Abstract
Objective
Fibromyalgia is a condition characterized by chronic widespread muscle pain and fatigue. The primary objective of this study was to determine if pain, perceived cognitive fatigue, and perceived physical fatigue were enhanced in participants with fibromyalgia compared to healthy controls during a cognitive fatigue task, a physical fatigue task and a dual fatigue task.
Methods
Twenty four people with fibromyalgia and 33 healthy controls completed pain, fatigue and function measures. A cognitive fatigue task (Controlled Oral Word Association Test) and physical fatigue task (Valpar peg test) were done individually and combined for a dual fatigue task. Resting pain, perceived cognitive fatigue and perceived physical fatigue were assessed during each task using visual analogue scales. Function was assessed with shoulder range of motion and grip.
Results
People with fibromyalgia had significantly higher increases in pain, cognitive fatigue and physical fatigue when compared to healthy controls after completion of a cognitive fatigue task, a physical fatigue task, or a dual fatigue task (p<0.01). People with fibromyalgia performed equivalently on measures of physical performance and cognitive performance on the physical and cognitive fatigue tasks, respectively.
Conclusions
These data show that people with fibromyalgia show larger increases in pain, perceived cognitive fatigue and perceived physical fatigue to both cognitive and physical fatigue tasks compared to healthy controls. The increases in pain and fatigue during cognitive and physical fatigue tasks could influence subject participation in daily activities and rehabilitation.
Fibromyalgia affects 4-10% of the US population affecting 3.4% of women and 0.5% of men in the US (1). The 1990 American College of Rheumatology diagnostic criteria for fibromyalgia includes chronic widespread pain on both sides of the body and above and below the waist as well as pain in 11 of 18 specified sites (tender points) with digital palpation (2). Fatigue is an extremely common symptom in fibromyalgia with up to 100% of people with fibromyalgia reporting fatigue and is greater in severity compared to other arthritic conditions (3). Fatigue, as described clinically, is a subjective experience and may have both physical and cognitive components that are related yet distinct (4). While the pain associated with fibromyalgia contributes to significantly reduced function, the relationship between pain, fatigue and function is currently not well understood.
Muscle fatigue has been previously measured in people with fibromyalgia using a variety of techniques. In general prior studies have examined static contractions of a single muscle in the upper extremity or the lower extremity, or a bicycle exercise task. Voluntary muscle strength and endurance are decreased in people with fibromyalgia (5-9). When compared to sedentary controls, the majority of studies show no differences in peripheral or central fatigue indices for a variety of muscle types (5, 9, 10). However, when compared to healthy controls, one study showed altered motor recruitment during voluntary contraction of the biceps muscle in people with fibromyalgia (11), and two showed greater superimposed twitches during quadriceps contraction in people with fibromyalgia (8, 12). On the other hand, people with fibromyalgia rate their perceived fatigue and exertion significantly higher before and during exercise tasks (bicycle task or single muscle contractions) compared to healthy controls (6, 10). People with fibromyalgia typically describe fatigue as an overall feeling of tiredness or exhaustion, fatigue while completing functional tasks (e.g. folding laundry, drying hair or getting dressed), decreased attention, sleepiness, or feeling of heaviness. Thus, examination of perceived physical fatigue in response to a functional task is multifactorial and may be distinctly different from responses to a fatiguing exercise task using a single muscle, or direct measures of muscle fatigue mechanisms.
Perceived cognitive fatigue is distinctly different from perceived physical fatigue. Perceived cognitive fatigue is a subjective self-report of cognitive fatigue often measured using a visual analog scale. Perceived cognitive fatigue is different from cognitive dyscognition (13) occurring when there is decreased performance during an acute task requiring sustained mental effort. Cognitive performance is measured by verbal fluency, memory, concentration, automatic processing (13, 14), and is commonly decreased in people with fibromyalgia (15). ‘Fibro fog” is a subjective report of cognitive difficulties such as mental confusion, memory difficulties, memory decline or speech difficulties (13, 15, 16) and could reflect both cognitive fatigue and/or cognitive performance. Performing both a physical and a cognitive fatigue task simultaneously exacerbates both physical and cognitive performance when done as individual tasks in people with neurological diseases, i.e. stroke, multiple sclerosis and Parkinson's (17, 18). However, it is unclear if a decrease in performance is observed in people with fibromyalgia during a dual fatigue task, and if there are alterations in cognitive fatigue in people with fibromyalgia.
The primary objective of this study was to determine if pain, perceived cognitive fatigue, and perceived physical fatigue were enhanced in participants with fibromyalgia compared to healthy controls during a cognitive fatigue task, a physical fatigue task and a dual fatigue task. We tested the following hypotheses: 1) perceived cognitive and physical fatigue are enhanced during a cognitive fatigue task, physical fatigue task and dual fatigue task in people with fibromyalgia compared to healthy controls and 2) physical and/or cognitive fatigue tasks will result in greater pain and worse function in people with fibromyalgia compared to healthy controls.
Methods
Subjects
Following approval by the Institutional Review Board at the University of Iowa, healthy controls and people with fibromyalgia were recruited through the University of Iowa. Inclusion criteria were ages 18-86, able to reach overhead, able to stand for at least 5 minutes, and no history of shoulder injury or surgery. Additional inclusion criteria for the people with fibromyalgia included diagnosis of fibromyalgia as described by the 1990 American College of Rheumatology (2). Criteria for exclusion were: uncontrolled hypertension, active inflammatory condition, cognitive deficits, shoulder injury or surgery, unable to reach overhead, pregnancy, or unable to stand for at least 5 minutes. In the current study 24 people with fibromyalgia (FM)(23 female, 1 male) aged 25-72 (female mean 51.87, SD11.11; male 65) and 33 healthy controls (HC) (33 female, 1 male) aged 25-77 years (female mean 45.03, SD 14.62, male 47) participated in the study.
