Biochimie. 2002;84(8):821–837. doi: 10.1016/s0300-9084(02)01445-1. [DOI] [PubMed] [Google Scholar]
- Walker MP, Jordan I, Briese T, Fischer N, Lipkin WI. Expression and characterization of the Borna disease virus polymerase. J. Virol. 2000;74(9):4425–4428. doi: 10.1128/jvi.74.9.4425-4428.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund L, Sokolowski M, Carlsson A, Rush M, Schwartz S. Inhibition of translation by UAUUUAU and UAUUUUUAU motifs of the AU-rich RNA instability element in the HPV-1 late 3′ untranslated region. J. Biol. Chem. 2002;277(43):40462–40471. doi: 10.1074/jbc.M205929200. [DOI] [PubMed] [Google Scholar]
- Wilson GM, Brewer G. The search for trans-acting factors controlling messenger RNA decay. Prog. Nucleic Acid Res. Mol. Biol. 1999;62:257–291. doi: 10.1016/s0079-6603(08)60510-3. [DOI] [PubMed] [Google Scholar]
- Yen TJ, Gay DA, Pachter JS, Cleveland DW. Autoregulated changes in stability of polyribosome-bound beta-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol. Cell. Biol. 1988;8(3):1224–1235. doi: 10.1128/mcb.8.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin AS, Michalowski D, Bear J, Smulevitch SV, Traish AM, Peng R, Patton J, Shatsky IN, Felber BK. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol. Cell. Biol. 2003;23(18):6618–6630. doi: 10.1128/MCB.23.18.6618-6630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]