Abstract
Background
It is well known that peroxisome proliferator-activated receptor gamma (PPARγ), a ligand-activated transcription factor, plays a protective role in anti-inflammatory responses in both acute and chronic central nerve system (CNS) insults. Emerging evidence in rats suggests that vagus nerve stimulation (VNS), while restraining inflammatory cytokine production in the peripheral nervous system, also exerts a significant CNS neuroprotective function against ischemic stroke injury. The aim of this study was to explore the role of PPARγ in VNS-mediated anti-inflammatory protection against ischemic stroke damage.
Material/Methods
Adult male Sprague-Dawley rats (total n=160) preconditioned through transfection with either PPARγ small interfering RNA (siRNA) or lentiviral vector without siRNA and surgically subjected to middle cerebral artery occlusion and reperfusion subsequently received VNS treatment at 30 min post-occlusion. The expression of PPARγ after VNS treatment was measured by real-time PCR and Western blotting, also supported by immunofluorescence staining. Subsequently, the neurological deficits scores, the infarct volume, and the brain histopathology were all evaluated. Additionally, the influence on the pro-inflammatory cytokines expression and neuro-immune cells activation was determined by ELISA and immunofluorescence staining.
Results
We found that VNS upregulated expression of PPARγ in ischemia penumbra, diminished the extent of ischemic infarct, alleviated neuronal injury, and suppressed pro-inflammatory cytokine expression and immune cell activation (P<0.05). However, rats with PPARγ silencing failed to manifest significant neuroprotection and anti-inflammatory effect induced by VNS treatment (p>0.05).
Conclusions
PPARγ may participate in the process by which VNS modulates the neuro-inflammatory response following ischemia/reperfusion in rats.
MeSH Keywords: Cholinergic Fibers; Hypoxia-Ischemia, Brain; Peroxisome Proliferator-Activated Receptors; Vagus Nerve Stimulation
Background
Despite the current effective clinical treatment for acute ischemic stroke – the introduction of recombinant tissue-type plasminogen activator (tPA) within 4.5 h of the event [1] – such rapid restoration of cerebral blood flow also has some drawbacks. It impairs auto-regulatory mechanisms and initiates an excessive inflammatory response that exacerbates ischemic injury, and is therefore potentially detrimental to the patient, negatively influencing prognosis [2,3]. Hence, a deeper understanding of the mechanisms of post-ischemic inflammation is needed to spur the development of improved therapeutic measures.
The parasympathetic nervous system regulates multiple aspects of physiology beyond the well-known control of heart rate, additionally affecting hormone secretion, gastrointestinal peristalsis, digestion during ingestion, and finally, control of immune responses [4]. Activating vagus nerve efferents (vagus nerve stimulation [VNS]) has been shown to significantly decrease systemic levels of pro-inflammatory mediators such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and the DNA-binding protein, high-mobility group box 1 (HMGB-1) in experimental models of endotoxemia. This immune-system modulation is mainly due to the binding of acetylcholine (Ach) to its alpha-7 nicotinic receptor (α7nAChR) expressed on macrophages [5], and essentially constitutes a physiological anti-inflammatory system. The clinical potential of VNS is not necessarily limited to systemic inflammation, as found, for example, in the experimental models of sepsis, ischemia/reperfusion, refractory partial epilepsy, hemorrhagic shock, myoarthritis, and pancreatitis cardial ischemia, ileus, experimental [6,7]. There is also emerging evidence that electrical stimulation of the vagus nerve has a potential neuroprotective action – reducing infarct volume during the acute phase of ischemic stroke [8]. To the best of our knowledge, the detailed mechanism of this action has not yet been well established.
The nuclear transcription factor peroxisome proliferator-activated receptor (PPARγ) traditionally plays an important role in regulating adipocyte differentiation, lipid metabolism, and insulin resistance [9]. It has also been shown to inhibit the synthesis and secretion of pro-inflammatory cytokines in multiple central nervous system diseases and brain renin-angiotensin system activity [10]. Although the PPARγ-specific ligand thiazolidinediones (TZD) can exert significant anti-inflammatory protection, the compounds also have adverse effects, including weight gain, peripheral edema, cardiac load, and hemodilution. Hence, a superior clinical approach that is both efficacious and better tolerated with respect to adverse-effects profile is needed for the treatment of stroke patients.