Results for the participant's clinical characteristics and demographics are presented in Table 1. To assist in the clinical presentation of the people with fibromyalgia in comparison to healthy controls, the following questionnaires were completed: 1) Mini-Mental State Exam (MMSE): The mini mental state exam is an 11-item measure of cognitive function. Test-retest reliability for the MMSE is r=0.64 to 0.85 (19). 2) Fibromyalgia Impact Questionnaire (FIQ): The FIQ was used to measure the subject's ability to complete functional tasks at home, work and social areas of life. The test-retest reliability for the FIQ is r=0.56-0.92 (20). 3) Center for Epidemiological Studies – Depression (CES-D): The CES-D is a 20 item self-report scale regarding the symptoms of depression in the last week. The CES-D has been shown to be a reliable measure of depressive symptom with high internal consistency with Cronbach's alpha 0.85-0.90 (21). 4) Multidimensional Assessment of Fatigue (MAF): The MAF is a 16-item self-report measure of fatigue that includes degree and severity, distress, timing-and impact on various activities of daily living. The MAF has been shown to have internal consistency r=0.93 (22). 5) Modified Fatigue Impact Scale (MFIS): This 21-item instrument provides an assessment of the effects of fatigue on the person and includes physical, cognitive, and psychosocial domains. Test retest reliability is 0.72-0.93 (23).
Table 1.
Demographics and clinical characteristics (n=57). Demographic information is in percentage of sample. Clinical characteristics data mean (M) ± S.E.M.
| Demographics2.14 | |||
|---|---|---|---|
| Fibromyalgia (n=24) | Healthy Subjects (n=33) | P Value | |
| Age (years) female and male | 52.41 ± 2.28 | 45.09 ± 2.50 | 0.02 |
| Female (% sample) | 23 (95.83%) | 32 (96.97%) | 0.82 |
| Ethnicity (%sample) | |||
| Caucasian | 21 (87.50%) | 26 (78.79%) | 0.39 |
| Others | 3 (12.50%) | 7 (21.21%) | 0.39 |
| Marital Status (% sample) | |||
| Married/co-habiting | 10 (41.67%) | 14 (42.42 %) | 0.95 |
| Single/widowed | 14 (58.83%) | 19 (57.58%) | 0.95 |
| Education (% sample) | |||
| High School or less | 6 (25.00%) | 6 (18.18%) | 0.53 |
| Some college or above | 18 (75.00%) | 27 (81.82%) | 0.53 |
| Income (% sample) | |||
| <$60,000 | 14 (41.67%) | 18 (54.55%) | 0.77 |
| >$60,000 | 10 (41.67%) | 15 (45.45%) | 0.77 |
| Fibromyalgia diagnosis (length in years) | 983 ± 152 | Not Applicable | Not Applicable |
| Clinical Characteristics | |||
| BMI | 34.21 ± 1.88 | 26.21 ± 1.26 | ≤0.01 |
| CESD | 20.58 ± 2.16 | 6.57 ± 0.98 | ≤0.01 |
| FIQ | 55.19 ± 2.87 | 9.70 ± 1.59 | ≤0.01 |
| MMSE | 28.41 ± 0.26 | 28.84 ± 0.29 | 0.30 |
| MAF | 65.50 ± 6.44 | 10.96 ± 1.83 | ≤0.01 |
| MFIS | 51.70 ± 3.81 | 11.56 ± 2.01 | ≤0.01 |
BMI=Body Mass Index, CESD=Center for Epidemiological Studies - Depression, FIQ=Fibromyalgia Impact Questionnaire, MMSE=Mini-Mental State Exam, MAF=Multidimensional Assessment of Fatigue, MFIS=Modified Fatigue Impact Scale
Fatigue Tasks
Cognitive fatigue task (CFT)
Controlled Oral Word Association Test (COWAT) was used to induce cognitive fatigue. The COWAT is a verbal fluency test. Participants are typically required to verbally generate as many words as possible in one minute for a given letter. Generally words are clustered in 3 letter sets (CFL, PRW or FAS), with letters given in three discrete trials, for a total of 3 minutes. For this study, the COWAT was modified to last 18 minutes to induce cognitive fatigue and match the average time for completion of the physical fatigue task. The subjects completed word listing for eighteen letters, constituting 18 trials, for a total of 18 minutes with the three letter sets repeated twice. The order of letters was as follows: C, F, L, P, R, W, F, A, S. The participant was instructed not to use words that were proper names or repeat words within each trial. The COWAT has test-retest reliability for total word score is r=0.84 and validity is 0.6 to 0.4 for scoring of clusters, and switch scores (24). For the purposes of our study, we scored the COWAT using the total number of words over the entire 18 minute time period.
Physical fatigue task (PFT)
The physical fatigue task involved an upper extremity task utilizing the Valpar component work sample 9 whole body range of motion work panels and pegs (Valpar International, Figure 1). Each panel has 22 pegs and 3 shapes. The heights of the panel are adjusted so that the subject must reach 4 to 6″ above his/her head height for transfer 1. The physical task was divided into two segments: 1) transferring pegs and shapes from shoulder height to overhead and 2) transferring pegs and shapes overhead to waist height. The subject utilized the dominant arm for the peg activity. The non-dominant arm was allowed to assist in moving the shapes or stabilize the panel. The subject was instructed to complete the task as fast as comfortable with the order of pegs from the black triangle, white square and red kidney. The ability to perform the physical fatigue task was assessed by the time to complete transfer one (shoulder height to overhead) and transfer two (overhead to waist height) and total time for both transfers.
Figure 1. Picture of Valpar Peg Task Equipment (VCWS 9, Valpar Whole Body Range of Motion, Valpar International Corporation, Tucson, Arizona).

Dual fatigue task (DFT)
The dual fatigue task involved a combined (dual) cognitive fatigue task and physical fatigue task completed at the same time as described above. The DFT was measured by total words and total time for transfer completion. The subject was allowed to stop the test if unable to continue due to pain or fatigue.
