Abstract
Objective
An increase in circulating branched-chain amino acids (BCAA) is associated with insulin resistance. Adipose tissue is a potentially important site for BCAA metabolism. We evaluated whether monomethyl branched chain fatty acids (mmBCFA) in adipose tissue, which are likely derived from BCAA catabolism, are associated with insulin sensitivity.
Design and Methods
Insulin-stimulated glucose disposal was determined by using the hyperinsulinemic-euglycemic clamp procedure with stable isotope glucose tracer infusion, in 9 lean and 9 obese subjects, and in a separate group of 9 obese subjects before and 1 year after Roux-en-Y gastric bypass (RYGB) surgery (38% weight loss). Adipose tissue mmBCFA content was measured in tissue biopsies taken in the basal state.
Results
Total adipose tissue mmBCFA content was ~30% lower in obese than lean subjects (P = 0.02), and increased by ~65% after weight loss in the RYGB group (P = 0.01). Adipose tissue mmBCFA content correlated positively with skeletal muscle insulin sensitivity (R2 = 35%, P = 0.01, n = 18).
Conclusions
These results demonstrate a novel association between adipose tissue mmBCFA content and obesity-related insulin resistance. Additional studies are needed to determine whether the association between adipose tissue mmBCFA and muscle insulin sensitivity is causal or a simple association.
Keywords: adipose tissue, monomethyl branched chain fatty acids, branched chain amino acids, muscle, insulin resistance, hyperinsulinemic-euglycemic clamp
Insulin resistance is a common metabolic complication of obesity and an important risk factor for the development of type 2 diabetes, the metabolic syndrome, and coronary heart disease (1, 2). It has been proposed that an increase in circulating branched-chain amino acids (BCAA), valine, leucine, and isoleucine, is involved in the pathogenesis of insulin resistance, because increased plasma BCAA concentrations are often observed in obese and insulin resistant states (3, 4), and weight loss leads to decreased plasma BCAA concentrations and improved insulin action (5, 6). However, the underlying mechanism(s) responsible for the relationship between BCAA metabolism and insulin resistance is not known.
Monomethyl branched chain fatty acids (mmBCFA) could provide a link between BCAA metabolism and metabolic dysfunction. In most peripheral tissues, BCAA are deaminated by mitochondrial branched chain aminotransferase (BCAT2 or BCATm) to generate branched-chain α-ketoacids (7), which are then decarboxylated by the branched-chain α-ketoacid dehydrogenase complex (8). The resulting short-chain branched acyl moieties can be exported out of mitochondria (9) and undergo conventional de novo fatty acid biosynthesis, catalyzed by fatty acid synthase (FAS), to produce mmBCFA (10). Alternatively, the fatty acyl chain could be extended within mitochondria by using the mitochondrial fatty acid synthesis (FAS II) system (11) (Supplementary Figure S1).
The predominant branching in mmBCFA is near the terminal end of the carbon chain with an isopropyl or isobutyl group denoted as iso- or anteiso-BCFA, respectively. mmBCFA are present in a large range of organisms from bacteria to mammals, indicating conserved metabolic pathways for their synthesis and function. The enzymes involved in BCAA metabolism are key regulators of both the degradation of BCAA and the synthesis of mmBCFA. Skeletal muscle and adipose tissue are the primary sites for BCAA degradation (12), whereas BCAA catabolism in the liver is minimal because of low levels of BCATm (7). A study conducted in a rodent model demonstrated that adipose tissue BCAA metabolism can modulate circulating BCAA concentrations (13), presumably because adipose tissue is a major site for plasma BCAA uptake and conversion to lipids (14). Whole tissue assessments of BCAA catabolic activities and kinetics also suggest that adipose tissue could play an important role in regulating whole body BCAA homeostasis in people (15, 16).