Recent research has shown that nicotine modulates the dendritic cell (DC) phenotype by up-regulation of PPARγ gene expression via α7nAchR, and that DCs influence inflammatory cytokine synthesis and co-inhibitory molecule expression in vitro [11]. Importantly, the distribution of PPARγ expression includes the hippocampus, corpus striatum, hypothalamic paraventricular nucleus, basal ganglia, thalamus, and piriform cortex [12]; some of these brain regions are also innervated by afferent fibers of the vagus nerve. Studies also suggested that PPARγagonists exert anticonvulsive effects in epilepsy, possibly through inhibiting oxidative stress and inflammation [13]. It had been reported that the antiepileptic effect induced by VNS might be related to the increased convulsive threshold of medial temporal structures [14]. To date there has been no in vivo study on the relationship between PPARγ and VNS in the brain. However, based on the brain distribution of PPARγ and these previous findings, a logical hypothesis associates the neuroprotective mechanisms of VNS with increasing PPARγ expression. To test this hypothesis, our study investigated the role of endogenous PPARγ in anti-inflammatory actions induced by VNS during reperfusion after stroke, a mechanism thought to reduce neuronal injury in the brain.
Material and Methods
Animal preparation
Adult male Sprague-Dawley rats weighing 250–300 g (n=160) were obtained from the Experimental Animal Center of the Chongqing Medical University. Experimental protocols followed the Guidelines for the Care and Use of Laboratory Animals approved by the Institutional Ethics Committee of Chongqing Medical University (Permit No. SCXK [Chongqing] 2007-0001) and the State Science and Technology Commission of China. Rats were housed in standard conditions of temperature (22–24°C), humidity (60%) and a 12-h light/dark cycle, with food and water freely available. The experiments were divided into 2 parts and rats were randomly assigned to the experimental groups required for each experiment. The first experiment included 3 groups of animals: (1) ischemia/reperfusion group (I/R group), (2) ischemia/reperfusion + sham stimulation group (I/R+SS group), and (3) ischemia/reperfusion + vagus nerve stimulation group (I/R+VNS group). The second experiment, utilizing PPARγ siRNA, was designed to detect the role of PPAR in the VNS-mediated anti-inflammatory response: (1) I/R+LV-control group, (2) I/R+VNS+LV-control group, (3) I/R+ LV-shPPARr group, and (4) I/R+VNS+LV-shPPARr group.
In vivo lentivirus injection
Lenti-PPARγ (NM_013124) or its vector was constructed by a reagent company (Neuronbiotech, Shanghai, China). The sequence of PPARγ siRNA was the following: sense – 5′-CCUCCC UGAU GAAUAAAGATT-3′; antisense – 5′-UCUUUAUUCAUCAGGG AGGT T-3′. Seven days before subjecting animals to middle cerebral artery occlusion (MCAO), rats (n=8 per group) were anesthetized and subsequently placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The total volume 4 μL of concentrated viral solution or its control was delivered bilaterally into the lateral ventricle using a cannula (outer diameter, 0.2 mm) connected to a micro-syringe (Hamilton Instruments, Bordentown, NJ, USA), at an injection rate of 0.2 μL/min (coordinates from bregma: anteroposterior, 1.1 mm, mediolateral, 0.8 mm, and dorsoventral, 4.2 mm). At the end of injection, the needle was left in place for an additional 10 min to reduce backflow of the liquid along the injection void.
Right middle cerebral artery occlusion /reperfusion and electrical stimulation of the vagus nerve
Rats were deeply anesthetized and normothermia was maintained with a heating plate, and with continuous monitoring blood pressure and heart rate obtained during the experimental process, as reported in a previous study [15]. Transient focal cerebral ischemia was induced by the intraluminal filament occlusion model using a silicon-coated 4-0 nylon filament, as described previously [16]. To ensure the success of the MCAO model, regional cerebral blood flow was monitored by using continuous laser Doppler flowmetry (LDF) (Perimed, North Royalton, OH, USA) during occlusion and early reperfusion. Rats with an occluded blood flow reduction of less than 70% or a rapid restoration of the LDF signal during reperfusion were excluded from the study.
During transient MCAO of 30 min, animals were maintained under anesthesia and received right cervical vagal nerve stimulation, delivered by a Grass Model S48 stimulator and constant current unit according to methods of our previous study [17]. The stimulating electrodes were self-constructed using the method of Smith, and were composed of 2 curved silver wires covered with polyethylene and fixed 1.5 mm apart by a solid bar. Electrodes were sutured to the sternocleidomastoid muscle and wrapped around the right cervical nerve under a microscope controller, and a 30-s train of 0.5-ms square pulses (0.5 mA, 20 HZ), was repeatedly delivered every 5 min for 60 min [8]. At 2 h after MCAO surgery, reperfusion was performed. After recovery from anesthesia, rats were returned to their cages and allowed free access to food and water.