Outcome Measures
Pain was assessed using a visual analogue scale (VAS) and measured at baseline and after each fatigue task for a total of four measurements. The scale consisted of a 10-cm horizontal line with anchor descriptors of “no pain” and “worst pain imaginable”. The subject was instructed to place a single mark through the continuous line at the appropriate point on the scale. Pain VAS has good test-retest reliability with ICC 0.71-0.99 (25).
Perceived cognitive fatigue and perceived physical fatigue were assessed using a 10 cm visual analogue scale (VAS). For perceived cognitive fatigue anchors were “no mental fatigue” and “worst mental fatigue imaginable”. For perceived physical fatigue anchors were “no physical fatigue” and “worst physical fatigue imaginable”. No specific definitions were given to the subject for rating cognitive fatigue or physical fatigue. Fatigue VAS has internal consistency with Cronbach's alpha 0.91-0.96 (26).
Physical function was measured with 3 tests: 1) physical performance on the fatigue task (see above) and was self-paced as fast as comfortable allowing for a measure of performance, 2) Range of motion (ROM): Standing active shoulder flexion was measured for both shoulders with a goniometer before and after each task for the cognitive fatigue task, physical fatigue task and dual fatigue task for a total of six measurements. Shoulder flexion range of motion has an inter-rater reliability of 0.69 and r=0.53-0.58 with a standard error of measurement of 17 degrees (27). 3) Grip strength: Bilateral grip strength measurements (pounds) were taken for both hands with a hand dynamometer at setting 2 (Jamar®) before and after the cognitive fatigue task, physical fatigue task and dual fatigue task. For grip strength, test-retest reliability is 0.82-0.85 (28).
Cognitive performance was measured with the COWAT as a single task and as part of the dual fatigue task when performed at the same time as the VALPAR. The COWAT was self-paced allowing for a measure of performance and was measured by total number of words completed during each task.
Protocol
Each session began by obtaining consent, completing questionnaires, and measuring height and weight. Visual analogue scales were completed for pain, cognitive fatigue and physical fatigue at baseline before starting the tasks and repeated after each task. Subjects were then randomized to a testing order by selecting one of two testing orders for the three fatigue tasks. The testing order was selected by pulling a slip of paper of testing out of an envelope, each containing one of the two testing orders. Fatigue task testing orders were: 1) CFT/PFT/DFT (11 FM, 16HC) or (2) PFT/CFT/DFT. (13FM, 18 HC). Pre- and post-task measurements were taken for grip strength and ROM. A 10 minute rest was given between each fatigue task.
Statistical Analysis
Descriptive statistics (mean, standard error) were determined for each study variable. Normality of data was confirmed with Kolmogorov-Smirnov goodness of fit test (p< 0.05). Clinical characteristics and demographics are reported in Table 1. For normally distributed data, t-tests compared differences in demographic data between groups (age, BMI, MMSE, FIQ, CES-D, MAF, and MFIS). Because of multiple comparisons, p<0.01 was considered statistically significant. We therefore controlled for BMI and depression (CES-D) in the subsequent analysis as they had a p<0.01 but not for age as the p=0.02. For demographic data (nonparametric) presented as percent of the population a Mann-Whitney U Test for differences between groups was completed.
For outcome measures (pain, fatigue, grip and range of motion), data are represented as the mean ± standard error of mean (S.E.M) and 95% confidence intervals (CI). Summary data for measures before and after completion of each fatigue task for both healthy controls and people with fibromyalgia are shown in Table 2. Difference scores between baseline and after each fatigue tasks were calculated for each outcome measure. These difference scores are shown in Figure 2 for healthy controls and people with fibromyalgia. Since all subjects performed each fatigue task, differences between groups were analyzed using the difference scores with a repeated measures analysis of covariance (ANCOVA) for each individual outcome measure (pain, fatigue, grip and range of motion) controlling for baseline values, BMI, and depression. Post-hoc testing with t-tests examined for differences between healthy controls and people with fibromyalgia for each outcome measure at baseline, after fatigue task and difference scores.
Table 2.
Data for all outcome measures for pain, fatigue and function represented baseline to after completion of the task. Data are mean (M) ± S.E.M and 95% confidence intervals (CI).