Adipose tissue gene expression of enzymes involved in BCAA catabolism is lower in obese and insulin resistant mice and people than in their lean counterparts (17, 18). Therefore, it is possible that increased catabolism of BCAA and conversion to mmBCFA in adipose tissue could improve insulin sensitivity by clearing BCAA from plasma. The purpose of the present study was to evaluate the possibility that adipose tissue mmBCFA metabolism is associated with whole-body (primarily skeletal muscle) insulin sensitivity in obese subjects. Accordingly, we conducted: i) a cross-sectional study to assess the relationship between adipose tissue mmBCFA content and insulin sensitivity in lean and obese subjects, and ii) a longitudinal study to assess the effects of marked weight loss on adipose tissue mmBCFA metabolism and insulin sensitivity.
METHODS AND PROCEDURES
Study subjects
A total of 27 subjects (33 to 61 yrs old) participated in two studies. Study 1 was a cross-sectional study that involved 9 lean (BMI = 23.0 ± 0.5 kg/m2, 7 women and 2 men) and 9 obese (BMI = 45.6 ± 1.6 kg/m2, 7 women and 2 men) subjects. Study 2 was a longitudinal study that involved 9 obese subjects (BMI = 48.3 ± 3.2 kg/m2, 8 women and 1 man), who were studied before and 1 year after Roux-en-Y gastric bypass (RYGB) surgery. All subjects provided written informed consent before participating in this study, which was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis, MO. All subjects completed a comprehensive medical evaluation, including a detailed history, physical examination, blood tests, and a 2-hour oral glucose tolerance test. No subject had type 2 diabetes, consumed more than 20 g of alcohol/day, smoked cigarettes, engaged in regular exercise (>1 h/wk), or were taking medications known to alter glucose or lipid metabolism.
Experimental procedures and analyses
Hyperinsulinemic-euglycemic clamp procedure and adipose tissue biopsy
Insulin sensitivity was assessed by using the hyperinsulinemic-euglycemic clamp (HEC) procedure, in conjunction with stable isotopically-labeled glucose tracer infusion. Subjects were admitted to the Washington University School of Medicine Clinical Research Unit in the evening and consumed a standard dinner. The next morning, after subjects fasted for 12 hours overnight, a catheter was inserted into a forearm vein for infusion of tracers, dextrose, and insulin, and a second catheter was inserted into a radial artery for blood sampling. A primed-continuous infusion of [6,6-2H2]glucose (priming dose: 22.0 μmol/kg body weight; infusion rate: 0.22 μmol/kg body weight/min; Cambridge Isotope Laboratories, Andover, MA) was started and maintained until the end of the clamp procedure. After a basal period of 3.5 hours, insulin was infused at a rate of 50 mU/m2 body surface area/min for 4 hours. During insulin infusion, euglycemia (plasma glucose concentration ~100 mg/dL) was maintained by infusing 20% dextrose solution enriched to 2.5% with [6,6-2H2]glucose. Blood samples were obtained immediately before starting the glucose tracer infusion and during the final 30 min of the basal period and the insulin clamp to determine plasma glucose concentration and tracer-to-tracee ratio (TTR). Subcutaneous abdominal tissue biopsies were obtained from the periumbilcal area 60–90 minutes after starting the glucose tracer infusion during the basal period of the clamp procedure, as previously described (19).
After the HEC procedure was completed, the 9 obese subjects who participated in Study 2 had RYGB surgery. This procedure involved constructing a small (~20 mL) proximal gastric pouch by stapling across the stomach. A 150-cm Roux-Y limb was constructed by transecting the jejunum 30 cm distal to the ligament of Treitz and creating a jejunojejunostomy, 150 cm distal to the transection. The Roux limb was then anastomosed in a retrocolic fashion to the proximal gastric pouch by using either a hand-sewn or circular stapled technique. No subject experienced serious postoperative complications. The HEC procedure and abdominal fat biopsy were repeated 1 year after RYGB surgery, when subjects had lost a significant amount of weight and had maintained their body weight stable (<2% change) for at least 2 weeks before repeat studies were performed.