Neurological deficits scores and evaluation of cerebral infarct volume
At 24 h post-reperfusion, all rats were subjected to a neurological scores assessment by a blinded assessor, using a 12-point scale that included assessment of postural reflex, visual placing in forward and sideways directions, tactile placing of the dorsal and lateral paw surfaces, and proprioceptive placing [18]. After this neurological assessment, rats n=8 per group were sacrificed using an anesthesia overdose and their brains were rapidly excised and frozen for 15 min. The freshly frozen brains were coronally sectioned into five 2-mm-thick slices using a brain matrix, stained with a 2% solution of 2,3,5-triphenyltetrazolium chloride in phosphate-buffered saline (PBS) at 37°C for 15 min, and then fixed with 10% formalin in PBS. The stained slices were captured for quantification of infarct volume, which was calculated by multiplying infarct area by slice thickness.
Histological examination
At 24 h post-reperfusion, rats (n=6 per group) were deeply anesthetized and transcardially perfused with 0.9% normal saline and then with ice-cold 4% paraformaldehyde (Sigma-Aldrich, Inc., St. Louis, MO, USA) in 0.1-M phosphate buffer (PB; pH 7.5); brains were incubated in 10% formalin for 24 h at room temperature, dehydrated through a series of graded ethanols, and embedded in paraffin. Brains were sectioned into 5-μm thickness, dewaxed in xylene, passed through a decreasing alcohol gradient, and further stained with hematoxylin for 5–10 min and eosin for 2–3 min (H&E staining) as described previously [19]. Using 6 images from 6 randomly selected visual fields, brain histopathology was observed under a light microscope at 400× magnification.
Real-time reverse transcriptase (RT)-polymerase chain reaction (PCR)
The following primers were used. For PPARγ, forward: 5′-CAC AAT GCC ATC AGG TTT GG-3′; reverse: 5′-GCT GGT CGA TAT CAC TGG AGA TG-3′. For βactin, forward: 5′-AGA GGG AAA TCG TGC GTG AC-3′; reverse: 5′-CCA TAG TGA TGA CCT GTC CGT-3′. Total RNA (n=8 per group) was isolated from brain tissue using Trizol reagent (Gibco BRL, Rockville MD, USA), according to the manufacturer’s instructions. cDNA was synthesized using 2 μg of total RNA in a 25 μL total reaction volume using a reverse transcription kit (Takara, Japan). The conditions for amplification were as follows: 95°C for 5 min; 40 cycles of 10 s at 95°C and 30 s at 60°C. Each amplification was repeated 3 times from different RT reactions. Relative gene expression was calculated using the 2−ΔΔCt method.
Western blot analysis
The regions of ischemia and penumbra in the right side of each MCAO brain (n=8 per group) were isolated on ice and homogenized in lysis buffer. Protein concentrations were measured with a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equivalent amounts of protein were separated on 10% SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed with rabbit anti-PPARγ (1:1000 Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight and subsequently incubated with anti-rabbit peroxidase-conjugated secondary antibody (1:2000, Sigma-Aldrich) for 1 h at room temperature. The blots were quantified and normalized with glyceraldehyde-3-phosphate dehydrogenase (GADPH) by scanning with a ScanJet scanner (Hewlett Packard, Inc. Palo Alto, CA, USA).
Immunofluorescence staining
Rats (n=8 per group) were anesthetized and perfused transcardially with 4% paraformaldehyde (PFA). Twelve-μm-thick frozen brain sections were harvested after fixation in 4% PFA for 24 h. Briefly, slices were blocked in donkey serum for 30 min, then incubated overnight at 4°C with primary antibody (PPARγ 1:200, Cell Signaling Technology Novus, Cambridge, UK), GFAP (glial fibrillary acidic protein) 1:200 (Cell Signaling Technology), and microglia-specific Iba-1 (ionized calcium binding adaptor molecule 1, Cell Signaling Technology), at 1:500. Secondary antibody incubation occurred sequentially for 1 h at room temperature and slices were subsequently washed with PBS for 30 min. Images were captured at 400× magnification using a Laser Scanning Confocal Microscope (LEICA, TCS SP2, Germany).
Measurement of inflammatory markers by enzyme-linked immunosorbent assay (ELISA)
To measure inflammatory cytokine levels in the ischemic penumbra, rat brain tissues (n=8 per group) were homogenized in sodium phosphate buffer (pH=7.4) and centrifuged at 14 000 g for 5 min to extract the supernatant. Assays for TNF-α and IL-1β were carried out according to manufacturer’s instructions (Hushing, Shanghai, China) and read at 450 nm in a microplate reader (Bio-Tek, Inc., Winooski, VT, USA).