| Variable | Baseline | Cognitive Fatigue Task (COWAT) | Physical Fatigue Task (VALPAR) |
Dual Fatigue Task (COWAT and VALPAR) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| FM | HC | FM | HC | FM | HC | FM | HC | ||
| Pain | M | 3.26 ± .42** | 0.42 ± 0.24 | 4.39 ± 0.46** | 0.22 ± 0.07 | 6.27 ± 0.43** | 1.07 ± 0.21 | 5.87 ± 0.48** | 0.96 ± 0.23 |
| CI | 2.38 to 4.14 | -0.07 to 0.92 | 3.43 to 5.35 | 0.07 to 0.38 | 5.38 to 7.17 | 0.64 to 1.50 | 4.87 to 6.86 | 0.48 to 1.45 | |
| Perceived cognitive fatigue | M | 3.36 ± 0.47** | 0.73 ± 0.14 | 5.70 ± 0.44** | 2.15 ± 0.43 | 5.06 ± 0.51** | 0.78 ± 0.23 | 5.84 ± 0.45** | 1.72 ± 0.33 |
| CI | 2.37 to 4.35 | 0.45 to 1.02 | 4.78 to 6.62 | 1.27 to 3.04 | 4.00 to 6.11 | 0.30 to 1.26 | 4.91 to 6.77 | 1.03 to 2.40 | |
| Perceived physical fatigue | M | 3.25 ± 0.43** | 0.89 ± 0.28 | 4.96 ± 0.53** | 0.45 ± 0.13 | 6.66 ± 0.40** | 2.05 ± 0.35 | 6.65 ± 0.43** | 1.66 ± 0.30 |
| CI | 2.34 to 4.15 | 0.60 to 1.47 | 3.85 to 6.07 | 0.18 to 0.72 | 5.82 to 7.51 | 1.32 to 2.78 | 5.75 to 7.54 | 1.04 to 2.27 | |
| Grip right hand | M | 53.75 ± 3.94 | 60.06 ± 2.05 | 47.50 ± 4.40 | 61.75 ± 2.98 | 48.20 ± 4.61 | 62.18 ± 3.15 | 45.95 ± 4.54 | 60.45 ± 3.13 |
| CI | 45.57 to 61.92 | 55.87 to 64.24 | 38.39 to 57.75 | 55.67 to 67.84 | 38.65 to 58.61 | 55.76 to 68.60 | 36.50 to 55.36 | 54.06 to 66.84 | |
| Grip left hand | M | 47.75 ± 3.17 | 54.75 ± 2.20 | 45.00 ± 3.79 | 55.42 ± 2.57 | 44.79 ± 3.99 | 57.00 ± 2.71 | 44.16 ± 4.14 | 54.30 ± 2.98 |
| CI | 41.18 to 54.31 | 50.26 to 59.25 | 37.14 to 53.06 | 50.18 to 60.65 | 35.59 to 52.77 | 51.47 to 62.52 | 36.55 to 52.74 | 48.23 to 60.37 | |
| ROM right shoulder | M | 173.83 ± 1.59 | 176.33 ± 1.55 | 171.75 ± 1.87 | 174.93 ± 1.51 | 171.33 ± 2.14 | 179.27 ± 1.59 | 172.25 ± 1.48 | 177.00 ± 1.12 |
| CI | 170.83 to 177.13 | 173.17 to 179.49 | 167.88 to 175.61 | 171.85 to 178.02 | 166.89 to 175.76 | 176.06 to 182.75 | 169.18 to 175.31 | 174.70 to 179.29 | |
| ROM left shoulder | M | 170.53 ± 1.64 | 172.15 ±1.56 | 165.58 ± 1.66 | 170.81 ± 1.42 | 167.25 ± 2.04 | 173.75 ± 1.43 | 168.00 ± 1.27 | 172.78 ± 1.34 |
| CI | 165.48 to 172.26 | 168.96 to 175.34 | 162.13 to 169.03 | 170.82 to 176.68 | 163.02 to 171.47 | 170.69 to 176.74 | 165.36 to 170.63 | 170.04 to 175.52 | |
S.E.M =standard error of mean, COWAT=Controlled Oral Word Association Test, Valpar=Valpar Peg Test, VAS=visual analogue scale, ROM=range of motion, ROM=Range of motion, CFT=Cognitive fatigue performance task, PFT=physical fatigue performance task, DFT=dual fatigue performance task
<0.01 Significant difference between fibromyalgia group and healthy control
Figure 2.
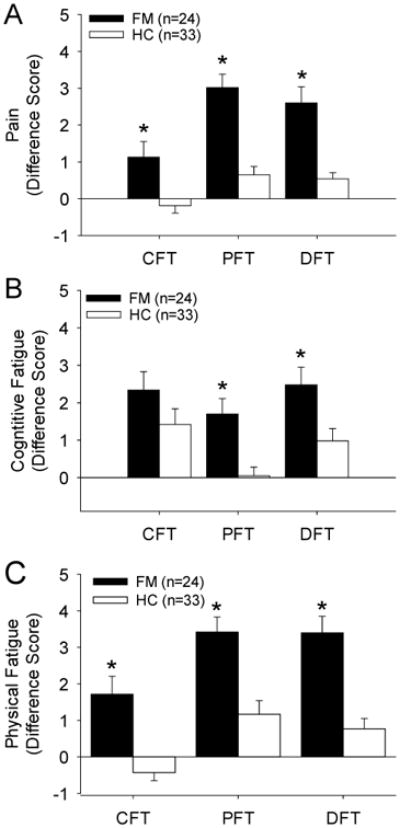
Graphs represent the difference scores for ratings of pain (A), cognitive fatigue (B), and physical fatigue (C) for people with fibromyalgia and healthy controls after performing the cognitive fatigue task (CFT), physical fatigue task (PFT) or dual fatigue task (DFT). Significant differences were observed between people with fibromyalgia and healthy controls across all measures and fatigue tasks (*, p<0.01).
Cognitive fatigue task performance was based on total number of words during the CFT or DFT. Physical fatigue task performance was based on total time for transfer of pegs during the PFT or DFT. Fatigue task performance (cognitive, physical, dual fatigue tasks) in individuals with fibromyalgia and healthy controls were compared using a general linear model multivariate analysis with covariates of BMI and depression (Table 3). Percent decrement in cognitive and physical performance (single task-dual task)/single task was calculated and compared between fibromyalgia and healthy controls with a multivariate analysis with covariates of BMI and depression (Table 4).
Table 3.
Fatigue Task Performance in people with fibromyalgia (n=24) compared to healthy controls (n=33) Data for fatigue task performance during single and dual fatigue tasks with covariates of BMI and Depression (CESD). Data are mean (M) ± S.E.M. (M) and 95% confidence intervals (CI).
| Performance Measure | All Subjects | FM | HC | P Value FM vs. HC | ||
|---|---|---|---|---|---|---|
| CFT | Total Words | M | 255.54 ± 6.88 | 255.04 ± 10.84 | 255.90 ± 9.03 | 0.65 |
| CI | 241.76 to 269.32 | 232.59 to 277.48 | 237.51 to 274.30 | |||
| PFT | Total Transfer (s) | M | 911.31 ± 25.99 | 980.91 ± 50.81 | 860.69 ± 22.39 | 0.01 |
| CI | 859.25 to 963.38 | 875.79 to 1086.04 | 815.06 to 906.32 | |||
| DFT | Total Words | M | 195.29 ± 8.65 | 207.58 ± 16.33 | 186.36 ± 9.01 | 0.30 |
| CI | 177.95 to 212.64 | 173.79 to 241.37 | 168.00 to 204.72 | |||
| Total Transfer (s) | M | 973.57 ± 34.42 | 1065.62 ± 58.82 | 906.63 ± 37.95 | 0.08 | |
| CI | 904.61 to 1042.82 | 943.93 to 1187.31 | 829.31 to 983.95 | |||
BMI=Body mass index, CESD=Center for Epidemiology – Depression, S.E.M. =standard error of measure, FM=fibromyalgia group, HC=healthy control group, CFT=Cognitive fatigue performance task, PFT=physical fatigue performance task, DFT=dual fatigue performance task
p<0.01 Significant difference between fibromyalgia group and healthy control group
Table 4.
