Abstract
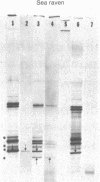
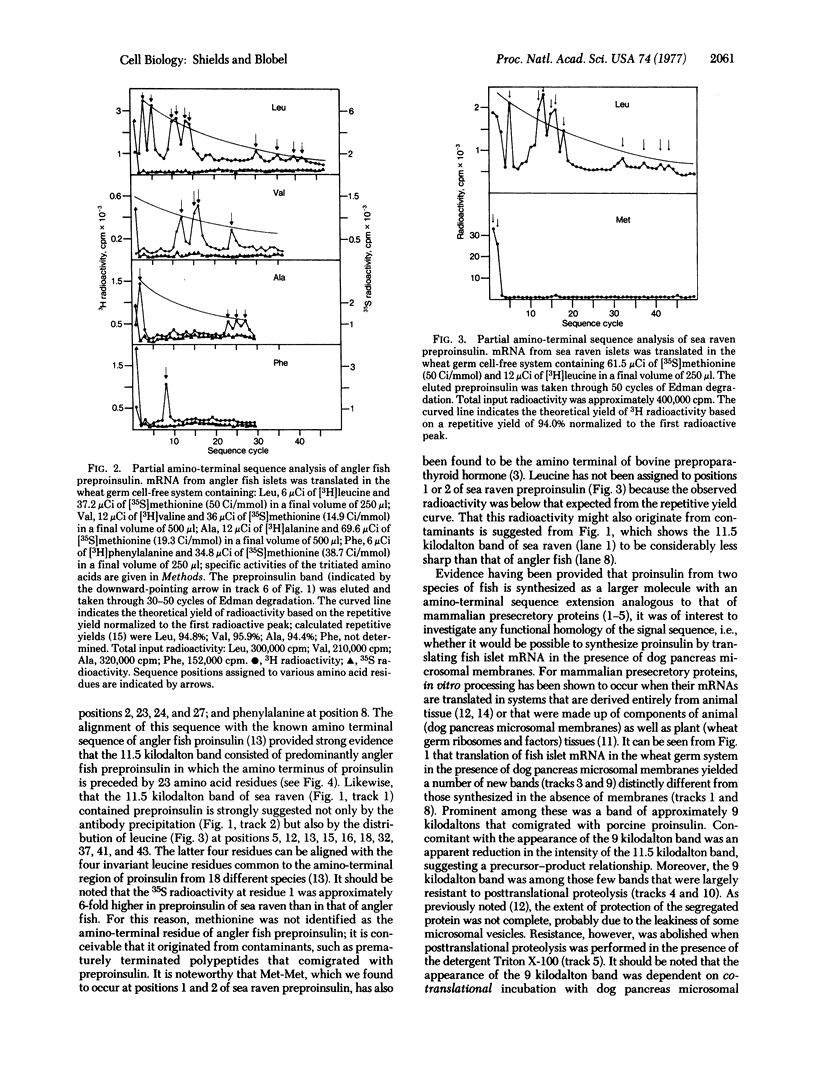
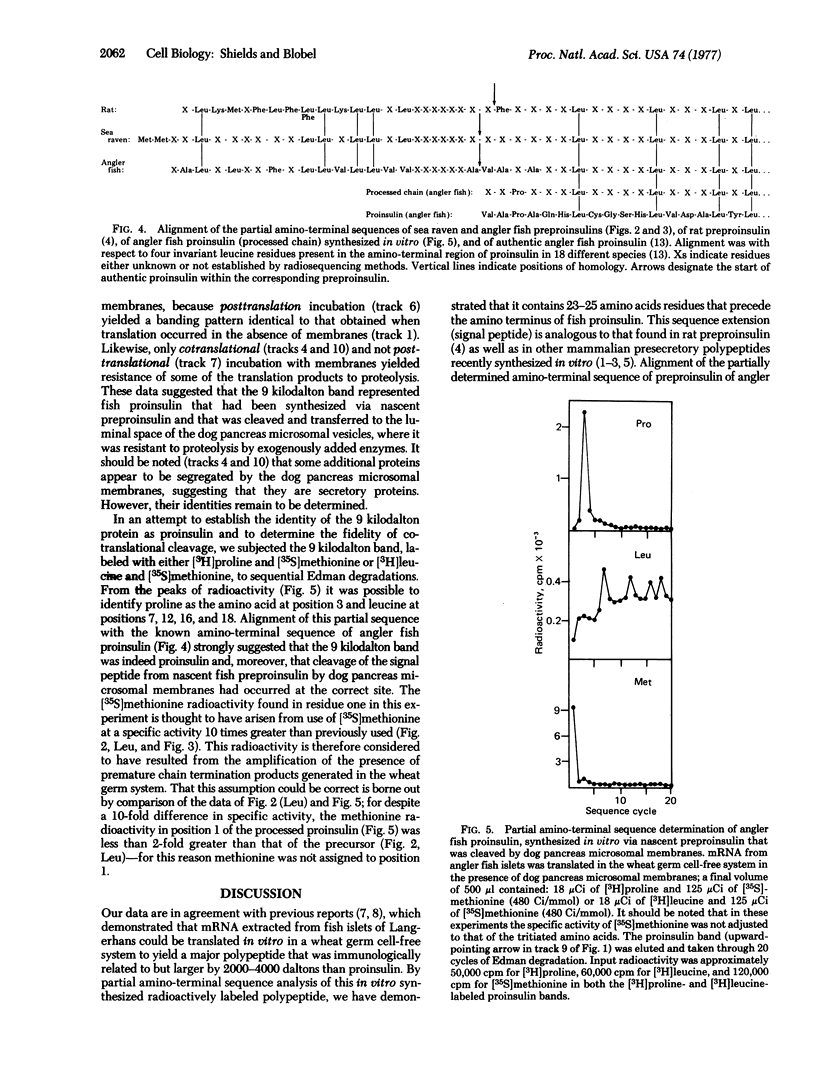
Poly(A)-containing mRNA isolated from the islets of Langerhans obtained from two species of fish, angler fish (Lophius americanus) and sea raven (Hemitripterus americanus), stimulated protein synthesis 16-fold in a wheat germ cell-free system. Characterization of the translation products by polyacrylamide gel electrophoresis in sodium dodecyl sulfate showed a major polypeptide weighing 11,500 daltons that was specifically precipitated by an antibody against angler fish insulin. Partial sequence analysis of the amino terminal revealed that this polypeptide is preproinsulin, in which the amino terminus of proinsulin is preceded by either 23 (angler fish) or 25 (sea raven) amino acid residues. Translation of fish islet mRNA in a wheat germ cell-free system in the presence of dog pancreas microsomal membranes led to the correct cleavage of the nascent preproinsulin, resulting in the synthesis of authentic fish proinsulin, as verified by partial sequence analysis. Moreover, the synthesized fish proinsulin was segregated, presumably into the luminal space of the dog pancreas microsomal vesicles, because it was found to be resistant to proteolysis by added trypsin and chymotrypsin. Our data thus suggest that the mechanisms and information for the transfer of secretory proteins across the microsomal membrane are highly conserved during evolution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Ernst M. D., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: analysis of radioactive tryptic peptides and amino acid sequence. Biochemistry. 1976 Jan 13;15(1):15–19. doi: 10.1021/bi00646a003. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Biesbroeck J., Chyn R., Boime I., Szczesna E., McWilliams D. Isolation of a biologically active messenger RNA: preparation from fish pancreatic islets by oligo(2'-deoxythymidylic acid) affinity chromatography. Ciba Found Symp. 1976;41:97–116. doi: 10.1002/9780470720233.ch6. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A., Klatt D., Prehn S., Hahn V., Höhne W. E. Evidence for the synthesis of a precursor of carp proinsulin in a cell-free translation system. FEBS Lett. 1976 Oct 15;69(1):32–36. doi: 10.1016/0014-5793(76)80647-3. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Marked hydrophobicity of the NH2-terminal extra piece of immunoglobulin light-chain precursors: possible physiological functions of the extra piece. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3273–3277. doi: 10.1073/pnas.73.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]