Assessment of insulin sensitivity
Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT plus; Yellow Springs Instruments, Yellow Springs, OH). Glucose TTR in plasma was determined by using gas chromatography-mass spectrometry (GC-MS) (20, 21). Glucose rate of appearance (Ra) in plasma was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 minutes of the basal and insulin infusion periods (22). Glucose rate of disappearance (Rd) from plasma was equal to endogenous glucose Ra plus the rate of exogenously infused dextrose and glucose tracer. Insulin-stimulated glucose Rd in μmol per kg of body weight per minute and the percent increase above basal were used as indices of skeletal muscle insulin sensitivity (21).
Adipose tissue fatty acid analyses
Adipose tissue fatty acids (including mmBCFA) were determined by using GC-MS (Hewlett-Packard 6890N series GC interfaced to an Agilent 5973N mass spectrometer with DB-5 MS 30 m × 0.25 mm × 0.25 m column) in electron ionization mode, as previously described (23). Both the intra- and inter-assay coefficients of variation for these analyses are below 5%.
Isolation of adipose tissue mRNA and quantitative polymerase chain reaction (qPCR)
Frozen adipose tissue samples were homogenized in TRIzol® reagent (Life Technologies, Foster City, CA) and the total RNA was isolated according to the manufacturer’s protocol. cDNA was synthesized and gene expression was determined with real time qPCR by using SybrGreen reagent and the ABI 7500 thermal cycler (Life Technologies), as previously described (24). The primer sequences used are listed in Supplementary Table S1.
Statistical analyses
All datasets were tested for normality according to the Shapiro-Wilks criteria, and appropriate statistical tests were used for normally and non-normally distributed variables (parametric and non-parametric, respectively). Differences between lean and obese subjects were evaluated by using the Student’s independent t test or the Mann-Whitney U test (for normally and non-normally distributed variables, respectively), and the effects of weight loss were evaluated by using the Student’s paired t test or Wilcoxon’s signed-rank test (for normally and non-normally distributed variables, respectively). The relationship between adipose tissue mmBCFA and insulin sensitivity was assessed by calculating Pearson’s or Spearman’s correlation coefficients (for normally and non-normally distributed variables, respectively). A P-value < 0.05 was considered statistically significant. Results are presented as means ± SEM. All analyses were conducted with SPSS version 20 for Windows (IBM SPSS, Chicago, IL).
RESULTS
Insulin sensitivity
Study 1
Glucose Rd increased from 11.7 ± 0.5 μmol/kg/min in the basal state to 48.9 ± 3.3 μmol/kg/min during insulin infusion (a 320 ± 30 % increase) in the lean subjects, and from 8.2 ± 0.5 μmol/kg/min in the basal state to 18.0 ± 2.1 μmol/kg/min during insulin infusion in the obese subjects (a 123 ± 27 % increase). Both glucose Rd and the percent increase in glucose Rd during insulin infusion were significantly lower in obese than lean subjects (P < 0.001).
Study 2
Body weight decreased by 38 ± 1 %, from 132 ± 10 kg before to 82 ± 7 kg, 1 year after RYGB surgery (P < 0.001). Glucose Rd increased from 7.9 ± 0.3 μmol/kg/min in the basal state to 10.5 ± 0.8 μmol/kg/min during insulin infusion before weight loss (a 35 ± 11 % increase), and from 11.1 ± 1.7 in the basal state to 20.2 ± 2.3 μmol/kg/min during insulin infusion after weight loss (a 92 ± 18 % increase). Both glucose Rd and the percent increase in glucose Rd during insulin infusion were significantly greater after than before RYGB surgery (P < 0.005).
Adipose tissue mmBCFA levels in lean and obese subjects
Adipose tissue mmBCFA content as a molar percentage of total adipose tissue fatty acids was ~30% lower in obese than lean subjects (P = 0.02) (Figure 1A), but the percentages of major fatty acids (palmitic acid, C16:0 and oleic acid, C18:1(n-9)) were not significantly different between groups (Figure 1B). Individual adipose tissue mmBCFA in lean and obese subjects are shown in Table 1.
Figure 1.
