Abstract
Rationale
The psychoactive substance, caffeine may improve cognitive performance, but its direct impact on learning and memory remains ill-defined. Conflicting reports suggest that caffeine may impair as well as enhance Pavlovian fear conditioning in animals, and its effect may vary across different phases of learning.
Objectives
To dissect the effect of a motor-stimulant dose of caffeine (30 mg/kg i.p.) on acquisition, retrieval or consolidation of conditioned fear in C57BL/6 mice.
Methods
Fear conditioning was evaluated in a conditioned freezing paradigm comprising 3 tone-shock pairings and a two-way active avoidance paradigm lasting two consecutive days with 80 conditioning trials per test session.
Results
Conditioning to both the discrete tone conditioned stimulus (CS) and the context was markedly impaired by caffeine. The deficits were similarly evident when caffeine was administered prior to acquisition or retrieval (48 and 72 h after conditioning); and the most severe impairment was seen in animals given caffeine before acquisition and before retrieval. A comparable deficit was observed in the conditioned active avoidance test. By contrast, caffeine administered immediately following acquisition neither affected the expression of tone freezing nor context freezing.
Conclusions
The present study challenges the previous report that caffeine primarily disrupts hippocampus-dependent conditioning to the context. At the relevant dose range, acute caffeine likely exerts more widespread impacts beyond the hippocampus, including amygdala and striatum that are anatomically connected to the hippocampus; and together they support the acquisition and retention of fear memories to discrete stimuli as well as diffused contextual cues.
Keywords: adenosine, fear conditioning, learning, memory, mice, state-dependency
Introduction
Caffeine is a mixed antagonist at the adenosine receptors A1 and A2A, and it is the world's most widely used psycho-stimulant drug (Fredholm et al. 1999; Ferré 2008). It is commonly used to sustain wakefulness, raise alertness and enhance mood, with often positive impact, reported by the users, on cognitive performance (Nehlig 2010). However, it remains debatable whether caffeine directly interferes with the memory processes or alters performance indirectly by its arousal effect on attention, vigilance and reaction time speed (Nehlig 2010). Indeed, a literature survey reveals that caffeine may facilitate as well as impair memory performance across a variety of memory tests that depend on Pavlovian associative learning in laboratory rodents (Table 1). Only two studies have reported a null effect, suggesting that caffeine may robustly affect learning and memory performance. Yet, the direction of the effect is difficult to predict although impairment is reported about twice as often as enhancement.
Table 1.
Summary of reported effects of caffeine on Pavlovian conditioning in mice and rats.
| Effects of caffeine on Pavlovian conditioning in mice and rats | ||||||
|---|---|---|---|---|---|---|
| Paradigm | Species | Drug regime | Dosage | Effects | References | |
| Inhibitory/ passive avoidance | Mouse | 30 min pre-training | 1, 3, 10, 30, 100 mg/kg | 10-100 mg/kg impaired retention | Angelucci et al. 1999 | |
|
| ||||||
| 5,10, 20, 40 mg/kg | 40 mg/kg impairs retention | Gulick and Gould 2009 | ||||
|
| ||||||
| 20 mg/kg | Impaired retention | Sanday et al. 2013 | ||||
|
| ||||||
| 60 min pre-training | 12.5, 25, 50, 100 mg/kg | 50 and 100 mg/kg impaired retention | Zarrindast et al. 1994 | |||
|
| ||||||
| Rat | 45 min pre-training | 1, 2, 20 mg/kg | 20 mg/kg impaired retention | Dall'Olio et al. 1978 | ||
|
| ||||||
| Mouse | Immediately post-training | 0.25, 0.5, 1.0 mg/kg | Enhanced retention | Cestari et al. 1996 | ||
|
| ||||||
| 1, 3, 10, 30, 100 mg/kg | 1-30 mg/kg enhanced retention | Angelucci et al, 1999 | ||||
|
| ||||||
| 0.1, 0.3, 1.0, 3.0 mg/kg | 0.3 mg/kg enhanced retention | Kopf et al. 1999 | ||||
|
| ||||||
| 180 min post-training | 0.1, 0.3, 1.0, 3.0 mg/kg | No effect | ||||
|
| ||||||
| Mouse | 30 min pre-test | 1, 3, 10, 30, 100 mg/kg | 3-10 mg/kg enhanced retention | Angelucci et al. 1999 | ||
|
| ||||||
| 20 mg/kg | No effect | Sanday et al. 2013 | ||||
|
| ||||||
| Mouse | 30 min pre-training and pretest | 40 mg/kg | 40 mg/kg impairs retention | Gulick and Gould 2009 | ||
|
| ||||||
| 1, 3, 10, 30, 100 mg/kg | 3-100 mg/kg impaired retention | Angelucci et al. 1999 | ||||
|
| ||||||
| 20 mg/kg | No effect | Sanday et al. 2013 | ||||
|
| ||||||
| Rat | 45 min pre-training and pretest | 20 mg/kg | Impaired retention | Dall'Olio et al. 1978 | ||
|
| ||||||
| Mouse | Added to drinking water in home cage for 7 days | 0.1, 0.3, and 1.0 g/l | No effect | de Oliveira et al. 2005 | ||
|
| ||||||
| Rat | Added to drinking water in home cage for 30 days | 1 g/l | Enhanced retention in 12 months but not 3 months old animals | Sallaberry et al. 2013 | ||
|
| ||||||
| Active avoidance | Mouse | Immediately pre-training | 5, 10, 20 mg/kg | 5-20 mg/kg enhanced learning | Oka et al. 1980 | |
|
| ||||||
| Pre-training | 2.5, 5, 10 mg | 10 mg/kg impaired learning | Sansone et al. 1994 | |||
|
| ||||||
| Rat | 5 min pre-training | 10 mg | Enhanced learning | Bakshi et al. 1995 | ||
|
|
|
|||||
| 15 min pre-training | 75 mg/kg | Shen et al. 1990 | ||||
|
|
|
|||||
| 60 min pre-training | 40 mg/kg | Petkov and Rousseva 1984 | ||||
|
| ||||||
| 15 min pre-training | 120 mg/kg | Impaired learning | Shen et al. 1990 | |||
|
| ||||||
| Rat | Immediately post-training | 20 mg/kg | Enhanced long-term retention | Yonkov et al. 1984 | ||
|
| ||||||
| Conditioned Freezing | Rat | 15 min preconditioning | 10, 20, 30 mg/kg | 20 and 30 mg/kg impaired context conditioning | Corodimas et al. 2000 | |
|
| ||||||
| 15 min pre-test | 30 m/kg | Impaired context conditioning | ||||
|
| ||||||
| 15 min preconditioning and pre-test | 30 mg/kg | Impaired context conditioning | ||||
|
| ||||||
| Subcutaneously implanted caffeine pellets for 7 days | 5, 10, 25 mg/kg per animal | No effect | ||||