Statistical analysis
All data are expressed as mean ±SEM in triplicate. Comparisons between multiple groups were conducted with one-way ANOVA followed by a post-hoc Tukey multiple-comparison test. Neurological scores were analyzed with the Kruskal-Wallis test. Two group comparisons were analyzed by the two-tailed t-test. Differences in values were considered significant at p<0.05.
Results
Physiological parameters
Physiological parameters exhibited no significant differences among experimental groups. Body temperatures were also within normal ranges during the experiment. Likewise, the mean values of blood pressure and heart rate during the experiment were not significantly different between groups, consistent with our previous study [20].
PPARr expression in ischemic penumbra was enhanced by VNS treatment
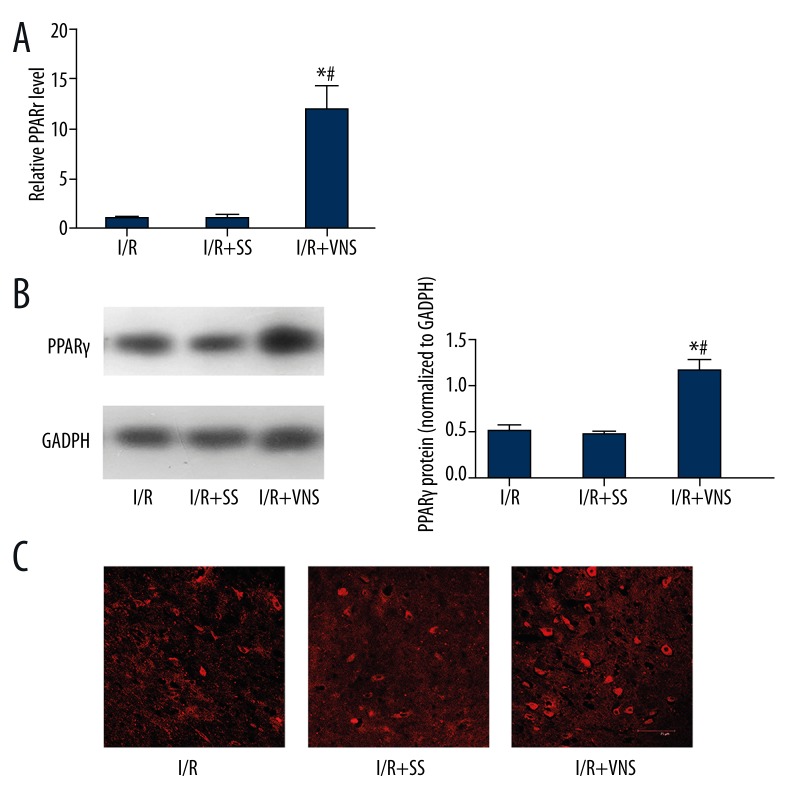
To explore the possible relationship between the protective mechanisms of VNS and PPARγ, we detected PPARγ expression in the cortical ischemic penumbra at 24 h post-I/R in rats treated with VNS compared with untreated animals. As shown in Figure 1, PPARγ mRNA levels were significantly upregulated in the I/R+VNS group as compared to the I/R and I/R+SS groups (p<0.05). Correspondingly, stimulation also enhanced PPARγprotein expression around the ischemic boundary in the I/R+VNS group but not in the I/R and I/R+SS group. Thus, it seemed that a dramatic increase of PPARγ expression in the brain was due to a brief and repeated VNS. To confirm the localization of PPARγ expression, immunofluorescence staining was also performed. As shown in Figure 1, PPARγ was expressed in a perinuclear location in neurons in the cerebral cortex and its expression was elevated after subjected to VNS. These results indicate that at least a portion of the PPARγ expression up-regulation after ischemic insult in the rat brain is induced by VNS.
Figure 1.
Upregulation of PPARγ expression induced by VNS at 24 h post-reperfusion in the cerebral ischemic cortex. (A) PPARγ gene expression induced by VNS in rat brain measured by RT-PCR. Data are presented as mean ±SEM. (*p<0.05 compared with I/R group and #p<0.05 compared with I/R+SS group.) (B) PPARγ protein level after VNS assessed by Western blot at 24 h post-MCAO. Data are presented as mean ±SEM. A: I/R group B: I/R+SS group C: I/R+VNS group. (*p<0.05 vs. I/R group, #p<0.05 vs. I/R+SS group). (C) Immunofluorescence staining showing peri-nuclear PPARγ expression in the ischemic penumbra, and VNS treatment leading to an increase in PPARγ protein expression, A: I/R group; B: I/R+SS group; C: I/R+VNS group. Scale bar=75 μm.