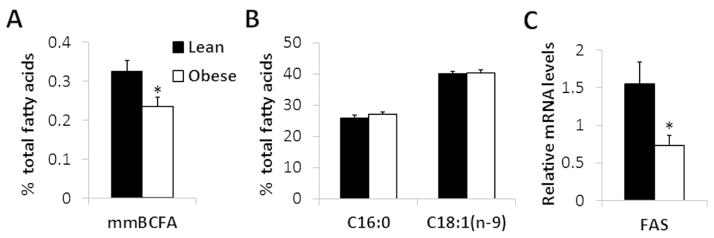
Subcutaneous abdominal adipose tissue total mmBCFA content (A) and C16:0 and C18:1 (n-9) contents (B) (as percentages of total fatty acids), and FAS gene expression (C) in lean (black) and obese (white) subjects. *Value is significantly different from corresponding value in lean subjects, P = 0.02. Values are means ± SEM (n = 9).
Table 1.
Individual adipose tissue mmBCFA content in lean and obese subjects.
| mmBCFA | Lean (n=9) | Obese (n=9) | P-value | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| C15ISO | 0.0204 | 0.0013 | 0.0131 | 0.0017 | 0.0588 |
| C15anteISO | 0.0182 | 0.0012 | 0.0103 | 0.0018 | 0.0223 |
| C17ISO | 0.0850 | 0.0044 | 0.0570 | 0.0070 | 0.0470 |
| C17anteISO | 0.1354 | 0.0073 | 0.1014 | 0.0103 | 0.1665 |
| C19ISO | 0.0646 | 0.0055 | 0.0353 | 0.0090 | 0.0090 |
| C21ISO | 0.0019 | 0.0001 | 0.0009 | 0.0003 | 0.0179 |
| Total | 0.3255 | 0.0274 | 0.2357 | 0.0232 | 0.0235 |
Units are percentages of total adipose tissue fatty acids.
De novo synthesis of mmBCFA in adipose tissue requires both BCAA catabolism and lipogenesis catalyzed by FAS, whose gene expression was lower in adipose tissue from obese than lean subjects (P = 0.02) (Figure 1C).
Effect of bariatric surgery-induced weight loss on adipose tissue mmBCFA levels
The percentage of total adipose tissue mmBCFA increased by ~65% after marked weight loss (P = 0.01) (Figure 2A). The percentages of individual adipose tissue mmBCFA are shown in Table 2. Adipose tissue FAS gene expression increased after weight loss (P = 0.05) (Figure 2B).
Figure 2.
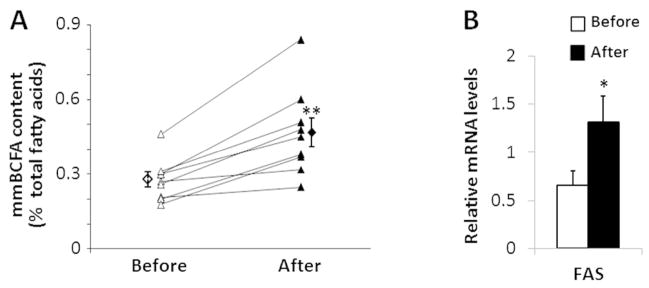
Individual and group means of subcutaneous abdominal adipose tissue total mmBCFA content (A) and FAS gene expression (B) in obese subjects before (white) and 1 year after (black) weight loss induced by Roux-en-Y gastric bypass surgery. Value is significantly different from corresponding value before surgery, *P = 0.05, **P = 0.01. Values are means ± SEM (n = 9).
Table 2.
