Abstract
Tamoxifen, a selective estrogen receptor modulator, is widely used in chemotherapy of estrogen receptor-positive breast cancer. Recent studies have indicated that tamoxifen might have potential chemotherapeutic effect on glioma. In the present study, we determined the chemotherapeutic action of tamoxifen on human glioma cell lines. Methylation of 06-methylguanine-DNA methyltransferase expression was identified in A172, U251 and BT325 glioma cell lines, but not U87 cell line. Consistently, A172, U251 and BT325 cell lines are resistant to temozolomide. Tamoxifen induced significant cytotoxic action in A172, U251, BT325, and U87 cell lines. Further Hoechst 33342 staining and apoptosis flow cytometric analysis demonstrated that tamoxifen induced apoptosis in BT325 cell line. Mitochondrial complex analysis indicated that tamoxifen, but not other estrogen receptor modulators, dose dependently inhibit complex I activity. In summary, our study suggests that tamoxifen might have chemotherapeutic effect on temozolomide resistant glioma through its direct action on mitochondrial complex I inhibition and could provide further evidence to support future clinical trial of tamoxifen for the treatment of glioblastoma.
Keywords: Glioma, Temozolomide, Tamoxifen, Mitochondria
Introduction
Glioblastoma multiforme (GBM) are a class of tumor that develops from glial cells, which are the most common type of primary brain tumor and one of the most aggressive of all malignancies. The current standard therapy for GBM includes surgery, radiotherapy and chemotherapy with the alkylating agent temozolomide (TMZ) [1]. However, despite the current standard treatment regimen, the average survival expectancy is 14.6 months and the overall 5-year survival rate for GBM is 9.8% [2, 3]. Much of the TMZ resistance observed clinically is due to high expression of O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair enzyme that removes the O6-alkylguanine and contributes to alkylating agent resistance in tumors [4, 5]. There are currently few alternate treatment options for patients with TMZ resistant gliomas and new treatments targeting TMZ-resistant tumors are thus desperately needed.
Tamoxifen is an estrogen receptor (ER) modulator commonly used for the treatment of ER-positive breast cancer [6]. Because of its antitumor effects predominantly observed in patients with ER-positive tumors, it is generally accepted that the primary actions of tamoxifen are mediated through the inhibition of ER pathway. It has also been clear that tamoxifen has many other anti-tumor actions in addition to the ER pathway, including increase in calcium influx [7], activation of protein kinase C [8], TGF-β2 [9], bcl-2 [10] JNK-1 and caspase-3 signal pathways [11]. Given that tamoxifen has been extensively used as an anti-tumor drug without significant toxic side effects and that it can penetrate blood brain barrier, tamoxifen might be a potentially treatment for brain tumors. Indeed, tamoxifen has been shown to have potent anti-tumor activity in a variety of tumor types, including glioma [11-13]. However, the mechanism underlying the action of tamoxifen on glioma remains unclear.
In the current study, we evaluated the effect of tamoxifen on TMZ-resistant gliomas, which express high level of MGMT activity. We found that tamoxifen significantly inhibited the viability of TMZ-resistant human glioma cells, and induced apoptosis of glioma cells in vitro. We further identified that the actions of tamoxifen was mediated through inhibition of mitochondrial complexes I.
Materials and methods
Cell culture and reagents
The human glioma cell lines U87-MG, U251 and A172 used in this study were purchased from the American Type Culture Collection, BT325 was obtained by Beijing Neurosurgical Institute. Tamoxifen, 4-hydroxytamoxifen, ICI182,780, 1,3,5-Tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT, selective estrogen receptor α agonist), 2,3-bis(4-hydroxyphenyl) propionitrile (DPN, selective estrogen receptor β agonist), DMSO, TMZ, tamoxifen and hoechest 33342 were purchase from Sigma (Sigma Aldrich, USA), Cell Counting Kit-8 (CCK-8) (Dojindo, Japanese), Annexin V-FITC & PI apoptosis detection kit (Jiamei, Beijing, China), ZR Genomic DNA Kit, EZ DNA Methylation-Gold Ki, ZymoTaq DNA Polymerase and Human Methylated & Un-methylated DNA Set (ZYMO Research, USA), Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA).