Decrease of neuroprotection induced by VNS in cerebral I/R rats by PPARr silencing
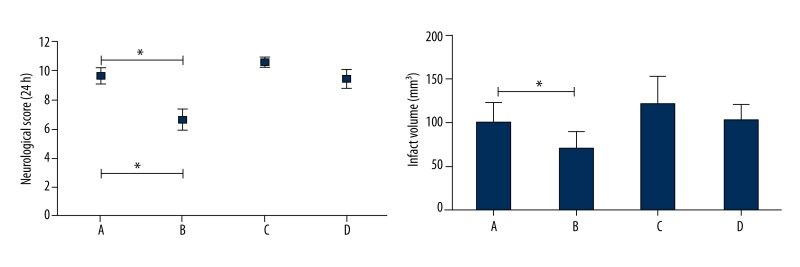
Rats with PPARr silencing or without were pretreated as described in the Methods section above. Subsequent to transient MCAO and VNS treatment at 24 h, neurological-deficits scores and infarct sizes were evaluated. Results showed that VNS significantly improved neurological scores and decreased infarct volume compared to that in the I/R+LV-control group (p<0.05), while after PPARr silencing there were no significant differences in neurological scores and brain infarct size when comparing the I/R+VNS+ PPARrsiRNA group with compared I/R+VNS+ LV-shPPARr with I/R+LV-shPPARr group. As shown in Figure 2, these results suggest that PPARr was involved in the beneficial effects of VNS on transient cerebral I/R+LV-control group in rat.
Figure 2.
Neuroprotective effect induced by VNS was reduced after PPARr silencing during cerebral I/R insult. VNS improved the neurological deficits scores and reduced the cerebral infarct volume at 24 h post-reperfusion in rats. However, after PPRAr silencing, there were no significant differences in neurological recovery and infarct volume between I/R+LV-shPPARr and I/R+VNS+LV-shPPARr group. Data are mean ± SEM. *p<0.05 compared with I/R+ LV-control group. A: I/R+ LV-control group, B: I/R+VNS+LV-control group, C: I/R+LV-shPPARr group, D: I/R+VNS+ LV-shPPARr group.
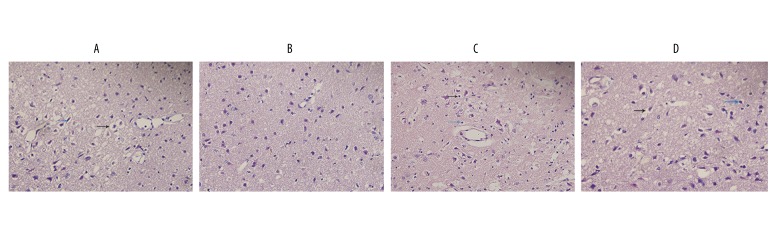
Exacerbation of neuronal necrosis in the ischemic penumbra after PPARr silencing
At 24 h post-reperfusion, brain histopathology was evaluated in HE-stained sections. In normal, untreated animals (data not shown), neuron morphology exhibits regular cellular and organelle structure with a regular neuronal arrangement. As shown in Figure 3, in the I/R group neurons were sparse (presumably the outcome of widespread necrosis) and these neurons that did survive exhibited vacuolization, disordered structure, swelling, interstitial edema, and severe cell deformation, with karyopyknotic nuclei; additionally, the neuropil exhibited a loosening compared to the normally tightly packed nerve fibers, indicating disintegration due to necrosis. Compared with the I/R+LV-control group, the scope of brain damage after VNS treatment (I/R+VNS+LV-control group) appeared to be diminished, with denser neuropil and a greater density of surviving neurons, with relatively distinct cell outlines and relatively intact internal structure. After PPARr silencing, there were no significant differences in brain histopathology between I/R+LV-shPPARr and I/R+VNS+LV-shPPARr group. These results show that PPARr has an important role in the neuroprotective effect of VNS in mediating cerebral ischemia injury.
Figure 3.
Histopathological changes at 24 h post-reperfusion after VNS treatment in rat with PPARr silencing or vehicle control. Brain slices were examined at a magnification of 400×. A: I/R+ LV-control group, B: I/R+VNS+LV-control group, C: I/R+LV-shPPARr group, D: I/R+VNS+ LV-shPPARr group. Black arrows represent perinuclear vacuolization and blue arrows represent pyknotic nuclei.