Individual adipose tissue mmBCFA content in obese subjects before and 1 year after bariatric surgery-induced weight loss.
| mmBCFA | Before weight loss (n=9) | After weight loss (n=9) | P-value | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| C15ISO | 0.0191 | 0.0024 | 0.0844 | 0.0131 | 0.0002 |
| C15anteISO | 0.0162 | 0.0022 | 0.0761 | 0.0123 | 0.0002 |
| C17ISO | 0.0865 | 0.0093 | 0.1099 | 0.0133 | 0.1686 |
| C17anteISO | 0.1524 | 0.0148 | 0.1910 | 0.0227 | 0.1730 |
| C19ISO | 0.0020 | 0.0003 | 0.0028 | 0.0015 | 0.5921 |
| C21ISO | 0.0006 | 0.0001 | 0.0011 | 0.0003 | 0.1152 |
| Total | 0.2767 | 0.0281 | 0.4652 | 0.0581 | 0.0100 |
Units are percentages of total adipose tissue fatty acids.
Relationship between adipose tissue mmBCFA and skeletal muscle insulin sensitivity
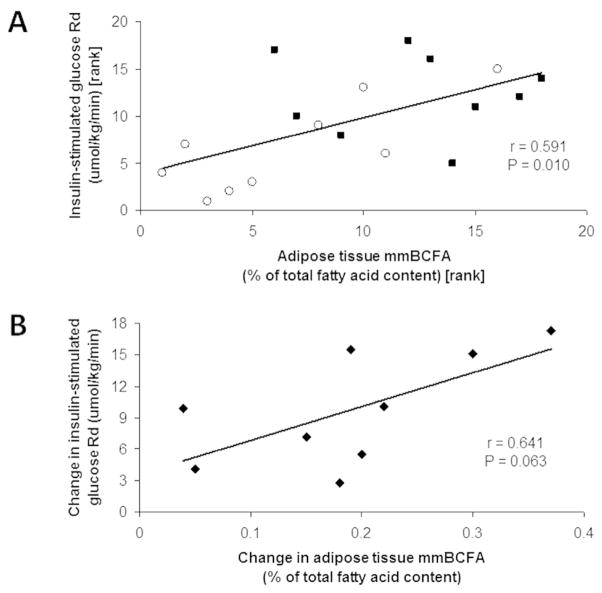
There was a positive correlation between adipose tissue mmBCFA content and insulin sensitivity (i.e. insulin-stimulated glucose Rd) among the group of 18 lean and obese subjects in Study 1 (Spearman’s ρ = 0.591, P = 0.010) (Figure 3A). There was also a trend toward a positive correlation between the change in adipose tissue mmBCFA content and the change in insulin sensitivity induced by weight loss in the 9 obese subjects who had RYGB surgery, but this association did not reach statistical significance (Pearson’s r = 0.641, P = 0.063) (Figure 3B).
Figure 3.
Relationship between skeletal muscle insulin sensitivity, assessed as insulin-stimulated glucose rate of disappearance (Rd) from plasma during a hyperinsulinemic-euglycemic clamp procedure and subcutaneous abdominal adipose tissue total mmBCFA content in 18 lean (black squares) and obese (white circles) subjects (A), and relationship between weight loss-induced changes in adipose tissue total mmBCFA content and insulin-stimulated glucose Rd in a separate group of 9 extremely obese subjects (B).
DISCUSSION
In the present study, we tested the hypothesis that BCAA-derived lipids (i.e. saturated fatty acids with one methyl branch near the terminal end of the carbon chain) are associated with insulin action. Insulin sensitivity in skeletal muscle (the major site of insulin-mediated glucose disposal) and mmBCFA content in adipose tissue (presumably a major site for de novo synthesis of mmBCFA) were determined in lean and obese subjects and in obese subjects before and after marked weight loss. We found that adipose tissue mmBCFA content was lower in obese than in lean subjects, and increased in extremely obese subjects after bariatric surgery-induced weight loss. Moreover, adipose tissue mmBCFA content correlated directly with skeletal muscle insulin sensitivity, assessed by using the HEC procedure, in conjunction with stable isotopically-labeled glucose tracer infusion. These results indicate that adipose tissue mmBCFA could be involved in the pathogenesis of obesity-related insulin resistance and provide a novel potential mechanistic link between BCAA metabolism and insulin action.