The U87MG, U251, A172 and BT325 cells were grown in DMEM supplemented with 10% FBS, and 1% penicillin-streptomycin and incubated at 37 °C in a humidified 5% CO2 incubator. Medium was changed 2-3 times/wk. Cells were observed with a phase-contrast microscope (Olympus).
Cell viability assay
Glioma tumor cell viability was evaluated by Cell Counting Kit-8 (CCK-8) assay. In brief, cells were seeded into 96-well plates at 5×104 cells/well and allowed to adhere for 24hr. Then culture media was removed and the test agents, tamoxifen (dissolved in ethanol or DMSO) and TMZ (dissolved in DMSO), were added to wells at indicated concentrations. Control cultures were treated with vehicle solution containing 0.5% ethanol or 0.1% DMSO, respectively. At 48 hr after treatment, 10 μl of CCK-8 was added to each well and the plates were incubated for additional 2 hr. Optical Density (OD) was measured at a wave length of 450 nm on Multimode Plate Readers (Tecan, Switzerland). These values are represented as OD values in percentage change compared with the control.
Isolation of DNA and Methylation-specific PCR
The DNA was extracted using a ZR Genomic DNA Kit and then 1 μg of extracted DNA underwent bisulfite modification using an EZ DNA Methylation-Gold Kit™ according to the manufacturer's instructions. For PCR amplification, The methylation-specific PCR (MS-PCR) was performed with primers specific for either methylated or modified unmethylated DNA (Table-1) [14]. The MS-PCR was performed on 1 ml of bisulfite-treated DNA under the following conditions: 95°C for 10 minutes; 35 cycles of 95°C for 30seconds, the annealing temperature 56.5°C for 30 seconds, and 72°C for 30 seconds; and a final extension of 5 minutes at 72°C. Human methylated DNA Set was used as a positive control for methylated and non-methylated alleles of MGMT. Ten microliters of each PCR product was directly loaded onto 10% polyacrylamide gels, stained with Good View™ Nucleic Acid Stain, and visualized under UV light and photographed by Bio-spectrum System (UVP, USA). A 100 bp DNA ladder was used for molecular size standards.
Table 1. Primers for un-methylated MGMT and methylated MGMT MS-PCR.
| un-methylated MGMT | sence | TTTGTGTTTTGATGTTTGTAGGTTTTTGT |
| antisence | AACTCCACACACTCTTCCAAAAACAAAACA | |
| methylated MGMT | sence | TTTCGACGTTCGTAGGTTTTCGC |
| antisence | GCACTCTTCCGAAAACGAAACG |
Apoptosis assay
Nuclear DNA was stained with Hoechst 33342 at a final concentration of 5 μg/ml. Cells were observed immediately with a fluorescence microscope (Carl Zeiss, Germany). For flow cytometry, cells were detached and labeled by Annexin V-FITC/PI apoptosis detection kit according to the manufacturer's instruction. Apoptotic and necrotic cells were quantified using a FACSCalibur cytometer (Becton Dickinson, USA) and analysis by the Cell Quest software. At least 10,000 cells were analyzed for each sample.
Mitochondrial isolation and mitochondrial respiration chain activity assays
Male Sprague Dawley rats (adult, 300g) were purchase from Charles River. They were maintained under controlled light cycle (12 hr day / night) and humidity with free access to water and rodent chow. All procedures were approved by the Institutional Animal Care and Use Committee. The animals were euthanized and fresh rat heart was removed and placed in ice-cold mitochondrial isolation buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES, and 1 mM EDTA, pH 7. The heart was homogenized immediately, and the mitochondrial fraction was isolated by differential centrifugation as described previously. Mitochondria were stored at −80 °C until subsequent analysis.
All mitochondrial activity assays were performed according to previous publications with minimal modifications: complex I, Complex I-III, Complex II, Complex III, and II-III. All assays used a similar master mix that contained potassium phosphate pH 7.4 (50 mM), BSA (2.5 mg/ml), MgCl2 (5 mM), and KCN (2 mM). Various inhibitors or substrates, including rotenone (6 μM), antimycin (2 μM), NADH (150 μM), CoQ1 60 μM, cyt c (50 μM), succinate (8 mM), and DBH2 (100 μM) were tested. Complex I activity was monitored by adding NADH to the mix with or without rotenone/antimycin. The final data were normalized to blank values at time zero. The reaction was monitored in a kinetic spectrophotometer at 340 nm. Complex I activity was measured in arbitrary units and normalized to control levels for statistical analysis. Isolated mitochondria equivalent to 40-200 μg mitochondrial protein was used for each assay. For complex I-III activity, the reaction was initiated with the addition of NADH, cyt c was used as final substrate and its reduction was monitored at 550 nm. For assay of complex II-III activity, succinate was added instead of NADH, and the electron transfer to cytochrome c was performed according to previous publications.
Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was evaluated using the Student's two-tailed t-test and where appropriate ANOVA was used. A p value ≤ 0.05 was considered to denote statistical significance.
Results
Methylation status of the MGMT promoter region in human glioma cell lines and their response to TMZ treatment
Methylation of the CpG islands in gene promoter region is correlated with transcriptional silencing. MGMT promoter methylation is the key mechanism of MGMT gene silencing and predicts a favorable TMZ chemotherapy outcome in GBM patients. To confirm the methylation status of the MGMT promoter, DNA was isolated from A172, BT325, U251 and U87MG cells and MGMT methylation was determined by MS-PCR. Methylated DNA was only observed in the promoter region of U87-MG cell line, but not A172, U251, and BT325 (Figure 1A). We further determined the chemotherapeutic effect of TMZ on A172, BT325, U251 and U87-MG cells. No chemotherapeutic action of TMZ was observed in A172 and BT325 cells with concentrations up to 40 μM (p>0.05, Figure 1B). A mild but significant reduction of cell viability in U251 cells was observed when treated with TMZ at 40 μM, but not at lower concentrations. On the other hand, chemotherapeutic effect of TMZ was observed in U87-MG cells at the concentrations of 4 and 40 μM (p < 0.05, Figure 1B). The therapeutic action of TMZ on U87MG cells is consistent with its MGMT methylation, which contribute to the MGMT silencing and TMZ sensitivity.
Figure 1.
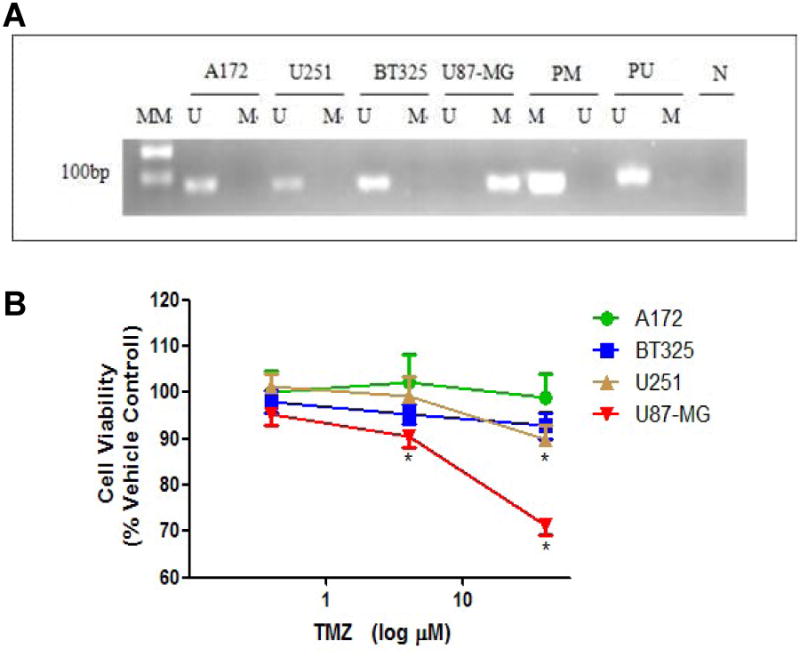
A. MS-PCR analysis depicts the methylation status of the MGMT promoter in A172, U251, BT325 and U87-MG glioma cells. Methylated DNA was only observed in U87-MG cell line. MGMT unmethylated: 93 bp; MGMT methylated: 8l bp; U: unmethylated; M: methylated; PM: Positive control of methylated PCR reaction; PU: Positive control of unmethylated PCR reaction; N: Negative control; MM: molecular marker. B. Chemotherapeutic effect of temozolomide (TMZ) on cell viability of human glioma cell lines U87-MG, U251, A172 and BT325. Glioma cell lines were treated with TMZ at 0.4, 4, 40 μM, or vehicle controls (0.1% DMSO), and the cell viability was determined by CCK-8 assay. Depicted are mean ± S.E.M, n=10, *p<0.05 vs vehicle control.