As illustrated in Table 1, the majority of data has been collected in the passive/inhibitory avoidance test. This test is useful for assessing memory associated with a specific context that signals danger typically acquired in a single learning trial. Active avoidance by contrast can assess memory associated with a discrete stimulus but not contextual cues. Conditioned freezing is therefore unique in being able to evaluate both aspects of associative learning, but so far only one study has examined the effect of caffeine on conditioned freezing (Corodimas et al. 2000). These authors showed that caffeine (20 and 30 mg/kg, i.p.) selectively impaired conditioned freezing to the context (24 h later) but not a discrete tone stimulus (48 h later). The specificity of the effect led Corodimas et al. (2000) suggest that caffeine might preferentially affect hippocampus-dependent spatial memory processes. The present study is motivated to further address the reported null effect on conditioned tone freezing. In particular, there are concerns over the fact that tone freezing was assessed following context freezing in the experiment by Corodimas et al. (2000), and a training protocol with relatively high electric shock (1 mA) and a large number of tone-shock pairings during acquisition might have rendered the conditioned freezing response to the discrete conditioned tone stimulus (CS) resistant to the disruptive effect of caffeine. To this end, we introduced here (i) a less aversive conditioning procedure (with 0.3 mA shock and limited to three tone-shock pairings), and (ii) a procedural difference whereby the CS-test was performed before the context-test. Furthermore, we elected to focus on the dose of 30 mg/kg, which was reported to be the most effective in the initial dose-response analysis by Corodimas et al. (2000). This is sufficient to provide a meaningful comparison, even though a dose-response approach would certainly generate additional data in the expense of additional animals.
In the conditioned freezing experiments, caffeine was administered shortly before conditioning (i.e., pre-training) or before CS and context tests. To control for state dependency (Overton 1983), one group of animals was administered caffeine on both (i.e., training and test) stages of the experiment. To distinguish the effect on acquisition from that on early consolidation following conditioning, a separate experiment was performed to examine the impact of post-training caffeine treatment. This represents the first attempt to evaluate the effect of caffeine on memory consolidation in the conditioned freezing paradigm, with a procedure similar to that used in several passive avoidance studies (Cestari et al. 1996; Angelucci et al. 1999; Kopf et al. 1999) suggesting that immediate post-training caffeine enhances memory consolidation – a suggestion that may be linked to a similar finding recently reported in humans (Borota et al. 2014).
To follow up our finding on pre-training caffeine treatment revealed in the conditioned freezing experiment, a conditioned active avoidance test was also performed. Comparing the expression of the conditioned freezing response and the conditioned avoidance response further allowed us to dispel specific concerns over measurement confounds potentially attributable to the motor-stimulant effects of caffeine (Fredholm et al. 1999).
The present study is an important addition to the literature on the cognitive effects of caffeine and further addresses the possibility to modulate performance on a fundamental memory process via disruption of adenosinergic signals in the brain.
Methods
Animals
Adult male C57BL/6J mice (approximately 8-week old) were obtained from The Jackson Laboratory, ME, USA. They were caged in groups of five in Sealsafe Plus Mouse IVC Greenline ventilated cages (Tecniplast, Milan, Italy) connected to a Smart Flow air handling unit (Tecniplast, Milan, Italy). Temperature and relative humidity inside the cages were maintained at 23°C and 42%, respectively. The lighting inside the animal vivarium followed a 12:12h light-dark circadian cycle with lights on from 0700–1900 hrs. Food and water were available ad libitum. Testing commenced following two weeks of acclimatization to their new home when the animals reached approximately 10 weeks of age. All procedures were conducted in the light phase of the circadian cycle in an AAALAC-accredited facility, and were in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Legacy Research Institute in compliance to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Drugs
Caffeine was obtained from Fisher Scientific (USA) and dissolved in sterile saline to obtain the desired concentration of 30 mg/kg. Caffeine or vehicle (saline) was administered via the intraperitoneal (i.p.) route at a volume of 5 ml/kg. All solutions for injection were freshly prepared on the days of injection.
Conditioned freezing
Four fear conditioning chambers (Model VFC-008, Med-Associates; see http://mail.med-associates.com/activity/fear.htm#menudrop) were used allowing four mice to be tested concurrently. They were positioned in sound attenuating cubicles (NIR-022MD). Each chamber consisted of three white acrylic sidewalls and a transparent Plexiglas front door. All chambers were equipped with a stainless steel grid floor (VFC-005A) through which electric shock could be delivered by a current shock generator (ENV-414S). A 1-s shock set at 0.3 mA served as the unconditioned stimulus (US). The inside of the chambers was illuminated by a house light positioned on the left side wall, 18 cm above the grid floor. A speaker (ENV-224AM) was mounted on the right sidewall of the chamber, 13 cm above the gird floor. It was connected to a programmable tone generator that produced the CS, a 2.9 KHz tone with an intensity of 85 dB (A-scale). Each chamber was equipped with a monochrome digital video camera (VID-CAM-MONO-2A) mounted on the transparent front door facing the testing area. Videos of the animals during testing were recorded at 30 frames per second and freezing was scored by the NIR software from Med-Associates. To provide a distinct experimental context to be used in the CS-test, the inside of the conditioning chambers could be modified by different types of chamber inserts including Curved Wall Inserts (VFC-008-CWI) and Smooth Floor Covers (ENV-005-GFCW). In addition, the illumination inside the box was reduced by covering the house light with semi-transparent tape.