The exact biochemical pathway(s) for de novo synthesis of adipose tissue mmBCFA from BCAA is not clear and requires additional studies to evaluate the incorporation of labeled BCAA into mmBCFA. However, data from a series of studies suggest de novo synthesis of mmBCFA could occur in mammalian cells. First, the enzymatic machinery needed for the conversion of BCAA to mmBCFA exists. The generation of short branched-chain acyl-CoAs by deamination and decarboxylation of BCAA occurs within the mitochondrial matrix (7, 8). In order to utilize the conventional cytosolic FAS I pathway for chain length extension, these branched chain acyl-CoAs are converted to acyl carnitines prior to transport out of the mitochondria (9). Branched chain acyl-CoAs can then be recovered from their carnitine esters in the cytosol either by carnitine acyltransferases (25) or via consecutive reactions catalysed by short chain acylcarnitine hydrolase (26) and acyl CoA synthetase. Alternatively, the corresponding short-chain branched acyl-acyl carrier protein (ACP) could be used by the mitochondrial FAS II for fatty acyl chain extension (11). Second, it has been shown that radiolabeled BCAA can be converted into fatty acids in adipose tissue (12). Finally, we recently found that the amount of C17ISO in 3T3-L1 cells before differentiation was below the detection limit, whereas C17ISO levels markedly increased during differentiation into adipocytes (Supplementary Figure S2).
We found adipose tissue FAS gene expression was lower in our obese than lean subjects, and increased in our extremely obese subjects after marked weight loss. These findings suggest that de novo lipogenesis, catalyzed by FAS, is an important regulator of adipose tissue mmBCFA production and content. Moreover, the increase in adipose tissue mmBCFA after weight loss in our subjects, despite a decrease in plasma BCAA observed by other investigators (5, 6), suggests that enzymatic activity is more important than substrate availability (plasma BCAA levels) in determining the rate of mmBCFA synthesis and adipose tissue mmBCFA content. In addition, it is also possible that adipose tissue mmBCFAs are derived from the diet (27) and gut bacteria (28). The relative contribution of the different potential sources to adipose tissue mmBCFA content in people is not known.
Although we observed a positive correlation between adipose tissue mmBCFA content and skeletal muscle insulin sensitivity, our study cannot determine whether this represents a true cause-and-effect relationship or a simple association. Several putative mechanisms could be responsible for the involvement of adipose tissue mmBCFA levels in the pathogenesis of skeletal muscle insulin sensitivity. A decrease in the conversion of BCAA to mmBCFA in obese compared with lean people could influence plasma BCAA clearance and contribute to higher plasma BCAA concentrations (3, 4), which can inhibit skeletal muscle insulin action (29). Although changes in adipose tissue BCAA metabolism can modulate circulating BCAA concentrations in rodents (13), the relative contribution of different tissues in regulating plasma BCAA concentrations in people is not known. It is also possible that mmBCFA and/or complex lipid species containing mmBCFA regulate the secretion of other active molecules from adipocytes (e.g. adipokines) which affect muscle insulin sensitivity. Future studies are needed to identify the major lipid species that contain mmBCFA in adipose tissue and evaluate the effects of these lipids on insulin sensitivity and metabolic function.
In summary, adipose tissue mmBCFA content is decreased in obese compared with lean people, increases after weight loss in obese people, and correlates positively with insulin sensitivity. These findings demonstrate a novel link between adipose tissue metabolism and skeletal muscle insulin action, suggest selective channeling of mmBCFA into specific lipid classes, and reinforce the notion that the specific chemical structure of adipose tissue lipids has important physiological effects. Additional studies are needed to understand the mechanism(s) responsible for these relationships and whether there is a causal association between adipose tissue mmBCFA and muscle insulin sensitivity.
Supplementary Material
What is already known about this subject
An increase in circulating branched chain amino acids (BCAA) is associated with insulin resistance.
Adipose tissue is involved in BCAA metabolism, and gene expression of adipose tissue enzymes involved in BCAA catabolism is lower in obese and insulin resistant people than in lean people.
What this study adds
This study demonstrates that adipose tissue mmBCFA content is lower in obese than in lean people, and increases after weight loss in obese people.