Chemotherapeutic effect of tamoxifen on TMZ-resistant glioma cells
We determined whether tamoxifen has chemotherapeutic effect on TMZ-resistant glioma cells. After 48 hr treatment, tamoxifen significantly decreased cell viability of BT325, A172, U251 and U87-MG cell lines with an IC50 at 12.87 μM, 16.99 μM, 32.05 μM, and 53.85 μM, respectively (Figure 2). We further investigated the pro-apoptotic action of tamoxifen in TMZ-resistance glioma cells. Hoechst 33342 staining indicated that tamoxifen induced DNA fragmentation in BT325 cells at the concentration of 15 μM (Figure 3A). Consistently, flow cytometry analysis demonstrated that tamoxifen (15 μM) significantly increased the number of BT325 cells undergoing early apoptosis (Annexin V+/PI-) (Figure 3B).
Figure 2.
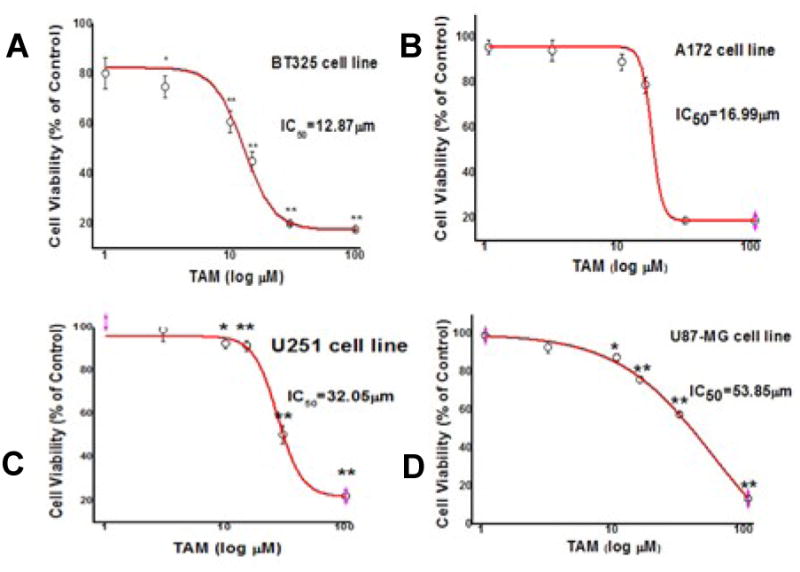
Chemotherapeutic effect of tamoxifen (TAM) on cell viability in human glioma cell lines BT-325 (A), A172 (B), U251 (C) and U87-MG (D). Glioma cell lines were treated with TAM at increasing concentrations or vehicle and the cell viability was determined by CCK-8 assay. Depicted are mean ± S.E.M, n=10, * p<0.05 vs control, ** p < 0.01 vs control.
Figure 3.
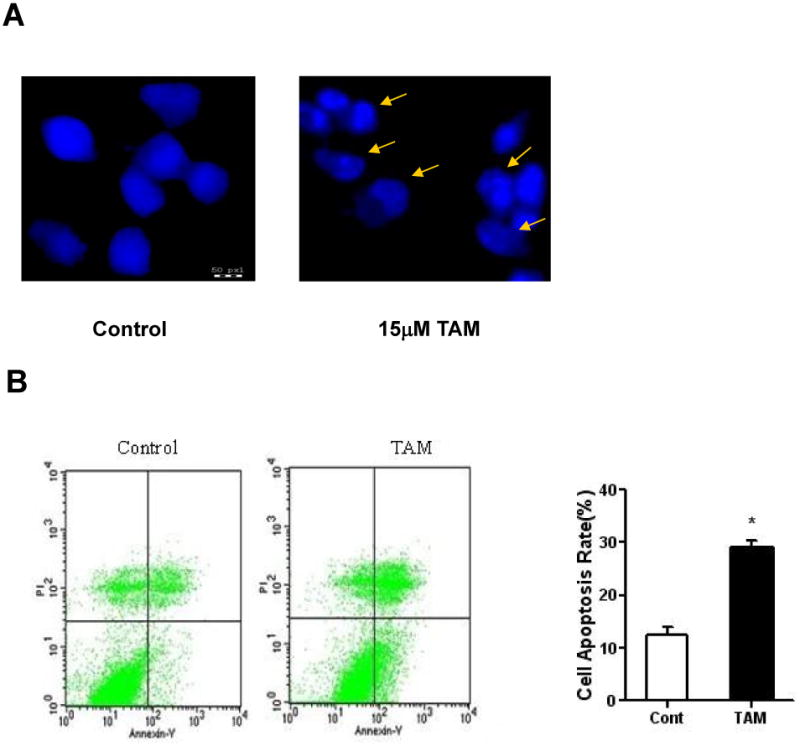
TAM induces apoptosis in BT-325 glioma cells. BT-325 glioma cells were treated with TAM (15 μM) for 48 hr. After treatment, cells were subjected to Hoechst 33342 staining (A) or Annexin V-FITC/propidium iodide staining followed by flow cytometric analysis (B). Data are means±S.E.M., *p < 0.05 vs control.