The experiment consisted of three phases as described in full detail elsewhere (Yee et al. 2006): conditioning, CS-test, and context test. The conditioning phase comprised three discrete trials of CS-US pairing. Each trial began with a 30 s tone CS followed immediately by the delivery of a 1-s foot-shock US. Each trial was preceded and followed by an inter-trial interval (ITI) of 180 s. 48 h later expression of CS-freezing was assessed in the modified context. Following a 120-s acclimatization period, the CS was presented for 8 min. The pre-CS and CS periods were evaluated separately. Another 24 h later, the animals were returned to conditioning context for a period of 8 min in the absence of any discrete stimulus to assess the conditioned freezing response evoked by the background contextual cues.
Two conditioned freezing experiments were conducted as depicted in Fig. 1. In the first experiment (Exp. 1), 30 animals were randomized into four groups in a 2 × 2 factorial design. In the V-V group (n = 8), vehicle was injected 10 min before conditioning (day 1) and 10 min before the CS test (day 3) and again before the context test (day 4). In the V-C group (n = 7), the animals were injected with vehicle before conditioning, but caffeine before the CS and context tests. In the C-V group (n = 7), the animals received only one injection of caffeine before conditioning, and vehicle before the CS and context tests. In the C-C group (n = 8), caffeine was injected before conditioning, the CS test and the context test. In the second conditioned freezing experiment (Exp. 2), caffeine or vehicle was injected 10 min after conditioning to assess the effect of caffeine on memory consolidation by testing the animals in the CS and context tests without any systemic injection (n = 8 per group).
Fig 1.
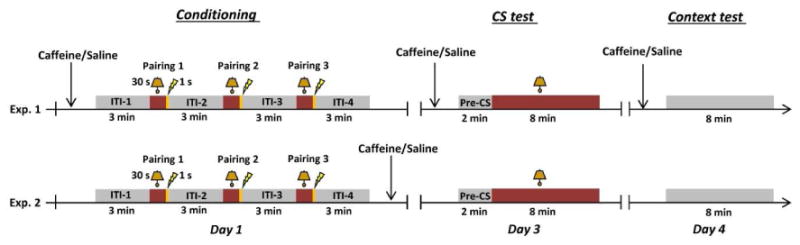
Timeline of the two conditioned freezing experiments. In Exp. 1, caffeine or saline was injected 10 min before conditioning on day 1, 10 min before the CS-test on day 3, and 10 min before the Context test on day 4. In Exp. 2, caffeine or saline was injected 10 min after completion of conditioning on day 1.
Two-way active avoidance conditioning
The apparatus consisted of four active-avoidance shuttle boxes for mice (Model ENV-010MC, Med-Associates) positioned inside sound attenuating cubicles (ENV-018MD, see http://mail.med-associates.com/behavior/packages/shuttleBox.htm). The boxes were separated into two identical compartments each providing a floor space of 20.3 cm × 15.9 cm. The two compartments were interconnected by a central opening allowing the animal to move freely from one compartment to the other. Illumination inside the box was provided by an external stimulus light (ENV-221M) attached to the back-wall of the sound attenuating cubicle. The stainless steel grid floor of the box (ENV-010MA-GF) was connected to a solid state scrambler module (ENV-412) delivering electric shocks at 0.3 mA which served as the US. The CS was a compound stimulus consisting of a tone (2.9 kHz, 85 dB) and a flashing light (0.5 s on and 0.5 s off). The tone was generated by a Sonalert module (ENV-223AM) attached to the back-wall of each shuttle box and the flashing light was produced by two stimulus lights (ENV-221M) with one mounted on the side-wall of each compartment, at 14.5 cm above the grid floor. Shuttle responses between the two compartments were detected by an array of photo-beams (ENV-256-8S) positioned on along the front wall of chamber.
Across 2 days, the animals underwent 80 conditioned avoidance trials per day administered at variable ITIs (mean of 40 s, ± 15 s). A trial began with the presentation of the CS. If the animal shuttled within 5 s from CS onset, the CS was terminated and the animal avoided the electric shock on that trial. Avoidance failure led immediately to an electric foot shock presented in coincidence to the CS. This could last for a maximum of 2 s but could be terminated by a shuttle response during this period (i.e., an escape response). If not, an escape failure was recorded on that trial. Two-way avoidance learning was indexed by the number of avoidance responses and the mean escape latency across successive blocks of 10 trials. To enhance data distribution and variance homogeneity the raw latency measure was subjected to a natural logarithmic transformation prior to statistical analysis. In addition, the number of spontaneous ITI shuttles was calculated as a measure of locomotor activity.
Statistical analysis
All data were analysed by parametric analysis of variance (ANOVA). To assist the interpretation of the statistical outcomes, significant effects were further investigated by confirmatory restricted analyses applied to subsets of the data included in the overall ANOVA, or Dunnett's post-hoc comparisons. All statistical analyses were carried out using IBM SPSS Statistics (version 18, SPSS Inc. Chicago IL, USA). A two-tailed criterion of p < 0.05 was taken as the yardstick for statistical significance. Data illustrated in figures always refer to mean ± standard error (SE).
Results
Caffeine impairs acquisition and retention of conditioned freezing
Conditioning (day 1)
The development of the conditioned freezing response to the three CS-US pairings was analysed by a 2 × 3 (drug × CS-presentations) split-plot ANOVA of percent time freezing across the three subsequent 30-s CS presentations. Freezing levels rapidly increased across CS presentations in the vehicle group the acquisition of the conditioned freezing response (Fig. 2a). Freezing levels were substantially lower in the caffeine group, and showed only a weak increase across CS-presentations. This yielded a significant main effect of drug [F(1,28) = 28.50, p < 0.001] and CS-presentations [F(2,56) = 65.00, p < 0.001], and the interaction between the two factors also attained statistical significance [F(2,56) = 26.39, p < 0.001].
Fig 2.