Fatigue Task Performance decrement in people with fibromyalgia (n=24) compared to healthy controls (n=33) Data for fatigue task performance during single and dual fatigue tasks with covariates of BMI and Depression (CESD). Data are mean (M)±S.E.M. and 95% confidence intervals (CI).
| Percent Decrement:(Single Task - Dual Task)/Single Task | All Subjects | FM | HC | P Value FM vs. HC | |
|---|---|---|---|---|---|
| Total Words | M | 23.64 ± 2.60% | 19.17± 4.77% | 26.89 ± 2.78% | 0.12 |
| CI | 18.43 to 28.85% | 9.29 to 29.05% | 8.27 ± 23.53% | ||
| Total Transfer(s) | M | -7.92 ± 2.87% | -11.04 ± 4.90% | -5.65 ± 3.47% | 0.47 |
| CI | -13.68 to -2.16% | -12.72 to 2.52% | -12.72 to 1.42% | ||
BMI=Body mass index, CESD=Center for Epidemiology – Depression, S.E.M. =standard error of measure, FM=fibromyalgia group, HC=healthy control group, CFT=Cognitive fatigue performance task, PFT=physical fatigue performance task, DFT=dual fatigue performance task
p<0.01 Significant difference between fibromyalgia group and healthy control group
Results
Demographics and subject characteristics are presented in Table 1. People with fibromyalgia showed a greater BMI and more depression than healthy controls. As expected, they had higher scores on the FIQ and fatigue questionnaires. Baseline pain, fatigue and function are presented in Table 2 and show that those with fibromyalgia had greater pain and fatigue ratings compared with healthy controls (p<0.01). However, grip strength and range of motion were not different between the groups. Overall there were group differences for pain (F1,52=34.6, p=0.0001), cognitive fatigue (F1,52=9.9, p=0.003), and physical fatigue (F1,52=30.6, p=0.001).
Cognitive Fatigue Task
During the cognitive fatigue task there was a significant increase in pain and physical fatigue, as measured with VAS, for people with fibromyalgia compared to healthy controls (Table 3). People with fibromyalgia showed an increase in pain of 1.13 ± 0.42 cm compared to -0.19 ± 0.20 cm for healthy controls (p=0.0001). Perceived cognitive fatigue during the cognitive fatigue task by 2.34 ± 0.49 cm in people with fibromyalgia which was similar to healthy controls (1.41± 0.42 cm, p=0.16). Perceived physical fatigue significantly increased during the cognitive fatigue task by 1.71 ± 0.41 cm compared to 0.048 ± 0.23 cm for healthy controls (p=0.0001). However, functional outcome measures (grip, range of motion) were unchanged by the cognitive fatigue task. Further the two groups did not differ in their ability to perform the CFT (Tables 2,3).
Physical Fatigue Task
During the physical fatigue task there was a significantly greater increase in pain, cognitive fatigue, and physical fatigue, measured by VAS, for people with fibromyalgia when compared to healthy controls (Table 2, Figure 2). People with fibromyalgia had a significantly greater increase in pain of 3.01 ± 0.36 cm compared to 0.65 ± 0.23 cm in healthy controls (p=0.0001). During the physical fatigue task, perceived cognitive fatigue increased 1.71 ± 0.48 cm in people with fibromyalgia compared to -0.43 ± 0.22 in the healthy controls (p=0.001), and perceived physical fatigue increased by 3.41 ± 0.41 cm in people with fibromyalgia 1.16 ± 0.37 cm in healthy controls (p=0.0001).
The functional outcome measures, grip and shoulder range of motion, and performance on the physical fatigue task were unchanged after the physical fatigue task. Total transfer time for was not significantly different in people with fibromyalgia (980.91 ± 50.81 s) compared to the healthy control group (860.69 ± 22.39 s) (p=0.09).
Dual Fatigue Task
Pain during the dual fatigue task demonstrated results similar to the single physical fatigue tasks with an increase of 2.60 ± 0.44 cm in the fibromyalgia group compared to 0.54 ± 0.17 cm in the healthy controls (p=0.0001). Perceived cognitive fatigue during the dual fatigue task demonstrated results similar to the single cognitive fatigue task with an increase of 2.48 ± 0.47 cm for people with fibromyalgia compared to 0.98 ± 0.33 cm for healthy controls (p=0.01). Perceived physical fatigue during the dual fatigue task demonstrated similar results to the single physical fatigue task with an increase of 3.40 ± 0.45 cm for people with fibromyalgia compared to 0.77 ± 0.28 cm for healthy controls (p=0.0001). Shoulder range of motion and grip during the dual fatigue task was not different between groups (Table 2). There was no significant difference in cognitive performance or physical performance in the dual fatigue task between people with fibromyalgia and healthy controls. Performance on the dual task was significantly diminished compared to the single task performance for fibromyalgia and healthy control groups for both cognitive and physical performance. Specifically, all subjects performed less well during the dual task with overall transfer time (p=0.01, paired t-test) (Table 3). However, a comparison of the magnitude of change in the dual task between participants with fibromyalgia and healthy controls was not significantly different (Table 4).