Tamoxifen inhibits mitochondrial electron transfer chain activities
We determined the effects of tamoxifen on mitochondrial electron transfer chain activities using mitochondria isolated from rat hearts. Tamoxifen inhibited overall complex I-III activity in a dose dependent manner (Figure 4A). While no significant effects of tamoxifen on complex II-III activity was observed (Figure 4B). Further analysis demonstrated that tamoxifen dose dependently inhibited mitochondrial complex I activity (Figure 5A, B). No inhibitory action of mitochondrial complex I was observed in other estrogen receptor modulators including ICI, DPN, PPT, and 4-OH tamoxifen (Figure 5C, D, E, F).
Figure 4.
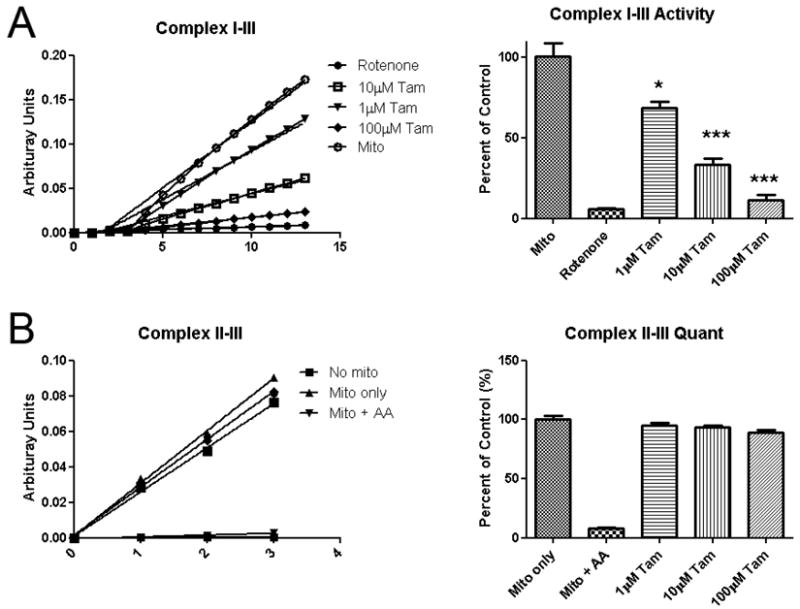
Effect of TAM on the activity of Complex I-III and II-III. TAM reduced overall complex I-III activity in a dose dependent manner (A), but had no significant effects on complex II-III activity (B).
Figure 5.