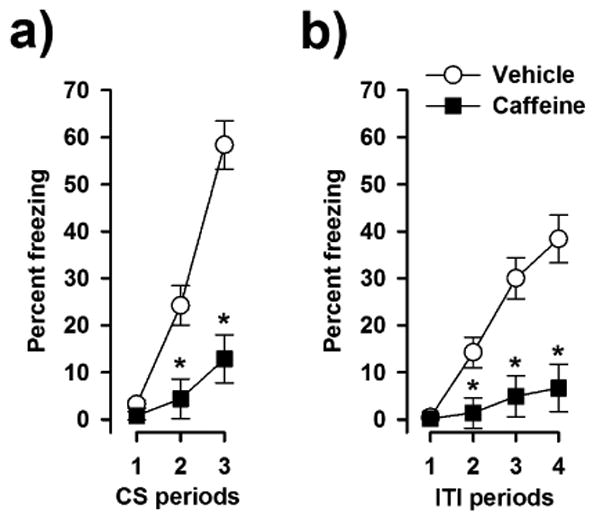
Effect of caffeine on the acquisition of conditioned freezing. (a) Caffeine attenuated the establishment of a conditioned freezing response to the tone CS across the three CS-US pairings. (b) Likewise, caffeine reduced the development of contextual fear across the four ITI periods. * denotes a significant group difference (p < 0.05) according to post-hoc analysis based on the error term associated with the overall ANOVA (n = 15 per group).
Additional pairwise comparisons between the two groups at each of the three CS presentations based on the error variance associated with the significant two-way interaction revealed a significant group difference at the second and third presentations of the CS (p's < 0.01) but not at the first one when the freezing levels were generally low since the CS was novel and had yet been paired with the US. This pattern of results indicates that caffeine impaired the development of the conditioned response to the tone CS.
Freezing behaviour during the ITI periods was separately analysed by a 2 × 4 (drug × ITI periods) split-plot ANOVA. The expression of spontaneous freezing behaviour as measured in the initial ITI period was low as expected and highly comparable between the two groups (Fig. 2b). ITI-freezing showed a linear increase across the following three ITI periods in the vehicle control group. This increase in freezing levels across subsequent ITI periods was substantially weaker in the caffeine group. Consistent with this observation, the ANOVA revealed a significant main effect of drug [F(1,28) = 15.82, p < 0.001], ITI-periods [F(3,84) = 36.28, p < 0.001] as well as their interaction [F(1,28) = 17.72, p < 0.001]. Pairwise comparisons between the two groups at successive ITI periods confirmed the presence of a significant difference (p's < 0.01) except the first ITI.
CS-test (day 3)
Expression of the conditioned freezing response to the tone CS was assessed 48 h in a neutral context after conditioning. As expected, the level of spontaneous freezing obtained in the first two minutes of pre-CS period was generally low, and also highly comparable among the four drug conditions (Fig. 3a). The analysis of pre-CS freezing did not yield any significant effects. Next, the onset of the CS led to an abrupt increase in freezing. Compared with the V-V group, this rise of freezing in response to the CS was substantially weaker in the other three groups, and was the lowest in the C-C group (Fig. 3a). Over the 8-min CS period, a reduction of freezing was evident in all groups suggesting extinction of the conditioned freezing response over time. This was the clearest in the V-V group given that their initial freezing response was also the highest. The pattern of between-groups difference was maintained across bins. A 2 × 2 × 4 (pre-training drug × pretest drug × 2-min bins) split-plot ANOVA of percent CS freezing revealed a significant effect of pre-training drug [F(1,26) = 5.64, p < 0.05] and pre-test drug [F(1,26) = 6.27, p < 0.05] in addition to a significant effect of bins [F(3,78) = 8.31, p < 0.001]. No higher order interaction terms attained statistical significance. Subsequent Dunnett's post-hoc test confirmed that the V-V group significantly differed from all other groups (p's < 0.05). These results indicated that 30 mg/kg caffeine impaired the expression of the conditioned freezing response to the tone CS irrespective of whether it was administered prior to conditioning or prior to CS-test. Caffeine therefore impaired the acquisition as well as the retention of conditioning to the CS. The visibly strongest freezing deficit was seen in the C-C group suggesting the effects of caffeine on acquisition and retention of conditioned freezing were additive. The lack of statistical support for this claim might be due to a floor effect.
Fig 3.
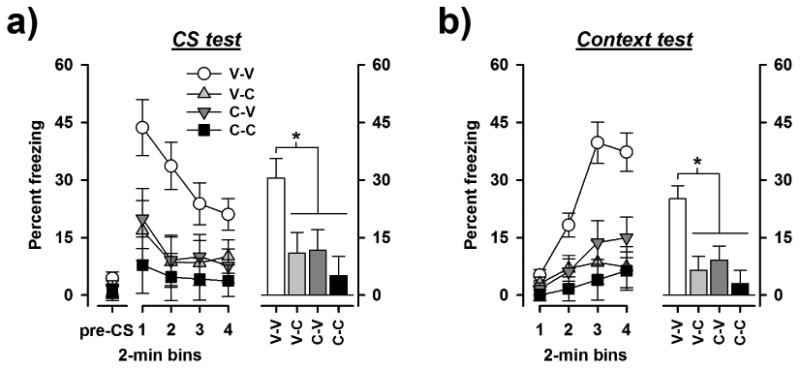
Effect of caffeine on the expression of conditioned freezing: (a) Pre-CS freezing and freezing to the tone CS is expressed across successive 2-min bin. The histogram on the right illustrates the mean freezing level per 2-min bin of the 8-min CS period. (b) Freezing to the training context 24 h after the CS-test is expressed in 2-min bins on the left and averaged across the four bins in the histogram on the right. Caffeine significantly impaired the expression of conditioned tone freezing and context freezing irrespective of the time of administration (i.e. before conditioning, before testing, or before both stages. * denotes that the V-V group froze significantly more than all other groups (p < 0.05) based on Dunnett's post-hoc tests. V-V: vehicle-vehicle (n = 8), V-C: vehicle-caffeine (n = 7), C-V: caffeine-vehicle (n = 7), V-V: caffeine-caffeine (n = 8).