Discussion
The current study shows a physical fatigue task produces a significantly larger increase in both perceived physical and cognitive fatigue in people with fibromyalgia when compared to healthy controls. Conversely a cognitive fatigue task produces a significantly larger increase in perceived physical fatigue in people with fibromyalgia when compared to healthy controls. We further show that both physical and cognitive fatigue tasks produce a significantly greater increase in pain in people with fibromyalgia when compared to healthy controls. These data suggest an interaction between pain and fatigue such that that individuals with chronic pain show enhanced fatigue to both physical and cognitive tasks, that the physical and cognitive fatigue tasks can increase pain.
The physical fatigue task (VALPAR) used in the current study involved multiple upper extremity joints in order to simulate a functional activity. This physical fatigue task would be similar to screwing in a light bulb, or reaching for dishes in a cupboard. While not previously used as a fatigue task, we show increases in perceived physical fatigue in healthy controls and those with fibromyalgia validating the task. Previous physical fatigue tasks in people with fibromyalgia show significant deficits in overall strength and endurance during single-joint tasks (5, 7-9, 12, 29). These studies have shown mixed results in muscle fatigue measures with some showing no changes in peripheral or central muscle fatigue indices, and some indicating deficits in central fatigue (5, 8-11). Interestingly, examination of EMG responses of the biceps muscle between healthy controls and fibromyalgia revealed changes consistent with muscle remodeling to a larger population of fatigue-resistant type I fibers (11) consistent with a sedentary population. Thus, muscle fatigue in people with fibromyalgia may have a central component but this is likely dependent on the task performed, the muscle examined, or muscle fiber type.
Prior studies show that perceived fatigue in people with fibromyalgia is increased in response to a physical task when compared to healthy controls (10, 30). The current study extended these prior findings by separately examining perceived cognitive and physical fatigue and showing significant increases in both fatigue measures in response to the physical fatigue task. Similarly, in people with chronic fatigue syndrome, a physical fatigue task (bicycling) significantly increased both perceived physical fatigue and perceived cognitive fatigue (31). People with central nervous system diseases such as multiple sclerosis, Parkinson's, stroke and traumatic brain injury, also show significant increases in both physical and perceived cognitive fatigue with a physical fatigue task (walking) (32-34). Thus, in people with conditions associated with fatigue, a physical fatiguing task enhances both physical and perceived cognitive fatigue.
Previous studies show that a single bout of exercise increases pain in people with fibromyalgia (6, 35). Our study similarly showed increases in pain with physical activity in people with fibromyalgia – these increases were 3/10 points on a VAS. The underlying mechanisms for this may relate to interactions in central nervous system pathways that mediate both motor and pain responses. Animal studies show that combining a fatiguing exercise task with a low-dose muscle insult enhances measures of hyperalgesia (pain-like behaviors) (36, 37) through activation of neurons in the caudal raphe nuclei of the brainstem (36). Basic research also shows that systemic increases in pro-inflammatory cytokines can initiate fatigue, and in parallel increase expression of pro-inflammatory cytokines (38). In a mouse model of chronic fatigue syndrome there is increased expression of cytokines in the central nervous system, both in the cortex and brainstem (39). People with fibromyalgia clearly have significant pain, pain with movement, and increases in circulating levels of pro-inflammatory cytokines (40). Similarly, there are increased levels of pro-inflammatory cytokines in other conditions associated with fatigue including multiple sclerosis and chronic fatigue syndrome, both of which also have pain (41-43). Thus, it is possible that the enhanced fatigue in response to physical fatigue is related to neuro-immune interactions that affect brain areas that modulate pain and motor responses.
Increases in pain during physical fatigue would be predicted to reduce functional performance. In fact, a recent study showed that pain explains 35-42% of the variance in functional performance in people with fibromyalgia (44). Surprisingly, in the current study, physical performance measured by grip force, range of motion or transfer time on the VALPAR in response to the physical fatigue task was similar in people with fibromyalgia compared to healthy controls. This is in direct contrast to prior studies showing reduced strength and reduced endurance during exercise tasks (8). However, some studies show similar aerobic capacity or maximal voluntary strength in people with fibromyalgia compared to healthy controls (5, 30). It is possible that differences between studies are task-dependent. The current study used a unique task to induce muscle fatigue that is not directly comparable to prior studies.
We also show that performing a cognitive fatiguing task increases pain, perceived physical fatigue, and perceived cognitive fatigue, in people with fibromyalgia, and that these increases are greater compared to healthy controls. Further, we show increases in perceived cognitive fatigue during a physical fatigue task in people with fibromyalgia that were greater than in healthy controls. Few studies have differentiated between physical and cognitive fatigue in fibromyalgia, and none have asked if cognitive fatigue can impact pain, perceived physical fatigue, and function. However, studies in other conditions with significant cognitive and mental fatigue have begun to evaluate these interactions. For people with chronic fatigue syndrome, physical fatigue increases perceived cognitive fatigue (31). One limitation in our study is that we did not screen our subjects for chronic fatigue syndrome, a common co-morbidity with fibromyalgia. In people with multiple sclerosis, traumatic brain injury, cancer and cognitive fatigue syndrome both cognitive fatiguing tasks and physical fatiguing tasks increase perceived cognitive fatigue (33, 45-47).
Despite enhanced cognitive fatigue induced by the COWAT in people with fibromyalgia, cognitive performance was similar to healthy controls. While not previously used as a fatigue task, we show increases in perceived cognitive fatigue in response to the COWAT validating the task. This is in contrast to prior studies that show reduced cognitive performance in people with fibromyalgia. Studies examining deficits in cognitive performance demonstrate altered working memory, episodic memory, attention, processing speed and verbal fluency using sophisticated cognitive function tests (14, 48-50). We specifically designed the cognitive task to induce cognitive fatigue. It is our understanding that using the COWAT for cognitive fatigue in studies of people with fibromyalgia is a novel approach but may also be a limitation in the study.