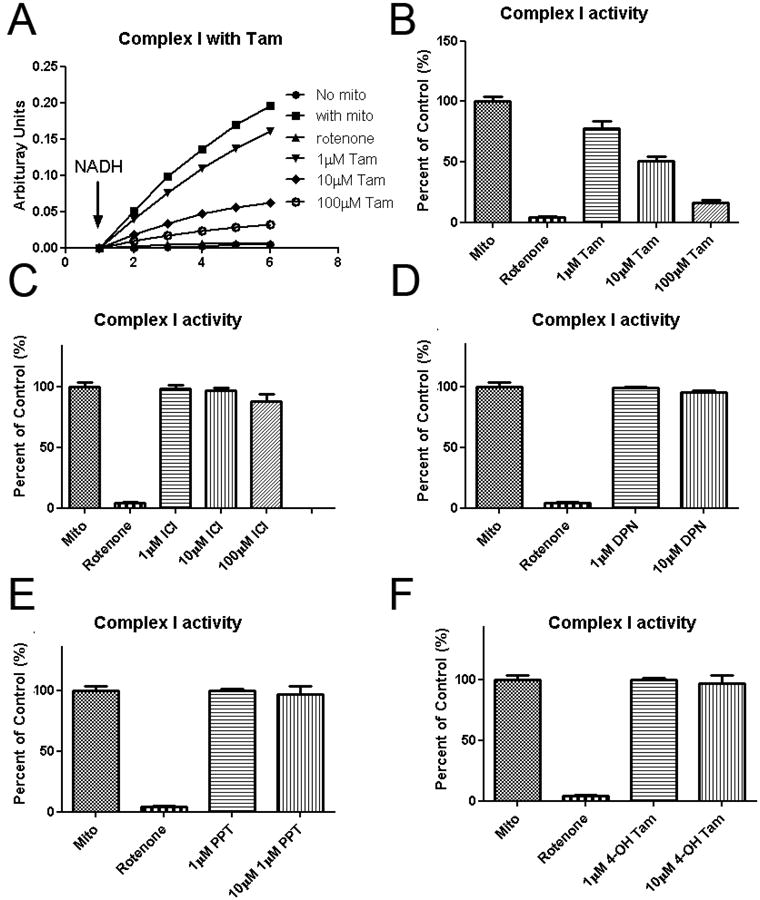
Effect of TAM and other SERM on mitochondrial complex I activity. Tamoxifen dose-dependently inhibits of complex I activity (A, B). None mitochondrial complex I inhibitory action was found in other tested selective estrogen receptor modulators ICI, DPN, PPT and 4-OH tamoxifen (C, D, E, F). Data are means±S.E.M. of value.
Discussion
Gliomas account for more than 50% of all brain tumors and are by far the most common primary brain tumors in adults. Malignant gliomas are associated with dismal prognoses due to their ability to infiltrate diffusely into normal brain parenchyma. Glioblastoma multiform (GBM) is the most aggressive of all malignancies and comprises of approximately half of all gliomas. For high- as well as low-grade gliomas, there is a clear reduction of the perioperative morbidity and mortality over the last 30 years due to the benefit of the current strategy for diagnosis (MRI) and treatment of gliomas (surgery, chemotherapy, radiotherapy) [1]. Nevertheless, the overall prognosis for patients with GBM has not changed from the 1970s until today.
TMZ is an imidazole chemotherapy tetrazine derivative of second-generation alkylating agent, which can lead to the covalent DNA strand cross-linking [15]. TMZ can be completely absorbed after oral administration and has become the first-line chemotherapy for gliomas, especially GBM or anaplastic tumor astrocytoma. However, the efficiency of TMZ on glioma was less than 45% due to MGMT conferred TMZ resistance [16]. Tamoxifen is a selective estrogen receptor modulator, which can be absorbed from gastrointestinal tract and cross blood-brain barrier with low side effects [17]. Tamoxifen was firstly clinically used as a therapeutic drug since 1969, and has become a standard treatment of estrogen receptor-positive breast cancer and endometrial cancer (6). In addition, chemotherapeutic action of tamoxifen has been tested in other cancers including glioma. Early clinical studies have indicated that tamoxifen might have effect in glioma [18]. In the current study, we determined the chemotherapeutic action of tamoxifen in TMZ resistant glioma cell lines. We first tested the effects of TMZ on U87-MG, U251, A172, and BT325 glioma cell lines, and found that U251, A172, and BT325 cell lines was not sensitive to TMZ. Methylation of MGMT promoter has been indicated as a common marker for predicting chemotherapy and prognosis of TMZ on glioma patient [19-21]. Consistently, U251, A172, and BT325 have unmethylated MGMT promoter, which rend them to TMZ resistance. We further determined the chemotherapeutic action of TAM on U87-MG, U251, A172, and BT325 glioma cell lines. We found that tamoxifen induces cytotoxicity in both TMZ sensitive and insensitive glioma cell lines. Interestingly, tamoxifen seems even have higher efficacy in TMZ insensitive glioma cells than TMZ sensitive glioma cells. Notably, our study indicated that chemotherapeutic effect of TAM was superior to TMZ on glioma. The peak plasma concentration of TMZ [22] and TAM [23] can be reached to 4 μM and 3.2 μM. We comparative analysis the effect of 10 times peak plasma concentrations of TMZ (40 μM) and TAM (30 μM) on each glioma cell lines. At 40 μM concentration, TMZ decreased U87-MG cell viability to about 70%. While, tamoxifen reduced cell viability of all glioma cell lines to 20% in TMZ insensitive glioma cell lines.