Context test (day 4)
Expression of the conditioned freezing response to the training context was evaluated 24 h following the CS test. Contextual freezing generally increased across the 8-min test period but the rate of increase was the most rapid in the V-V group (Fig. 3b). Again, animals in the C-C group exhibited the lowest level of freezing. A 2 × 2 × 4 (pre-training drug × pre-test drug × 2-min bins) split-plot ANOVA of percent CS freezing confirmed that pre-training [F(1,26) = 7.45, p < 0.05] as well as pre-test [F(1,26) = 12.08, p < 0.005] caffeine significantly reduced freezing to the context. Subsequent Dunnett's post-hoc tests showed that the vehicle control group differed from all other groups (p's < 0.05). This group difference was also captured in the rise of freezing over time [bins: F(3,78) = 30.13, p < 0.001] by the presence of the interactions terms: pre-training drug × bins [F(3,78) = 4.12, p < 0.05] and pre-test drug × bins [F(3,78) = 13.03, p < 0.001].
Post-training administration of caffeine does not affect the expression of conditioned freezing
Conditioning (day 1)
The animals performed in a similarly way as the V-V control group in the previous freezing experiment. As expected, the two groups were highly comparable across all measures because they did not receive any treatment (data not shown).
Caffeine administration after conditioning affected neither the expression of CS freezing (Fig. 4a) nor context freezing (Fig. 4b). Separate 2 × 4 (drug post-training × 2-min bins) ANOVAs of percent CS freezing and percent context freezing only revealed a significant main effect of bins [CS test: F(3,42) = 3.27, p < 0.05; context test: F(3,42) = 3.27, p < 0.05] reflecting the linear decrease and increase of the freezing response across blocks in the CS test and context text, respectively.
Fig. 4.
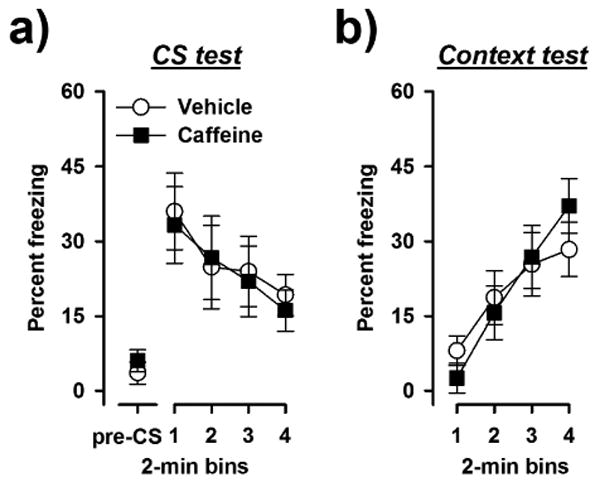
Effects of post-training caffeine treatment on conditioned freezing: (a) Pre-CS freezing and freezing to the tone CS is expressed across successive 2-min bin. (b) Freezing to the training context 24 h after the CS-test is expressed in 2-min bins. Post-training administration of caffeine neither affected the expression of conditioned tone freezing nor conditioned context freezing (n = 8 per group).
Caffeine impairs active avoidance conditioning
Active avoidance learning was indexed by the number of successful avoidance responses across successive blocks of 10 trials. Avoidance performance was relatively poor on day 1 with little indication of learning. On day 2, avoidance gradually improved across blocks reflecting the acquisition of the conditioned shuttle response (Fig. 5). Compared to the vehicle control group, caffeine treated animals learned at a slower pace and achieved a lower level of performance by the end of the test. In support of this, a 2 × 2 × 8 (drug × days × 10-trials blocks) split-plot ANOVA of avoidance responses per block revealed a significant main effect of drug [F(1,16) = 16.20, p < 0.001], and a significant drug × days interaction [F(1,16) = 36.18, p < 0.001]. The drug × blocks interaction approached statistical significance [F(7,112) = 2.06, p = 0.05]. In addition, there was a significant main effect of days [F(1,16) = 32.33, p < 0.001], blocks [F(7,112) = 6.86, p < 0.001], and their interaction [F(7,112) = 4.13, p < 0.001]. Similar analysis of the escape latency revealed essentially identical results (data not shown).
Fig. 5.
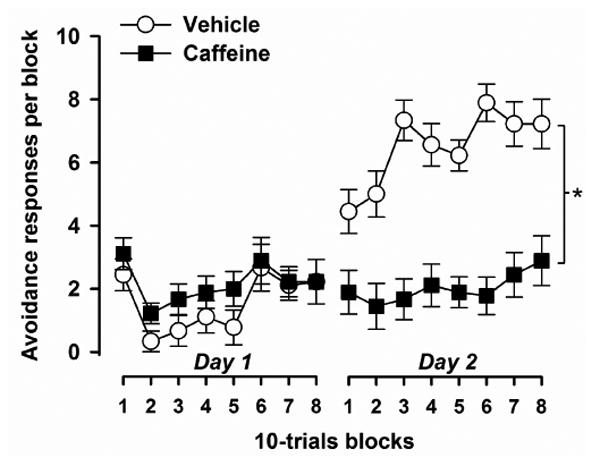
Effects of caffeine on the acquisition of active avoidance conditioning. Performance was indexed by the number of avoidance responses expressed as a function of successive blocks of 10 trials. Caffeine significantly impaired avoidance learning. * denotes the significant drug effect (p < 0.05) yielded by the 2 × 2 × 8 (drug × days × blocks) ANOVA of avoidance responses made per 10-trials block (n = 9 per group).
To ascertain that the performance deficit in the caffeine group was not due to lack of motivation to escape, or an inability to detect electric shock, we analysed the number of escape failures. The number of escape failures was low and highly comparable between groups supporting the impression that the performance deficit in the caffeine group was due to an effect on learning. Out of a total of 80 conditioning trials, the mean number of escape failures (± SE) per group was: vehicle = 13.44 ± 5.53; caffeine = 11.67 ± 5.53.
Because active avoidance performance may be influenced by the level of locomotor activity, we also analysed the number of ITI crossings as a measure of spontaneous activity. Caffeine marginally increased the number of ITI crossings but this was not statistically significant. A 2 × 2 × 8 (drug × days × 10-trials blocks) split-plot ANOVA of the number of ITI shuttles per 10-trials block neither yielded a significant main effect of drug (p > 0.12) nor any significant interaction involving the factor drug. The mean numbers of ITI crossing (± SE) per block and group was: vehicle = 15.12 ± 1.90, caffeine = 19.49 ± 1.90.