A PPARγ-mediated mechanism is responsible for VNS suppression of inflammatory cytokine secretion in the ischemic cerebral penumbra
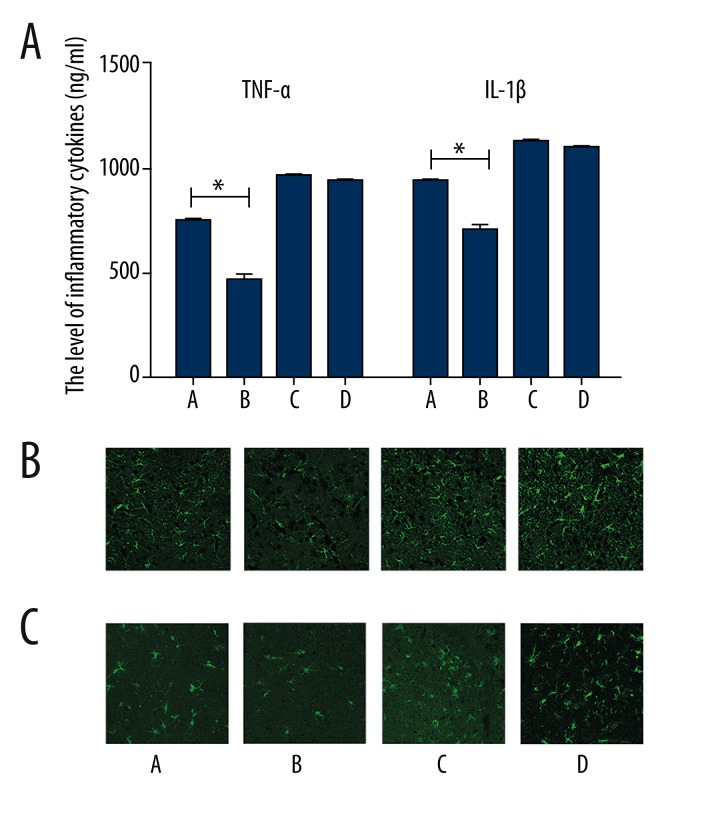
To investigate the role of PPARr in the VNS-mediated anti-inflammatory effect at the acute phase of cerebral I/R, ELISA was used to measure pro-inflammatory cytokine (TNF-α, IL-1β,) levels at 24 h post-I/R. As shown in Figure 4, results indicated that the levels of CNS inflammatory cytokines were downregulated in I/R+VNS+LV-control group than in I/R+LV-control group (p<0.05), supporting the idea that the neuroprotection induced by VNS is at least partly associated with a reduction in CNS inflammatory response. However, no significant differences in proinflammatory cytokine expression was shown I/R+VNS+LV-shPPARr and I/R +LV-shPPARr groups.
Figure 4.
The level of pro-inflammatory cytokines expression at 24 h post-reperfusion. (A) TNF-α and IL-1β in cerebral ischemic penumbra determined by ELISA. Data are presented as mean ±SEM. (*p<0.05 vs. I/R+ vehicle control group). (B) The number of activated astrocytes and microglia increased after transfection with PPARγ siRNA. Scale bar=75 μm. A: I/R+ LV-control group, B: I/R+VNS+LV-control group, C: I/R+LV-shPPARr group, D: I/R+VNS+ LV-shPPARr group.
After CNS insults, the proinflammatory cytokines could be rapidly generated by astrocytes and microglia, which are responsible for the anti-inflammatory response. We examined the changes of the immunoreactive cells by immunofluorescence staining and found that the density of the activated astrocytes and microglia was reduced in the ischemic penumbra in the I/R+VNS+LV-control group than I/R+ LV-control group. It should be noted that no significant differences exist between I/R+VNS+LV-shPPARr and I/R+LV-shPPARr group. These data suggest that the neuroprotective actions of VNS are partly due to suppression of inflammatory response and the inner anti-inflammatory mechanism was probably PPARr-dependent. PPARr deficit influenced the anti-inflammatory effect of VNS mediation.
Discussion
A number of potentially neuroprotective mechanisms appear to evoke upregulated gene transcription at the onset of brain ischemia, which could be a timely and effective mechanism for limiting brain tissue damage. Victor et al. demonstrated that PPARγexpression was markedly increased in neurons at 24 h post-reperfusion, representing a pro-survival mechanism for delaying neuronal death [21]. In our study, we found that after brain ischemia, PPARγmRNA and protein level were increased, and both were further upregulated after VNS treatment. Moreover, we noticed that the distribution of PPARr was mainly located in the cerebral cortex neuron.