Because its antitumor effect has been predominantly observed in patients with ER-positive tumors, it is generally accepted that the primary actions of tamoxifen are mediated through inhibition of ER pathway. However, ER-independent actions of tamoxifen have also been identified, including increase in reactive oxygen species production, caspase activation, increase in calcium influx, and activation of protein kinase C, TGF-β, and JNK-1 and caspase-3 signal pathway [7-9, 11]. In addition, direct actions of tamoxifen on mitochondrial function have been identified in isolated rat live mitochondria, MCF-7, and HeLa cells [24, 25]. Our study demonstrated that tamoxifen directly inhibit mitochondrial respiratory chain complex I. We speculated that the inhibitory action of tamoxifen on mitochondrial complex I contribute its pro-apoptotic action in glioma cells independent to TMZ sensitivity. Our study warrant further studies to determine the mechanism underlying the inhibitory action of tamoxifen on mitochondrial complex I which might provide additional insight for the application of tamoxifen in glioma patients.
In summary, the current study demonstrated that tamoxifen exert chemotherapeutic effect in both TMZ sensitive and insensitive glioma cell lines superior to TMZ. Our study indicated that the direct mitochondrial complex I inhibition might underlie the pro-apoptoitc action of tamoxifen in glioma cells. Given that tamoxifen does not have significant overlapping toxicities with most other chemotherapy [26], tamoxifen might be an alternative combined chemotherapy for GBM. Indeed, combinatorial administration of tamoxifen and TMZ appeared to be well-tolerated and potentially effective in increasing the efficacy of dose-dense TMZ schedule as a second-line therapeutic strategy [27]. Although the translational significance of the current study is limited by the lack of in vivo human GBM xenograft study, our results provided further evidence to support future in vivo studies and clinical trials of tamoxifen for the treatment of GBM.
Table 2. Analysis and comparison of the effects of 10-fold peak plasma concentrations of drugs on cell viability.
| Cell lines | TMZ (10PPC) | TAM (10PPC) |
|---|---|---|
| U87-MG | 69.58% | 57.11% |
| U251 | 90.00% | 53.06% |
| A172 | 92.55% | 20.56% |
| BT325 | 93.45% | 20.11% |
Acknowledgments
The research was supported by the Natural Science Foundation of Beijing (7102028), high-level training program for health staff of Beijing health system (2009-3-23), and Natural Science Foundation of China (81228009).
Footnotes
Conflicts of Interest: There are no conflict of interest
References
- 1.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Re. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Jhaveri N, Cho H, Torres S, Wang W, Schonthal AH, Petasis NA, et al. Noscapine inhibits tumor growth in TMZ-resistant gliomas. Cancer Lett. 2011;312:245–252. doi: 10.1016/j.canlet.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50:6119–6129. [PubMed] [Google Scholar]
- 5.Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Jan CR, Cheng JS, Chou KJ, Wang SP, Lee KC, Tang KY, et al. Dual effect of tamoxifen, an anti-breast-cancer drug, on intracellular Ca(2+) and cytotoxicity in intact cells. Toxicol Appl Pharmacol. 2000;168:58–63. doi: 10.1006/taap.2000.9011. [DOI] [PubMed] [Google Scholar]
- 8.Couldwell WT, Hinton DR, He S, Chen TC, Sebat I, Weiss MH, et al. Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett. 1994;345:43–46. doi: 10.1016/0014-5793(94)00415-3. [DOI] [PubMed] [Google Scholar]
- 9.Puchner MJ, Koppen JA, Zapf S, Knabbe C, Westphal M. The influence of tamoxifen on the secretion of transforming growth factor-beta2 (TGF-beta2) in glioblastomas: in vitro and in vivo findings. Anticancer Res. 2002;22:45–51. [PubMed] [Google Scholar]