Discussion
The present study showed that systemic caffeine (30 mg/kg) administered acutely just before learning or testing significantly impaired memory expression in the form of a conditioned freezing response to a discrete CS or the training context. Hence, the acquisition as well as the retrieval of associative learning in the conditioned freezing paradigm is sensitive to the acute effect of caffeine. The magnitude of the two effects on the final expression of conditioned freezing (to tone or context) also appeared similar when performance between the C-V group and V-C group is compared. Importantly, neither the pre-training nor pre-testing effect of caffeine could be solely attributed to state-dependent learning (Overton 1991) because animals in the C-C group that did not experience a change of drug state also exhibited a deficit as clear as that seen in the C-V and V-C groups. If anything, C-C subjects showed the strongest deficits numerically (albeit not statistically significant), which suggests that the pre-training and pre-testing effects of caffeine might be independent and additive. We might have been prevented from demonstrating the full extent of the additive effect in the C-C group due to a floor effect (Fig. 3). In sharp contrast to these clear effects of caffeine is the lack of a post-training caffeine effect on tone or context freezing, when the same dose of caffeine was administered immediately upon the completion of conditioning (Fig. 4).
The present study is only the second attempt to characterize the effects of acute caffeine on conditioned freezing. In comparison with the previous report by Corodimas et al. (2000), there are notable differences but also similarities. First, the observed deficit over the course of conditioning here is novel. The effect was demonstrated with considerable statistical power (n = 15 per group), and it was clearly dependent on trials (Fig. 2). Vehicle treated animals (V-V and V-C groups) rapidly developed a strong conditioned freezing response to the tone CS across the three CS-shock pairings reaching a freezing level of 60% by the third CS presentation while caffeine treated animals (C-C and C-V groups) only exhibited a modest (albeit statistically significant, p < 0.001) increase in percent freezing to just above 10% by the last CS presentation. It is not known if Corodimas et al. (2000) had observed a similar effect because no data were reported. This is unfortunate because it would be instructive to see if the deficit observed here (limited to three trials) could have persisted all through the ten CS-US pairings in Corodimas et al.'s study. If instead the caffeine-treated and control subjects had reached the same asymptotic performance by the tenth trial, then the lack of a caffeine effect on the subsequent test of conditioned freezing to the tone CS reported by Corodimas et al. (2000) may warrant re-consideration. For instance, the training parameters (10 trials and a very strong US) adopted by Corodimas et al. (2000) should have fostered the development of a very strong tone-shock association so as to limit their ability to detect the impact of pre-training as well as pre-test caffeine. Indeed, Corodimas et al. (2000) referred to the effect of caffeine on tone freezing as modest rather than absent.
Another procedural modification we introduced was to perform the CS test before the context test while maintaining the retention interval from acquisition to CS-test at 48 h (i.e. similar to the study by Corodimas et al. 2000). This change was expected to enhance our ability to detect an effect on conditioned freezing to the CS. Our animals thus did not receive any extinction experience with the context prior to the CS test, which is a potential confound that was also pointed out by Corodimas et al. (2000). In addition, while our V-C subjects underwent the CS-test following their first injection of caffeine, the equivalent group in Corodimas et al.'s experiment would be receiving their second caffeine injection just before their CS-test. Although we cannot ascertain that this particular procedural difference is crucial in deciding whether an effect of caffeine in CS freezing may emerge, the same effect of caffeine on context freezing (shown by Corodimas et al. and the present study) was clearly indifferent to this procedural difference.
The apparent lack of an effect on CS freezing in Corodimas et al.'s (2000) study is the major disagreement with our present study. On the other hand, caffeine's effect on the expression of conditioned context freezing demonstrated here essentially replicated Corodimas et al.'s observation at similar doses. Corodimas et al. (2000) went on to speculate that caffeine preferentially disrupts the normal function of the hippocampus in Pavlovian fear conditioning, since the hippocampus is closely involved in spatial and contextual learning (Seldon et al. 1991; Kim and Fanselow 1992; Phillips and LeDoux 1992). By showing that CS freezing was also severely compromised by pre-training as well as pretesting caffeine administration, the present study further suggests a critical action of caffeine in the amygdala and/or the amygdalar connections to the ventral striatum (Seldon et al. 1991; Phillips and LeDoux 1992; Ferreira et al. 2003, 2008). Hence, caffeine may modify Pavlovian conditioned freezing via multiple brain pathways. Moreover, to isolate the precise contribution of dissociable regional mechanisms, local infusion of caffeine into the critical brain regions would be necessary.
Although the observed freezing deficit in the CS- and context tests in the C-V group is essentially free from interference of the known motor effects of acute caffeine, interpretation of the freezing behaviour in the V-C and C-C groups must take into consideration the concomitant motor-stimulating effect of caffeine. First, in our experiments, caffeine was injected 10 min before testing. Hence, our data indicated that caffeine's acute motor effect did not influence the expression of baseline freezing behaviour (in the V-C and C-C groups) on the CS test day 12 min post-injection. Yet, as soon as the CS was turned on, a clear and substantial deficit in CS freezing was observed in both the V-C and C-C groups. It is therefore unlikely that this immediate effect of caffeine could be solely accounted by its motor effect. Furthermore, it has been shown that the motor-stimulant effect of 30 mg/kg caffeine peaked after 10 min and remained relatively stable over the next 50 min in C57BL/6 mice (Hsu et al. 2009; see their figure 1a). Hence, our baseline measure on the CS test session should provide a good estimate of the potential confounding effect of caffeine on the measurement of freezing. The interpretation that the observed reduction of conditioned freezing might primarily reflect impairment in the expression of Pavlovian learning therefore deserves serious consideration, in particular in the beginning of the CS period when the magnitude of the effect was at its strongest.