It is well known that VNS is an effective FDA-approved clinical treatment for refractory partial-onset seizures and treatment-resistant depression [22], and there is emerging evidences of its therapeutic potential for migraine, Alzheimer disease, and brain ischemia and infarct [23–25]. Ilknur et al. demonstrated that VNS initiated within 30 min following cerebral MCAO is capable of reducing infarct volume by nearly 50%, as well as demonstrating an improved neurological score in rats [26]. In a cardiac study in rats [27], protection afforded by VNS against myocardial ischemia depended on inhibition of cytokine synthesis through activation of α7nAchR. Such results raise concerns with respect to the mechanism of VNS’s beneficial effect in ischemic stroke. Consistent with previous studies, the rats treated with VNS in our study had better neurological scores and reduced infarct volume at 24 h post-ischemia, but we also found that rats with PPARr siRNA were more sensitive to cerebral ischemia. Although in rats with PPARr siRNA subjected to VNS treatment, the improvement was not significant after PPARr silencing. Brain histopathology findings also supported the above results at the neuronal level. All these data clearly indicate that VNS can protect neurons from ischemic stroke insult, and it appears that PPARr might participate in the VNS-induced neuroprotection.
More recently, abundant evidence has shown that PPARγ activation appears to be involved in inhibiting inflammation in animal models of inflammatory bowel disease, multiple sclerosis, and arthritis. In addition, PPARγ agonists likewise exert anti-inflammatory effects in experimental autoimmune encephalomyelitis (EAE) [28]. Rats treated pioglitazone to increase PPARγ activity showed decreased inflammatory response, reduced neuron death, and microglia and astrocyte activation, leading to an improvement of motor function [29]. Thus, the function of PPARγ activation exerted anti-inflammatory effects not only in peripheral organs, but also within the central nervous system (CNS) after acute and chronic insults.
VNS strongly reduces lipopolysaccharide (LPS)-induced serum TNF-α response and the related development of endotoxic shock, and there is speculation that the role of the vagus nerve in stroke may partly encompass this anti-inflammatory effect as well [5]. In our study, we found that proinflammatory cytokines expression were significantly downregulated after VNS treatment. After PPARγ silencing, there was no significant difference between VNS-treated and non-treated groups. Microglial and astrocyte activation were reinforced in the ischemic boundary as well. Considering these results, it appears that PPARγ is at least partially involved in mediating the anti-inflammatory effect of VNS. There may be several reasons for changes in PPARγ expression induced by VNS. There is a growing body of supportive evidence that activation of a7nAchR occurs to enhance neuronal survival in various CNS insults. Moreover, according to a previous study, nicotine strongly activated the dendritic cell (DC) – mediated immune response, accompanied by reduced inflammatory cytokine secretion via the upregulation of PPARγ [11]. Pretreatment of DCs with d-tubocurarine, a nonselective nAchR antagonist, could prevent the induction of PPARγ expression and inhibit inflammatory cytokine production. In addition, nicotine enhances PPARγ expression in human monocytes and monocyte-derived macrophages via α7nAchR inhibition by the selective antagonist α-bungarotoxin. These data show that the up-regulation of PPARγ expressions is partly associated with α7nAchR activity [30]. Therefore, VNS may upregulate PPARγ gene expression via activation of α7nAchR. This does not, of course, exclude other mechanisms that may be involved in VNS-mediated PPARγ upregulation. Serotonin, a brain neurotransmitter, caused enhanced secretion from dorsal raphe nucleus neurons under stimulation by norepinephrine, which in turn could result from VNS-mediated enhanced secretion from the locus coeruleus. This mechanism might also underlie the clinically relevant antidepressant effect of VNS. It has also been found that serotonin can promote PPARγ activation in fat cells, similar to the PPARγ agonist, rosiglitazone [31]. Based on these findings, we speculate that PPARγ upregulation may be associated to some extent with VNS-stimulated serotonin release. It will be of interest to test whether other mechanisms underlie such VNS actions.
Conclusions
Our study has found that VNS can suppress pro-inflammatory cytokine release via upregulation of PPARγ expression. These results strongly suggest that the anti-inflammatory mechanism of VNS following cerebral I/R might be at least partly due to such PPARγ upregulation. Therefore, appropriately timed VNS can be an effective therapy for mitigating post-ischemic brain damage.