Adipose tissue mmBCFA content correlates positively with insulin sensitivity, suggesting a potential link between adipose tissue BCAA metabolism and metabolic dysfunction.
Acknowledgments
The authors thank Melisa Moore and the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
Funding. This study was supported by National Institutes of Health grants DK037948, DK097608 and DK056341 (Nutrition Obesity Research Center), UL1 RR024992 (Clinical and Translational Science Award), the Longer Life Foundation, and the National Natural Science Foundation of China (31371437).
Footnotes
CONFLICTS OF INTEREST STATEMENT:
No potential conflicts of interest to disclose.
Author Contributions. X.S., F.M. and S.K. designed the study and wrote the manuscript. X.S., F.M., D.Z., E.F., J.C.E., and A.L.D. researched data. Every author reviewed and edited the manuscript. X.S. and S.K. are the guarantors of this work, had full access to all the data, and take full responsibility for the integrity of data and the accuracy of data analysis.
References
- 1.Fenske W, Athanasiou T, Harling L, Drechsler C, Darzi A, Ashrafian H. Obesity-related cardiorenal disease: the benefits of bariatric surgery. Nat Rev Nephrol. 2013;9:539–551. doi: 10.1038/nrneph.2013.145. [DOI] [PubMed] [Google Scholar]
- 2.Turer AT, Hill JA, Elmquist JK, Scherer PE. Adipose tissue biology and cardiomyopathy: translational implications. Circ Res. 2012;111:1565–1577. doi: 10.1161/CIRCRESAHA.111.262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013;62:2757–2761. doi: 10.2337/db13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976;57:987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2:445–456. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violante S, Ijlst L, Te Brinke H, Tavares de Almeida I, Wanders RJ, Ventura FV, et al. Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J. 2013;27:2039–2044. doi: 10.1096/fj.12-216689. [DOI] [PubMed] [Google Scholar]
- 10.Oku H, Kaneda T. Biosynthesis of branched-chain fatty acids in Bacillus subtilis. A decarboxylase is essential for branched-chain fatty acid synthetase. J Biol Chem. 1988;263:18386–18396. [PubMed] [Google Scholar]
- 11.Hiltunen JK, Chen Z, Haapalainen AM, Wierenga RK, Kastaniotis AJ. Mitochondrial fatty acid synthesis--an adopted set of enzymes making a pathway of major importance for the cellular metabolism. Prog Lipid Res. 2010;49:27–45. doi: 10.1016/j.plipres.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal J, Angel A, Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. Am J Physiol. 1974;226:411–418. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]
- 13.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285:11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 15.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- 16.Patterson BW, Horowitz JF, Wu G, Watford M, Coppack SW, Klein S. Regional muscle and adipose tissue amino acid metabolism in lean and obese women. Am J Physiol Endocrinol Metab. 2002;282:E931–936. doi: 10.1152/ajpendo.00359.2001. [DOI] [PubMed] [Google Scholar]
- 17.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–1187. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–374. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes (Lond) 2011;35:1233–1240. doi: 10.1038/ijo.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Zhou D, Abumrad NA, Su X. ADP-ribosylation factor 6 modulates adrenergic stimulated lipolysis in adipocytes. Am J Physiol Cell Physiol. 2010;298:C921–928. doi: 10.1152/ajpcell.00541.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122:4667–4674. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsay RR, Gandour RD, van der Leij FR. Molecular enzymology of carnitine transfer and transport. Biochim Biophys Acta. 2001;1546:21–43. doi: 10.1016/s0167-4838(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 26.Costa ND, Snoswell AM. Enzymic hydrolysis of acetylcarnitine in liver from rats, sheep and cows. Biochem J. 1975;152:161–166. doi: 10.1042/bj1520161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ran-Ressler RR, Bae S, Lawrence P, Wang DH, Brenna JT. Branched-chain fatty acid content of foods and estimated intake in the USA. Br J Nutr. 2014;112:565–572. doi: 10.1017/S0007114514001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.