Related to this issue is the possibility that the more pronounced freezing deficit in the C-C group (compared with the C-V and V-C groups) might reflect the combined action of caffeine's amnesic and motor-stimulant effects. This line of reasoning would assume that the freezing deficit in the V-C group solely stemmed from the motor effect of caffeine. This assumption may neither be refuted nor confirmed based on existing evidence (the present study and Corodimas et al., 2000), and therefore seems unnecessary as it would further suggest that the pure motor effect of caffeine and the amnesic effect of caffeine might generate such similar temporal profiles of impaired freezing between the V-C and the C-V groups.
Taken together, although the contribution of the acute motor stimulant effect caffeine (in the V-C and the C-C groups) cannot be completely ruled out, we believe that there is sufficient evidence to support the interpretation that the observed deficits likely reflect, at least in parts, a learning deficit. As an attempt to untangle the confounding influence between locomotor activity and freezing behaviour, we therefore moved on to study the effects of caffeine on active avoidance learning – a paradigm that also taxes the expression of conditioned fear. If the acute effect of caffeine on conditioned freezing solely stemmed from an increase in locomotor activity, then conditioned avoidance performance would have been enhanced rather than impaired. Our data does not support this prediction but reinforces our interpretation that caffeine negatively affects the retrieval as much as the acquisition of fear conditioning.
The development of the conditioned shuttle response to the shock-predicting CS was substantially impaired by caffeine, although the impairment only emerged on the second day of training. The impairment could not be attributed to a lack of motivation since caffeine did not affect the ability to escape when the animals failed to avoid the shock. Avoidance performance between the two groups began to separate as soon as they were tested again on the second day (Fig. 5). It was clear that despite weak performance on day 1, the vehicle controls were able to perform better immediately upon being returned to the shuttle boxes on day 2. This suggested that they were able to consolidate and retrieve their experience on day 1. Control performance on day 2 began at >40% successful avoidances and underwent further improvement, reaching >70% avoidance success as training progressed. By contrast, the performance of caffeine-treated animals was consistently poor across and within days, suggesting that caffeine might impair both the acquisition and retention of active avoidance conditioning. These conclusions are consistent with the insights gleaned from the conditioned freezing experiment.
Our avoidance experiment is at odds with a previous report in rats showing that caffeine (10 mg/kg) might enhance active avoidance learning (Bakshi et al. 1995). The fact that this was accompanied by a significant increase in spontaneous shuttle responses recorded during the inter-trial intervals suggests that the observed improvement in avoidance performance could be linked to the motor stimulant effect of caffeine. Here, we also observed a marginal increase in spontaneous ITI shuttles in the caffeine-treated mice as expected at a dose of 30 mg/kg (Hsu et al. 2009). However, this confounding effect simply cannot explain our observation of poor avoidance learning in the caffeine-treated mice. Taken together, the parallel (disruptive) effects of caffeine on conditioned freezing as well as active avoidance learning in the present study cannot be satisfactorily accounted for by the locomotor effect of caffeine. However, the biphasic, dose-dependent motor effect of caffeine – which activates locomotion at low doses but suppresses activity at high doses (Fredholm et al. 1999; El Yacoubi et al. 2000) – may still be partly responsible for the inconsistent effects of caffeine on active avoidance learning in the literature (see Table 1).
Because caffeine is not expected to be fully metabolized by the end of conditioning in the conditioned freezing experiment, pre-training caffeine administration (i.e., C-V and C-C group) might also influence memory consolidation of the relevant tone and context memory. This possibility was addressed here separately in an experiment that involved post-training caffeine injection, which did not affect subsequent expression of tone and context freezing (in the absence of further caffeine treatment). Hence, it is unlikely that the deficit caused by pre-training caffeine could stem from an effect on memory consolidation. Indeed, the literature suggests that post-training administration of caffeine is effective in enhancing the retention of passive avoidance learning, which was evident at doses much lower than 30 mg/kg (see Table 1). A similar effect of caffeine on consolidation has recently been reported in human recognition memory whereby a single dosing of 200 mg caffeine administered to healthy adults after training was sufficient to improve the detection of subtle changes made to the training items 24 h later (Borota et al. 2014). Therefore, we must caution that the present null effect of caffeine on the consolidation of Pavlovian conditioned freezing needs to be further evaluated across multiple doses. Thus, it cannot be excluded that lower doses of caffeine might be associated with a positive effect on Pavlovian fear conditioning while only sufficiently high doses of caffeine induce clear memory disruption. Parallel dose-response analyses of caffeine's effect on acquisition, retention, and consolidation of conditioned freezing may eventually be necessary to allow a comprehensive description of the acute effect of systemic caffeine on fear conditioning. However, the lack of a dose-response analysis here does not undermine our key finding that caffeine (at 30 mg/kg) impaired the development of conditioned fear to discrete cues and diffused contextual cues. It provides an important revision to Corodimas et al.'s (2000) original conclusion that caffeine might only affect contextual fear conditioning.
At this point, it is worth noting that 10–30 mg/kg caffeine also disrupted prepulse inhibition (PPI) of the acoustic startle response (Dubroqua et al. 2014). Disruption of PPI is a common effect of many psycho-stimulant drugs (Geyer et al. 2001) and caffeine is no exception. Although PPI is an operational measure of pre-attentive sensory gating rather than memory processing, deficiency in PPI might predict specific cognitive deficits (Geyer 2006a; 2006b). Hence, one may further speculate whether detrimental effect of caffeine on conditioned freezing may be mechanistically linked to the known psycho-stimulant effects of caffeine at the tested dose. Lastly, since Dubroqua et al. (2014) showed that caffeine did not reduce the direct reaction to the low-intensity prepulse stimulus in the PPI experiment, we may exclude the possibility of a hearing deficit as a potential explanation for caffeine's disruptive effect on PPI and the auditory fear conditioning deficit here.
Finally, we should emphasize that the present study does not provide a molecular pharmacological characterization of caffeine's effect on conditioned freezing. It focuses squarely on the systemic effects of caffeine on conditioned fear – an area that is clearly understudied. Hence, although the present study a priori cannot differentiate the relative involvement of the two subtypes of adenosine receptors that are blocked by caffeine, this limitation does not undermine the significance of the present findings in an arguably much understudied area of caffeine research. Instead, It will compliment and direct further dissections with subtype-specific antagonists as well as conditioned genetic knockout mouse models (e.g., Wei et al., 2014; Singer et al., 2014).