Abbreviations
- PPARγ
peroxisome proliferator-activated receptor γ
- VNS
vagus nerve stimulation
- I/R
Ischemia/reperfusion
- a7nAchRs
a7 – nicotinic acetylcholine receptor
- MCAO
middle cerebral artery occlusion
- TNF-α
tumor necrosis factor α
- IL-6
interleukin 6
- IL-1β
interleukin1β
- HMGB-1
High-mobility group box-1 protein
- TZD
thiazolidinediones
- ELISA
Enzyme-linked immunosorbent assay
- CNS
central nervous system
- tPA
tissue-type plasminogen activator
- RT-PCR
real-time reverse transcriptase polymerase chain reaction
- LDF
laser Doppler flowmetry
- GFAP
glial fibrillary acidic protein
- Iba-1
ionized calcium binding adaptor molecule 1
Footnotes
Source of support: This work was supported by the National Nature and Science Foundation of China under Grant No. 81271306
References
- 1.Harston GW, Sutherland BA, Kennedy J, Buchan AM. The contribution of L-arginine to the neurotoxicity of recombinant tissue plasminogen activator following cerebral ischemia: a review of rtPA neurotoxicity. J Cereb Blood Flow Metab. 2010;30:1804–16. doi: 10.1038/jcbfm.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung SY, Lin JY, Lin MC, et al. Synergistic efficacy of magnesium sulfate and FK506 on cerebral ischemia-induced infarct volume in gerbil. Med Sci Monit. 2004;10(4):BR105–8. [PubMed] [Google Scholar]
- 3.Gu L, Huang B, Shen W, et al. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi. J Neuroinflammation. 2013;10:109. doi: 10.1186/1742-2094-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheyuo C, Jacob A, Wu R, et al. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–95. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tecoma ES, Iragui VJ. Vagus nerve stimulation use and effect in epilepsy: what have we learned? Epilepsy Behav. 2006;8:127–36. doi: 10.1016/j.yebeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ay I, Lu J, Ay H, Sorensen AG. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–51. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Guo SW. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor agonists synergistically suppress proliferation of immortalized endometrial stromal cells. Fertil Steril. 2009;91:2142–47. doi: 10.1016/j.fertnstert.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–26. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagita M, Kobayashi R, Kojima Y, et al. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-γ upregulation. Cell Immunol. 2012;274:26–33. doi: 10.1016/j.cellimm.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Martin HL, Mounsey RB, Mustafa S, et al. Pharmacological manipulation of peroxisome proliferator-activated receptor γ (PPARγ) reveals a role for anti-oxidant protection in a model of Parkinson’s disease. Exp Neurol. 2012;235:528–38. doi: 10.1016/j.expneurol.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S, Xin Y, HaiQin W, et al. The PPARγ agonist rosiglitazone prevents neuronal loss and attenuates development of spontaneous recurrent seizures through BDNF/TrkB signaling following pilocarpine-induced status epilepticus. Neurochem Int. 2013;63:405–12. doi: 10.1016/j.neuint.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Rafael H, Moromizato P. Vagus stimulator for seizures. J Neurosurg. 1993;79:636–37. doi: 10.3171/jns.1993.79.4.0636. [DOI] [PubMed] [Google Scholar]
- 15.Hiraki T, Baker W, Greenberg JH. Effect of Vagus Nerve Stimulation During Transient Focal Cerebral Ischemia on Chronic Outcome in Rats. J Neurosci Res. 2012;90:887–94. doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–22. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 17.Smith DC, Modglin AA, Roosevelt RW, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Sanberg P, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–88. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 19.Tabassum R, Vaibhav K, Shrivastava P, et al. Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci. 2013;34:925–33. doi: 10.1007/s10072-012-1163-1. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Li L, Liu B, et al. vagus nerve stimulation alleviates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;9:e102342. doi: 10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor NA, Wanderi EW, Gamboa J, et al. PPARγamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–63. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- 22.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–38. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 23.Goadsby P, Grosberg B, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia. 2014 doi: 10.1177/0333102414524494. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Polak T, Dresler T, Zeller JB, et al. Vagus somatosensory evoked potentials are delayed in Alzheimer’s disease, but not in major depression. Eur Arch Psychiatry Clin Neurosci. 2014;264:263–67. doi: 10.1007/s00406-013-0415-2. [DOI] [PubMed] [Google Scholar]
- 25.Shinlapawittayatorn K, Chinda K, Palee S, et al. Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 2013;10:1700–7. doi: 10.1016/j.hrthm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: An unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–15. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mioni C, Bazzani C, Giuliani D, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–28. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 28.Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S. PPAR Regulation of Inflammatory Signaling in CNS Diseases. PPAR Res. 2008;2008:658520. doi: 10.1155/2008/658520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordet R, Ouk T, Petrault O, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–46. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 30.Amoruso A, Bardelli C, Gunella G, et al. Quantification of PPAR-gamma protein in monocyte/macrophages from healthy smokers and non-smokers: a possible direct effect of nicotine. Life Sci. 2007;81:906–15. doi: 10.1016/j.lfs.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Grès S, Gomez-Zorita S, Gomez-Ruiz A, Carpéné C. 5-hydroxytry ptamine actions in adipocytes: involvement of monoamine oxidase-dependent oxidation and subsequent PPARγ activation. J Neural Transm. 2013;120:919–26. doi: 10.1007/s00702-012-0959-8. [DOI] [PubMed] [Google Scholar]