The impact of cognitive and physical tasks on perceived pain and fatigue has clinical implications for guiding the design of activity-based treatment strategies such as exercise and pacing. Pain management interventions aimed at decreasing pain and fatigue, particularly during activity, could improve participation in regular activities and exercise. Cognitive fatiguing tasks such as education or verbal instruction may also increase pain and fatigue, both cognitive and physical. Instructions and education may need to be modified by giving in shorter intervals, giving written instruction, and giving homework that can be done at the patient discretion. Thus, clinicians should be aware of the impact potentially fatiguing tasks can have on pain, perceived cognitive fatigue, and perceived physical fatigue and modify both physical activity and also cognitive activity accordingly in this population.
In conclusion, these data show that people with fibromyalgia performing a fatiguing task, either physical or cognitive, show significant increases in pain and fatigue. Importantly, a physical fatigue task increases not only pain and perceived physical fatigue, but also perceived cognitive fatigue; conversely a cognitive fatigue task increases pain and perceived cognitive fatigue as well as physical fatigue. Despite the increases in pain and fatigue, there are no differences in physical or cognitive performance on the fatiguing tasks when compared to healthy controls. Thus, clinicians should be aware of the impact of fatiguing tasks on patient's disease severity and modify treatment plans to minimize this impact.
Significance and Innovations.
The current study shows that either a physical or a cognitive fatigue task increased both perceived physical and cognitive fatigue to a greater extent in people with fibromyalgia when compared to healthy controls.
People with fibromyalgia show larger increases in pain and fatigue in both cognitive and physical fatigue tasks compared to healthy controls.
Physical performance and cognitive performance was similar between people with fibromyalgia and healthy controls.
Acknowledgments
Supported by a grant from the Orthopedic Section of the American Physical Therapy Association and University of Iowa Carver College of Medicine and College of Nursing, NIH R34 Grant # AR060378 and NIH UM1 Grant #AR063381. Additional assistance from Ann Lawler for manuscript assistance and Genesis Medical Center for equipment assistance.
References
- 1.Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17:547–61. doi: 10.1016/s1521-6942(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F. Fibromyalgia. Rheum Dis Clin North Am. 1990;16:681–98. [PubMed] [Google Scholar]
- 3.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128:128–35. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.DeLuca J, editor. Fatigue as a window to the brain. Cambridge, Massachusetts 02142: The MIT Press Massachusetts Insititue of Technology; 2005. [Google Scholar]
- 5.Mengshoel AM, Saugen E, Forre O, Vollestad NK. Muscle fatigue in early fibromyalgia. J Rheumatol. 1995;22:143–50. [PubMed] [Google Scholar]
- 6.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin Exp Rheumatol. 1995;13:477–82. [PubMed] [Google Scholar]
- 7.Maquet D, Croisier JL, Dupont C, Moutschen M, Ansseau M, Zeevaert B, et al. Fibromyalgia and related conditions: Electromyogram profile during isometric muscle contraction. Joint Bone Spine. 2010;77:264–7. doi: 10.1016/j.jbspin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen S, Wildschiodtz G, Danneskiold-Samsoe B. Isokinetic and isometric muscle strength combined with transcutaneous electrical muscle stimulation in primary fibromyalgia syndrome. J Rheumatol. 1991;18:1390–3. [PubMed] [Google Scholar]
- 9.Norregaard J, Bulow PM, Danneskiold-Samsoe B. Muscle strength, voluntary activation, twitch properties, and endurance in patients with fibromyalgia. J Neurol Neurosurg Psychiatry. 1994;57:1106–11. doi: 10.1136/jnnp.57.9.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller TA, Allen GM, Gandevia SC. Muscle force, perceived effort, and voluntary activation of the elbow flexors assessed with sensitive twitch interpolation in fibromyalgia. J Rheumatol. 1996;23:1621–7. [PubMed] [Google Scholar]
- 11.Casale R, Sarzi-Puttini P, Atzeni F, Gazzoni M, Buskila D, Rainoldi A. Central motor control failure in fibromyalgia: A surface electromyography study. BMC Musculoskelet Disord. 2009;10:78. doi: 10.1186/1471-2474-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindh MH, Johansson LG, Hedberg M, Grimby GL. Studies on maximal voluntary muscle contraction in patients with fibromyalgia. Arch Phys Med Rehabil. 1994;75:1217–22. doi: 10.1016/0003-9993(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 13.Glass JM. Fibromyalgia and cognition. J Clin Psychiatry. 2008;69(Suppl 2):20–4. [PubMed] [Google Scholar]
- 14.Cherry BJ, Weiss J, Barakat BK, Rutledge DN, Jones CJ. Physical performance as a predictor of attention and processing speed in fibromyalgia. Arch Phys Med Rehabil. 2009;90:2066–73. doi: 10.1016/j.apmr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Etnier JL, Karper WB, Gapin JI, Barella LA, Chang YK, Murphy KJ. Exercise, fibromyalgia, and fibrofog: A pilot study. J Phys Act Health. 2009;6:239–46. doi: 10.1123/jpah.6.2.239. [DOI] [PubMed] [Google Scholar]
- 16.Ambrose KR, Gracely RH, Glass JM. Fibromyalgia dyscognition: Concepts and issues. Reumatismo. 2012;64:206–15. doi: 10.4081/reumatismo.2012.206. [DOI] [PubMed] [Google Scholar]
- 17.Bloem BR, Valkenburg VV, Slabbekoorn M, Willemsen MD. The multiple tasks test: Development and normal strategies. Gait Posture. 2001;14:191–202. doi: 10.1016/s0966-6362(01)00141-2. [DOI] [PubMed] [Google Scholar]