- 10.Moodbidri MS, Shirsat NV. Activated JNK brings about accelerated apoptosis of Bcl-2-overexpressing C6 glioma cells on treatment with tamoxifen. J Neurochem. 2005;92:1–9. doi: 10.1111/j.1471-4159.2004.02855.x. [DOI] [PubMed] [Google Scholar]
- 11.Tseng SH, Wang CH, Lin SM, Chen CK, Huang HY, Chen Y. Activation of c-Jun N-terminal kinase 1 and caspase 3 in the tamoxifen-induced apoptosis of rat glioma cells. J Cancer Res Clin Oncol. 2004;130:285–293. doi: 10.1007/s00432-004-0546-y. [DOI] [PubMed] [Google Scholar]
- 12.Couldwell WT, Antel JP, Yong VW. Protein kinase C activity correlates with the growth rate of malignant gliomas: Part II. Effects of glioma mitogens and modulators of protein kinase C. Neurosurgery. 1992;31:717–724. doi: 10.1227/00006123-199210000-00015. discussion 724. [DOI] [PubMed] [Google Scholar]
- 13.Misir Krpan A, Ivankovic S, Krajina Z, Ivankovic D, Stojkovic R. Tamoxifen in trimodal therapy with cytotoxic drugs and hyperthermia in vivo significantly enhance therapeutic efficacy against B16-F10 melanoma. Tumori. 2012;98:257–263. doi: 10.1177/030089161209800213. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161:1015–1022. doi: 10.1016/S0002-9440(10)64262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 16.Wei Z, Tao J, Fang Y, Guilin L, Liping D, Lixin X, et al. In vitro chemosensitivity test of glioma cells in tissue culture. Chinese Journal of Minimally Invasive Neurosurgery. 2007;12:163–166. [Google Scholar]
- 17.Grilli S. Tamoxifen (TAM): the dispute goes on. Ann Ist Super Sanita. 2006;42:170–173. [PubMed] [Google Scholar]
- 18.Couldwell WT, Hinton DR, Surnock AA, DeGiorgio CM, Weiner LP, Apuzzo ML, et al. Treatment of recurrent malignant gliomas with chronic oral high-dose tamoxifen. Clin Cancer Res. 1996;2:619–622. [PubMed] [Google Scholar]
- 19.Ueda S, Mineta T, Nakahara Y, Okamoto H, Shiraishi T, Tabuchi K. Induction of the DNA repair gene O6-methylguanine-DNA methyltransferase by dexamethasone in glioblastomas. J Neurosurg. 2004;101:659–663. doi: 10.3171/jns.2004.101.4.0659. [DOI] [PubMed] [Google Scholar]
- 20.Blough MD, Zlatescu MC, Cairncross JG. O6-methylguanine-DNA methyltransferase regulation by p53 in astrocytic cells. Cancer Res. 2007;67:580–584. doi: 10.1158/0008-5472.CAN-06-2782. [DOI] [PubMed] [Google Scholar]
- 21.Gradishar WJ. Adjuvant endocrine therapy for early breast cancer: the story so far. Cancer Invest. 2010;28:433–442. doi: 10.3109/07357901003631098. [DOI] [PubMed] [Google Scholar]
- 22.Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couldwell WT, Uhm JH, Antel JP, Yong VW. Enhanced protein kinase C activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery. 1991;29:880–886. doi: 10.1097/00006123-199112000-00013. discussion 886-887. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso CM, Custodio JB, Almeida LM, Moreno AJ. Mechanisms of the deleterious effects of tamoxifen on mitochondrial respiration rate and phosphorylation efficiency. Toxicol Appl Pharmacol. 2001;176:145–152. doi: 10.1006/taap.2001.9265. [DOI] [PubMed] [Google Scholar]
- 25.Lobaton CD, Vay L, Hernandez-Sanmiguel E, Santodomingo J, Moreno A, Montero M, et al. Modulation of mitochondrial Ca(2+) uptake by estrogen receptor agonists and antagonists. Br J Pharmacol. 2005;145:862–871. doi: 10.1038/sj.bjp.0706265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins HI, Won M, Seiferheld WF, Schultz CJ, Choucair AK, Brachman DG, et al. Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro Oncol. 2006;8:47–52. doi: 10.1215/S1522851705000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Cristofori A, Carrabba G, Lanfranchi G, Menghetti C, Rampini P, Caroli M. Continuous tamoxifen and dose-dense temozolomide in recurrent glioblastoma. Anticancer Res. 2013;33:3383–3389. [PubMed] [Google Scholar]