Acknowledgments
The authors would like to acknowledge the support from US National Institutes of Health (Grant R01MH083973), and the Legacy Foundation Grant awarded to BKY.
Source of Funding: Funding for the present study was provided by the US National Institutes of Health (NIH) (Grant R01MH083973), and a Legacy Foundation Grant awarded to BKY.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- Adan A, Serra-Grabulosa JM. Effects of caffeine and glucose, alone and combined, on cognitive performance. Hum Psychopharmacol. 2010;25:310–317. doi: 10.1002/hup.1115. [DOI] [PubMed] [Google Scholar]
- Angelucci ME, Vital MA, Cesário C, Zadusky CR, Rosalen PL, Da Cunha C. The effect of caffeine in animal models of learning and memory. Eur J Pharmacol. 1999;373:135–140. doi: 10.1016/s0014-2999(99)00225-3. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA, Taaid N, Swerdlow NR. A comparison of the effects of amphetamine, strychnine and caffeine on prepulse inhibition and latent inhibition. Behav Pharmacol. 1995;6:801–809. [PubMed] [Google Scholar]
- Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, Toscano JP, Yassa MA. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari V, Castellano C. Caffeine and cocaine interaction on memory consolidation in mice. Arch Int Pharmacodyn Ther. 1996;331:94–104. [PubMed] [Google Scholar]
- Corodimas KP, Pruitt JC, Stieg JM. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology (Berl) 2000;152:376–382. doi: 10.1007/s002130000557. [DOI] [PubMed] [Google Scholar]
- Dall'Olio R, Gandolfi O, Montanaro N. Effects of pre- and post-trial caffeine administrations upon “step-down” passive avoidance behavior in rats submitted or not to electroconvulsive shock. Pharmacol Res Commun. 1978;10:851–858. doi: 10.1016/s0031-6989(78)80104-0. [DOI] [PubMed] [Google Scholar]
- De Oliveira RV, Dall'Igna OP, Tort AB, Schuh JF, Neto PF, Santos Gomes MW, Souza DO, Lara DR. Effect of subchronic caffeine treatment on MK-801-induced changes in locomotion, cognition and ataxia in mice. Behav Pharmacol. 2005;16:79–84. doi: 10.1097/00008877-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Dubroqua S, Yee BK, Singer P. Sensorimotor gating is disrupted by acute but not chronic systemic exposure to caffeine in mice. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3548-8. in Press. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferreira TL, Moreira KM, Ikeda DC, Bueno OF, Oliveira MG. Effects of dorsal striatum lesions in tone fear conditioning and contextual fear conditioning. Brain Res. 2003;987:17–24. doi: 10.1016/s0006-8993(03)03217-7. [DOI] [PubMed] [Google Scholar]
- Ferreira TL, Shammah-Lagnado SJ, Bueno OF, Moreira KM, Fornari RV, Oliveira MG. The indirect amygdala-dorsal striatum pathway mediates conditioned freezing: insights on emotional memory networks. Neuroscience. 2008;153:84–94. doi: 10.1016/j.neuroscience.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive co-treatments for schizophrenia? Dialogues Clin Neurosci. 2006a;8:9–16. doi: 10.31887/DCNS.2006.8.1/mgeyer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006b;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Effects of ethanol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009;123:1271–1278. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology (Berl) 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kopf SR, Melani A, Pedata F, Pepeu G. Adenosine and memory storage: effect of A(1) and A(2) receptor antagonists. Psychopharmacology (Berl) 1999;146:214–219. doi: 10.1007/s002130051109. [DOI] [PubMed] [Google Scholar]
- Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20(Suppl 1):S85–94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- Oka M, Yamada K, Yoshida K, Shimizu M. Avoidance enhancement and discriminative response control by anxiolytics with drugs acting on the GABA system. Jpn J Pharmacol. 1980;30:325–336. doi: 10.1254/jjp.30.325. [DOI] [PubMed] [Google Scholar]
- Overton D. State-dependent learning and drug discrimination. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 18. Plenum Press; New York: 1983. pp. 59–127. [Google Scholar]
- Overton DA. Historical context of state dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2:253–264. [PubMed] [Google Scholar]
- Petkov VV, Rousseva S. Effects of caffeine on aggressive behavior and avoidance learning of rats with isolation syndrome. Methods Find Exp Clin Pharmacol. 1984;6:433–436. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- Sallaberry C, Nunes F, Costa MS, Fioreze GT, Ardais AP, Botton PH, Klaudat B, Forte T, Souza DO, Elisabetsky E, Porciúncula LO. Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunecontent in middle-aged rats. Neuropharmacology. 2013;64:153–159. doi: 10.1016/j.neuropharm.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Sanday L, Zanin KA, Patti CL, Fernandes-Santos L, Oliveira LC, Longo BM, Andersen ML, Tufik S, Frussa-Filho R. Role of state-dependent learning in the cognitive effects of caffeine in mice. Int J Neuropsychopharmacol. 2013;16:1547–1557. doi: 10.1017/S1461145712001551. [DOI] [PubMed] [Google Scholar]
- Sansone M, Battaglia M, Castellano C. Effect of caffeine and nicotine on avoidance learning in mice: lack of interaction. J Pharm Pharmacol. 1994;46:765–767. doi: 10.1111/j.2042-7158.1994.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Seldon N, Everitt B, Jarrard L, Robbins T. Complimentary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Shen Z, Wang G, Lin SZ. Two-way shuttlebox avoidance conditioning and brain NADH in rats. Physiol Behav. 1990;48:515–517. doi: 10.1016/0031-9384(90)90292-c. [DOI] [PubMed] [Google Scholar]
- Yonkov DI. Possible role of brain dopaminergic systems in the memory effects of central stimulants. Methods Find Exp Clin Pharmacol. 1984;6:235–239. [PubMed] [Google Scholar]
- Zarrindast MR, Shafaghi B. Effects of adenosine receptor agonists and antagonists on acquisition of passive avoidance learning. Eur J Pharmacol. 1994;256:233–239. doi: 10.1016/0014-2999(94)90548-7. [DOI] [PubMed] [Google Scholar]