- 18.Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructions on dual-task walking and cognitive task performance in people with parkinson's disease. Parkinsons Dis. 2012;2012:671261. doi: 10.1155/2012/671261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Andreu J, Ibanez-Bosch R, Portero-Vazquez A, Masramon X, Rejas J, Galvez R. Cognitive impairment in patients with fibromyalgia syndrome as assessed by the mini-mental state examination. BMC Musculoskelet Disord. 2009;10:162. doi: 10.1186/1471-2474-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett R. The fibromyalgia impact questionnaire (FIQ): A review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–62. [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. [CES-D] Applied Psychological Measurement. 1977;1:385. [Google Scholar]
- 22.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22:639–43. [PubMed] [Google Scholar]
- 23.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(Suppl 1):S79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 24.Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M. The reliability and validity of qualitative scores for the controlled oral word association test. Arch Clin Neuropsychol. 2007;22:475–88. doi: 10.1016/j.acn.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Kahl C, Cleland J. Visual analogue scale, numeric rating scale and the McGill pain quesitonniare: An overview of psychometric properties. Phys Ther. 2005;10:123. [Google Scholar]
- 26.Grant S, Aitchison T, Henderson E, Christie J, Zare S, McMurray J, et al. A comparison of the reproducibility and the sensitivity to change of visual analogue scales, borg scales, and likert scales in normal subjects during submaximal exercise. Chest. 1999;116:1208–17. doi: 10.1378/chest.116.5.1208. [DOI] [PubMed] [Google Scholar]
- 27.Hayes K, Walton JR, Szomor ZR, Murrell GA. Reliability of five methods for assessing shoulder range of motion. Aust J Physiother. 2001;47:289–94. doi: 10.1016/s0004-9514(14)60274-9. [DOI] [PubMed] [Google Scholar]
- 28.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74. [PubMed] [Google Scholar]
- 29.Jacobsen S, Danneskiold-Samsoe B. Dynamic muscular endurance in primary fibromyalgia compared with chronic myofascial pain syndrome. Arch Phys Med Rehabil. 1992;73:170–3. [PubMed] [Google Scholar]
- 30.Mengshoel AM, Forre O, Komnaes HB. Muscle strength and aerobic capacity in primary fibromyalgia. Clin Exp Rheumatol. 1990;8:475–9. [PubMed] [Google Scholar]
- 31.Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009;10:1099–112. doi: 10.1016/j.jpain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huolman S, Hamalainen P, Vorobyev V, Ruutiainen J, Parkkola R, Laine T, et al. The effects of rivastigmine on processing speed and brain activation in patients with multiple sclerosis and subjective cognitive fatigue. Mult Scler. 2011;17:1351–61. doi: 10.1177/1352458511412061. [DOI] [PubMed] [Google Scholar]
- 33.Johansson B, Berglund P, Ronnback L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 2009;23:1027–40. doi: 10.3109/02699050903421099. [DOI] [PubMed] [Google Scholar]
- 34.Lerdal A, Bakken LN, Kouwenhoven SE, Pedersen G, Kirkevold M, Finset A, et al. Poststroke fatigue--a review. J Pain Symptom Manage. 2009;38:928–49. doi: 10.1016/j.jpainsymman.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 36.Sluka KA, Danielson J, Rasmussen L, DaSilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44:420–7. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–97. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng R, Xu X, Tang Q, Bian D, Li Y, Qian C, et al. Polysaccharide of radix pseudostellariae improves chronic fatigue syndrome induced by poly I:C in mice. Evid Based Complement Alternat Med. 2011;2011:840516. doi: 10.1093/ecam/nep208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord. 2011;12:245, 2474–12-245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White AT, Light AR, Hughen RW, Vanhaitsma TA, Light KC. Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosom Med. 2012;74:46–54. doi: 10.1097/PSY.0b013e31824152ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantor JB, Ashman T, Gordon W, Ginsberg A, Engmann C, Egan M, et al. Fatigue after traumatic brain injury and its impact on participation and quality of life. J Head Trauma Rehabil. 2008;23:41–51. doi: 10.1097/01.HTR.0000308720.70288.af. [DOI] [PubMed] [Google Scholar]
- 43.Cantor F. Central and peripheral fatigue: Exemplified by multiple sclerosis and myasthenia gravis. PM R. 2010;2:399–405. doi: 10.1016/j.pmrj.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Goes SM, Leite N, Shay BL, Homann D, Stefanello JM, Rodacki AL. Functional capacity, muscle strength and falls in women with fibromyalgia. Clin Biomech (Bristol, Avon) 2012;27:578–83. doi: 10.1016/j.clinbiomech.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: An investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler. 2009;15:1215–27. doi: 10.1177/1352458509106712. [DOI] [PubMed] [Google Scholar]
- 46.Claros-Salinas D, Dittmer N, Neumann M, Sehle A, Spiteri S, Willmes K, et al. Induction of cognitive fatigue in MS patients through cognitive and physical load. Neuropsychol Rehabil. 2013;23:182–201. doi: 10.1080/09602011.2012.726925. [DOI] [PubMed] [Google Scholar]
- 47.Clanton NR, Klosky JL, Li C, Jain N, Srivastava DK, Mulrooney D, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the childhood cancer survivor study. Cancer. 2011 doi: 10.1002/cncr.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grace GM, Nielson WR, Hopkins M, Berg MA. Concentration and memory deficits in patients with fibromyalgia syndrome. J Clin Exp Neuropsychol. 1999;21:477–87. doi: 10.1076/jcen.21.4.477.876. [DOI] [PubMed] [Google Scholar]
- 49.Sletvold H, Stiles TC, Landro NI. Information processing in primary fibromyalgia, major depression and healthy controls. J Rheumatol. 1995;22:137–42. [PubMed] [Google Scholar]
- 50.Finan PH, Zautra AJ. Fibromyalgia and fatigue: Central processing, widespread dysfunction. PM R. 2010;2:431–7. doi: 10.1016/j.pmrj.2010.03.021. [DOI] [PubMed] [Google Scholar]


